Cutaneous metastases are relatively rare in clinical practice and their diagnosis requires a high index of suspicion because clinical findings can be subtle. These metastases reveal the presence of disseminated malignant disease and can lead to the diagnosis of unsuspected internal tumors or the spread or recurrence of an already diagnosed tumor. Early recognition of cutaneous metastases can facilitate prompt and accurate diagnosis resulting in early treatment; however, they are generally indicative of a poor prognosis. Some tumors have a predilection to metastasize to specific areas. Recognition of these patterns provides essential information that can guide the search for the underlying tumor.
Las metástasis cutáneas son relativamente raras en la práctica clínica. Su diagnóstico requiere un alto índice de sospecha, pues los hallazgos clínicos pueden ser sutiles. Las metástasis cutáneas ponen de manifiesto la presencia de un tumor maligno diseminado y pueden permitir el diagnóstico de neoplasias internas no conocida o indicar la diseminación o recurrencia de otras ya diagnosticadas. Su reconocimiento temprano puede llevar a un diagnóstico preciso y rápido, con el consiguiente tratamiento oportuno, aunque en la mayoría de los casos son indicativas de un pronóstico infausto. Algunos tumores tienen predilección por metastatizar en áreas específicas. El reconocimiento de esos patrones es esencial para dirigir la búsqueda del tumor subyacente.
The presence of metastases is one of the characteristics of malignant tumors that is a threat to the life of the patient and incontrovertibly signals the existence of a systemic disease.1 Considerable advances have been made in recent years in our understanding of how tumor cells circulating in the blood and in the lymphatic system are able to interact with and pass through the endothelium to reach distant sites, and of the properties that determine whether the cells of these disseminated tumors are able to survive and whether they will remain in a latent state or will be able to form macrometastases.2 New discoveries concerning early metastatic seeding, parallel progression, self-seeding of circulating tumor cells from the primary tumor, and the induction of premetastatic niches in organs at a distance from the primary tumor are now at the forefront of research.3
Skin metastases (SMs) are the result of infiltration of the skin by proliferations of cells from distant malignant tumors.4,5 The early detection of metastases within the body often requires sophisticated additional tests; however, SMs are usually easily observed on careful, targeted physical examination. Up to a third of SMs are diagnosed before or simultaneously with the primary tumor, and the role of the dermatologist in establishing an adequate clinical suspicion6,7 is essential.8 The early clinical recognition of SMs is crucial, as it can lead to the diagnosis of a previously unidentified primary malignant tumor, provide evidence of the dissemination of a previously known tumor, or be an early sign of recurrence of a malignant tumor apparently in remission. The diagnosis of SMs can therefore alter the staging of a malignant disease, with the consequent therapeutic and prognostic implications9; their presence will often lead to drastic changes in the management plan, particularly when the metastases indicate the persistence of a tumor thought to be in remission.10 Furthermore, SMs are easy to biopsy, which facilitates tests of sensitivity of the primary tumor to specific treatments, such as inhibitors of epidermal growth factor or of c-kit/CD117.11
Some tumors appear to have a predilection to metastasize to certain areas. Recognition of these patterns can help to target the search for an unknown underlying tumor.12
The recent presentation in various countries of a number of retrospective studies of SMs reflects current international interest in this subject.13
Etiology and PathogenesisMetastases arise when neoplastic cells break away from a primary tumor and disseminate to other sites.14,15 Several mechanisms involving various pathways are implicated in the development of metastases.16,17 Hematogenous and lymphatic spread are the most common, although separation of these 2 pathways can be difficult as they are interconnected. Lymphatic spread is the most common initial route of propagation of the majority of malignant tumors and its role in the determination of metastatic patterns is a subject of current research.18 Regional spread usually occurs through the body cavities, in particular the peritoneal cavity. Tumor-cell transplant due to the mechanical transport of fragments of tumor on surgical instruments during surgery or other invasive procedures can happen but is rare.19,20
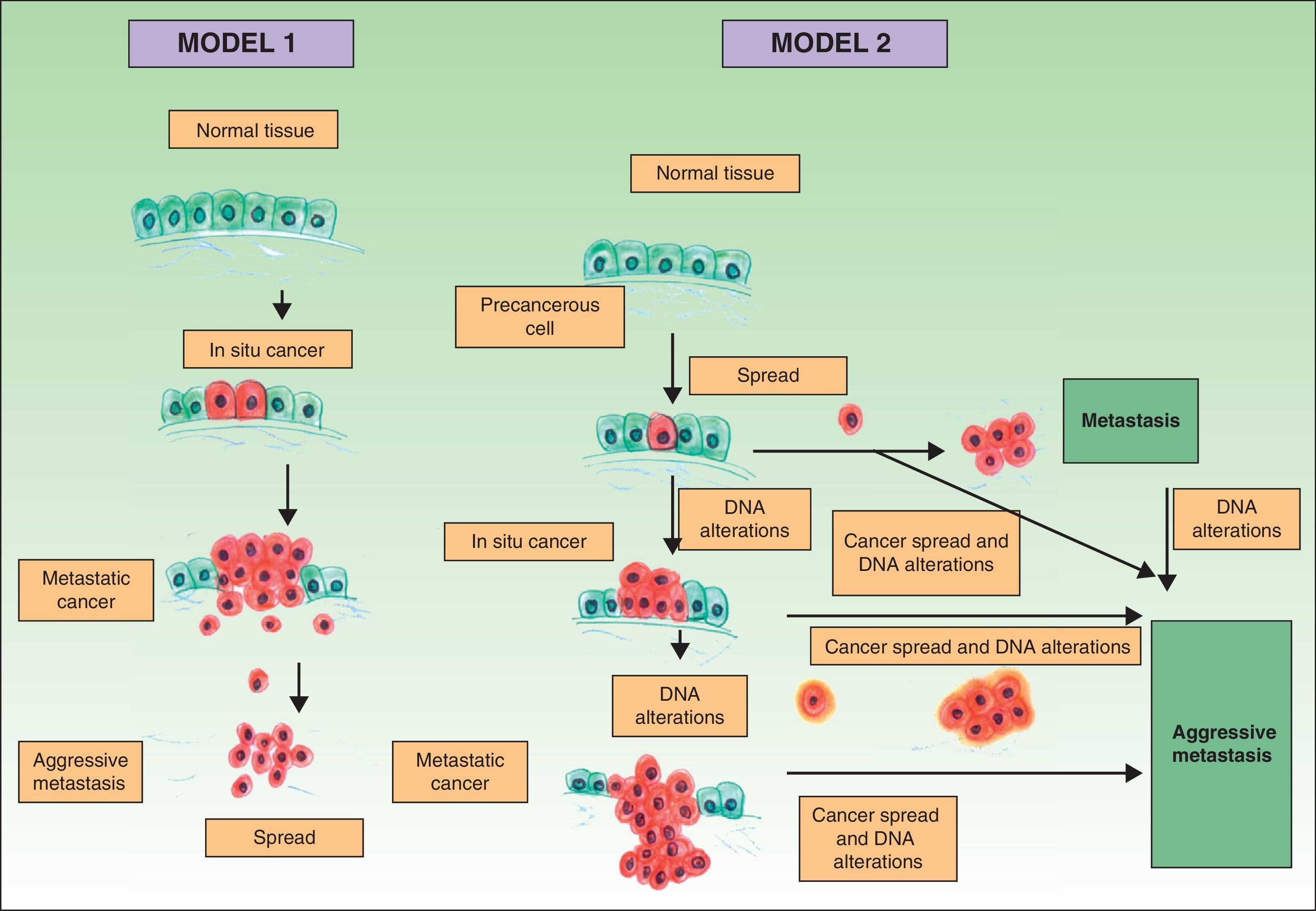
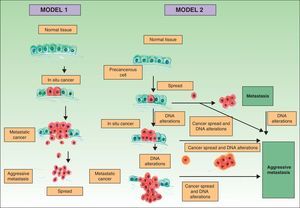
Traditionally it has been postulated that a series of steps must occur for a metastasis to develop. First, the primary tumor must be large enough to release a sufficient number of neoplastic cells into the circulatory or lymphatic systems. The majority of free neoplastic cells are destroyed by the immune system, whereas groups of 6 or 7 cells appear to have a greater likelihood of forming a metastasis.21 These cells, in turn, must have certain properties, such as cellular suspension and an adequate mitotic index, in order to survive.22 Development of a clone with metastatic potential is initially favored by the activation of specific oncogenes23,24 and the loss of tumor suppressor genes.25,26 For neoplastic cells in the circulatory system to become established, they must pass through the vessel walls. After the cells adhere to the vessel wall, a thrombus forms around them due to a lesion of the endothelial cells. This thrombus serves to protect the neoplastic cells. The metastasis becomes established and initially obtains its nutrients by means of diffusion phenomena27; later it will form its own vessels (angiogenesis).28,29 In this classical model of cancer development, the metastases correspond to the final stage of the metastatic cascade. However, recent studies suggest a different model, which predicts that the expression of proteins that regulate epithelial-mesenchymal transition promote oncogenesis concomitantly with metastatic spread. In this alternative model, cell spread from the primary tumor can occur at any time during development of the cancer30 (Fig. 1).
Pathogenesis of skin metastases. Diagram that compares the classical model of metastasis production with the most recent hypotheses. Source: Sánchez-García I.30
The true incidence of SMs is unknown. However, the incidence appears to be higher in some recent studies compared with historical series, although this may due to higher rates of biopsy and diagnosis rather than a true increase in the incidence.31 SMs are a rare finding in clinical practice, and their prevalence varies between 0.7% and 9% of patients with internal tumors, depending on the series.32
In theory, any malignant tumor can spread to the skin. However, in practice, a direct relationship has been found between the frequency of different malignant tumors and the appearance of SMs, with the most common malignant tumors in each sex being those that most frequently give rise to SMs. Thus, breast cancer in women, lung cancer in men, and adenocarcinomas of the digestive tract in both sexes are the most common origins of SMs.33
A meta-analysis published in 2003 reviewed 1080 cases of SM in 20 380 cancer patients, and reported an estimated SM rate of 5.3%.34 In a classic study from 1972, Brownstein and Helwig12 examined the distribution of SMs in 724 male and female patients. In men, the most common malignant tumors that metastasized to the skin were carcinoma of the lung (24%), colorectal carcinoma (19%), melanoma (13%), and oral squamous cell carcinoma (12%), whereas, in women, the most common tumors were breast cancer (69%), colorectal carcinoma (9%), melanoma (5%), and carcinoma of the ovary (4%). The area most commonly affected was the anterior chest wall; the lower limbs were the least common sites. In men, around 75% of SMs were observed on the head and neck, whereas, in women, 75% of cases were found on the anterior chest wall and on the abdomen. In general, the back was an uncommon site for SMs.35
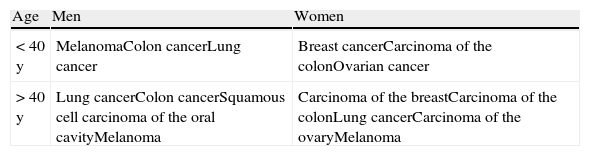
In women the most common site of SMs is the thorax, followed by the abdomen, the back, the upper limbs, the scalp, and the neck; in men, the thorax is also the area most commonly affected, followed, in descending order of frequency, by the abdomen, the back, the scalp, the neck, the face, the upper and lower limbs, and the pelvis. The frequency of SMs by age and sex is summarized in Table 1. SMs are very rare in children. The most common primary tumors that produce SMs in this age group are rhabdomyosarcoma and neuroblastoma.36
Frequency of Skin Metastases According to Age and Sex (in Decreasing Frequency).
| Age | Men | Women |
| < 40 y | MelanomaColon cancerLung cancer | Breast cancerCarcinoma of the colonOvarian cancer |
| > 40 y | Lung cancerColon cancerSquamous cell carcinoma of the oral cavityMelanoma | Carcinoma of the breastCarcinoma of the colonLung cancerCarcinoma of the ovaryMelanoma |
In the majority of cases, SMs develop after diagnosis of the primary tumor. However, in a notable proportion of patients (up to a third of cases), the metastases are discovered prior to37 or simultaneously with38 the primary tumor.
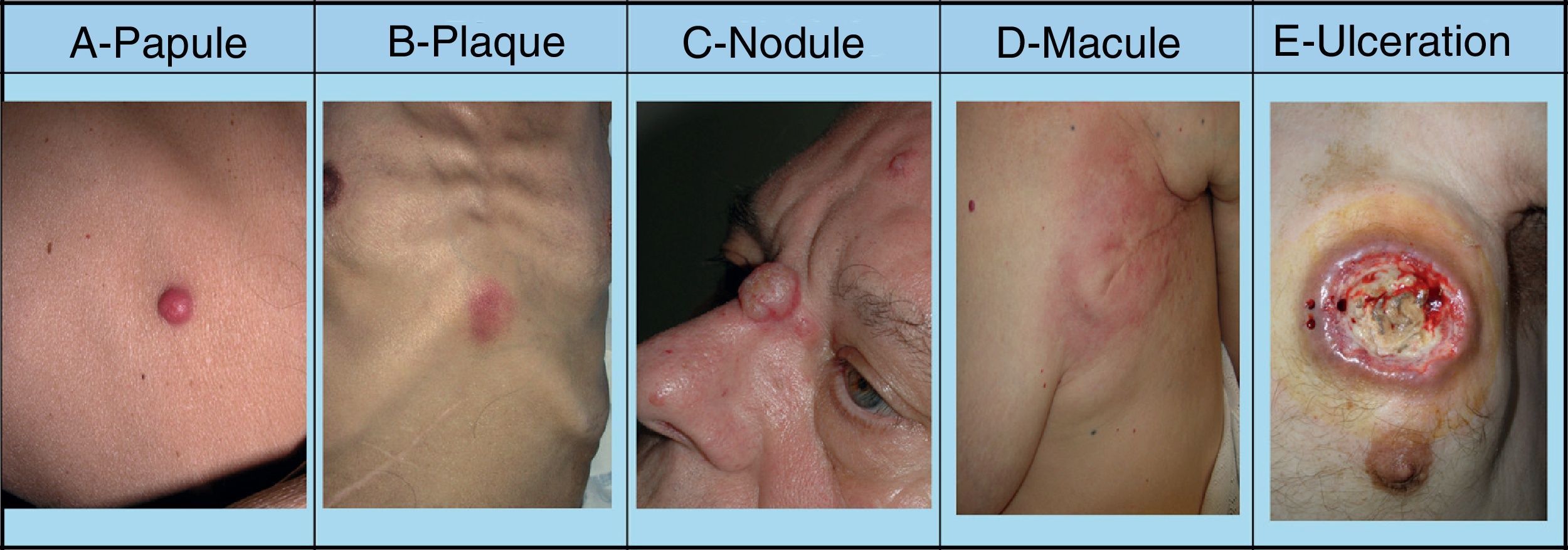
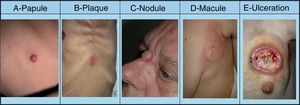
SMs tend to appear in a body region close to the primary tumor. Presentation is typically in the form of rapidly growing, mobile, round or oval nodules of firm or elastic consistency39; they may be ulcerated.40 However, they can present as any primary or secondary skin lesion41 (Fig. 2). The nodules are usually skin-colored, although metastatic nodules from renal cell or thyroid carcinoma often have a characteristic reddish or violaceous discoloration42,43 (Fig. 3). Some recent studies report that skin metastases present most often as a single nodular lesion, whereas older studies more commonly reported multiple nodules.44 This may be because more SMs are now diagnosed earlier.
Skin metastases can manifest as primary or secondary skin lesions. A, Metastasis from a gastric adenocarcinoma. Papule of 7mm diameter on the abdomen. B, Metastasis from an adenocarcinoma of the ovary presenting as a plaque. C, Metastasis from a urothelial carcinoma of the bladder presenting as a nodule. D, Metastasis from an adenocarcinoma of the breast. The lesion started as a macule over the mastectomy scar. E, Ulcerated infiltrated plaque corresponding to a metastasis from an adenocarcinoma of the lung.
Although most SMs are asymptomatic, patients may describe pain, particularly with lesions in certain areas, such as subungual metastases.45
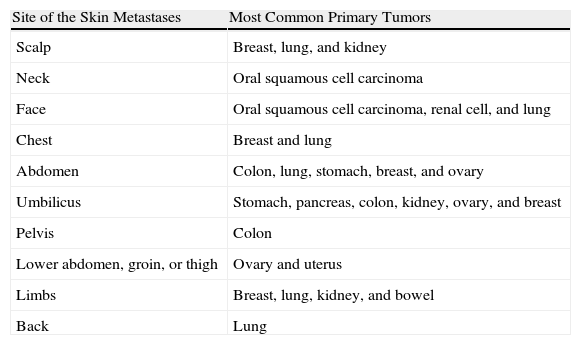
Gastrointestinal cancers (specifically, colorectal and gastric carcinomas) often give rise to metastases on the abdomen and pelvis. These carcinomas can spread along the urachus to produce umbilical nodules known as Sister Mary Joseph's nodules.46 SMs from squamous cell carcinoma of the oral cavity usually arise in the same body region, and most frequently affect the face and neck. Renal cell carcinoma, among others, typically metastasizes to the scalp and, because it is a highly vascular tumour, the lesions may be confused with hemangiomas or pyogenic granulomas. SMs from hepatocellular carcinoma often develop on the fingers, palms, soles, or the back, while those from gastric carcinoma tend to develop on the head and neck.47 Thus, the site of an SM can suggest a possible origin (Table 2).
Most Common Primary Tumors Giving Rise to Skin Metastases According to the Site of the Metastases.
| Site of the Skin Metastases | Most Common Primary Tumors |
| Scalp | Breast, lung, and kidney |
| Neck | Oral squamous cell carcinoma |
| Face | Oral squamous cell carcinoma, renal cell, and lung |
| Chest | Breast and lung |
| Abdomen | Colon, lung, stomach, breast, and ovary |
| Umbilicus | Stomach, pancreas, colon, kidney, ovary, and breast |
| Pelvis | Colon |
| Lower abdomen, groin, or thigh | Ovary and uterus |
| Limbs | Breast, lung, kidney, and bowel |
| Back | Lung |
A very large number of specific clinical forms of SMs have been described. Erysipeloid or inflammatory carcinoma is often observed in patients with adenocarcinoma of the breast and is not uncommon in other types of cancer (pancreas, rectum, lung, ovary, and parotid).48,49 It presents as well-defined erythematous lesions that are hot and tender, similar to erysipelas.50 Lymphatic obstruction by the neoplastic cells can give rise to localized lymphedema with peau d’orange. Lymphedema can cause the skin to become fibrous and yellowish, with a similar appearance to carcinoma en cuirasse51 or scirrhous carcinoma. This clinical form presents as indurated erythematous plaques that infiltrate the chest wall. It is usually observed in metastases from breast cancer, although it can be a form of presentation of a primary breast tumor or of SMs of other origins.52 The main differential diagnosis of this presentation is with infectious disorders. Erysipeloid carcinoma localized to the breast can be difficult to distinguish from mastitis. Persistent inflammation that does not respond to conventional therapy must therefore be carefully evaluated to exclude metastatic infiltration, particularly when there is no fever or leukocytosis.53
Telangiectatic carcinoma, described by Weber54 in 1933 in a patient with metastatic breast cancer, is characterized by the appearance of nodules, papules, or purpuric plaques on the chest wall,55 usually in the proximity of a surgical scar.56,57
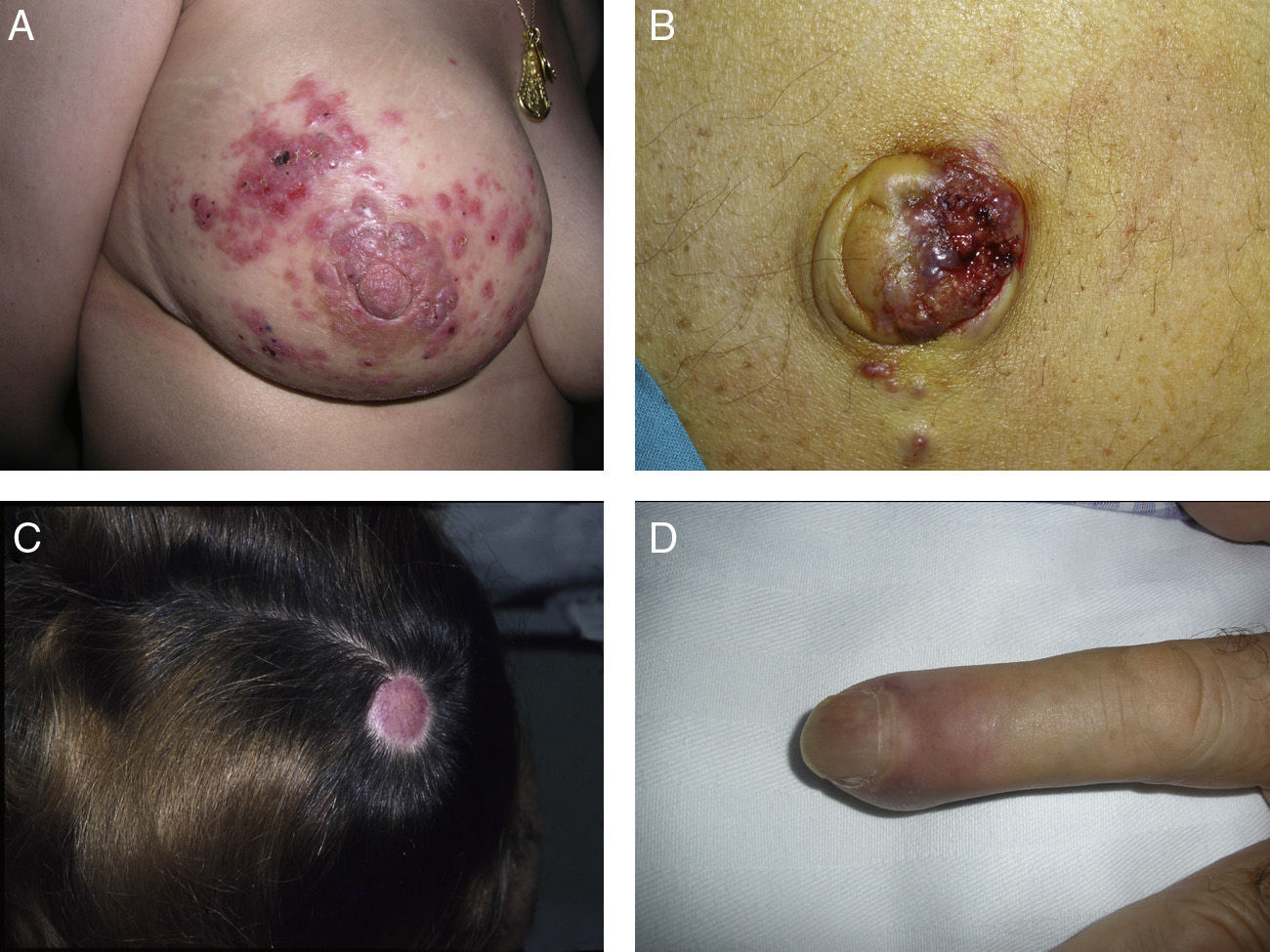
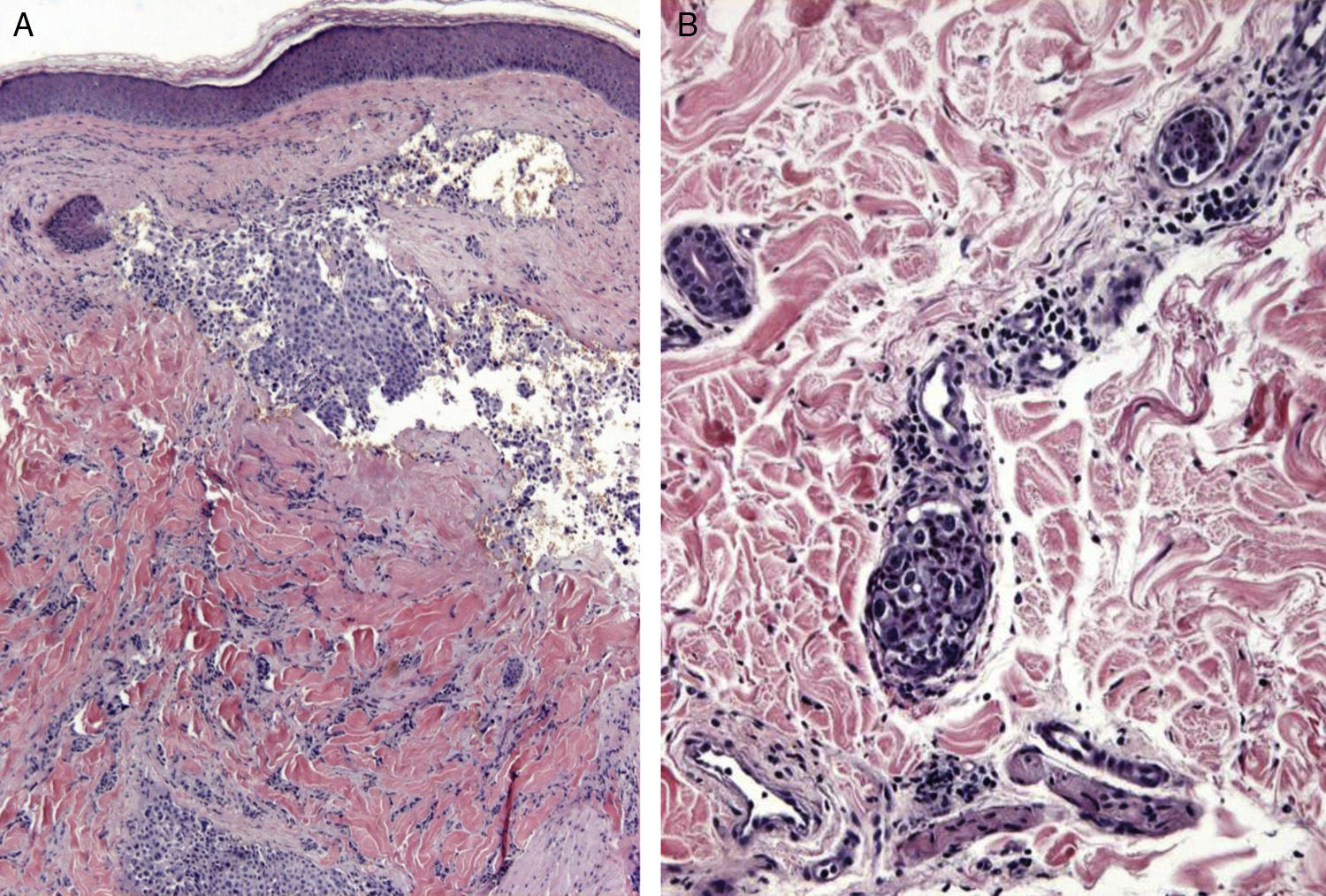
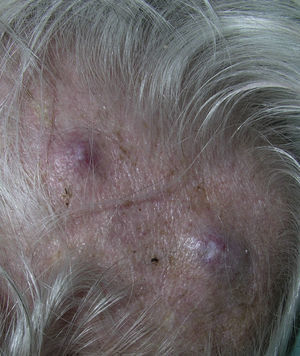
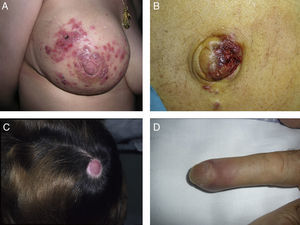
Zosteriform or herpetiform metastases present as papules, vesicles, nodules, or blisters affecting individual dermatomes,58,59 mimicking herpes zoster60,61 (Fig. 4). Although their etiology and pathogenesis are not fully understood, most hypotheses suggest there is spread of the tumor cells from the cutaneous lymph vessels to the sensory nerves, via which they reach the dorsal root ganglia.62,63
Unusual skin metastases. A, Zosteriform metastases from a ductal adenocarcinoma of the breast. Clusters of infiltrated papules, some confluent and some ulcerated, in a metameric distribution. B, Umbilical metastasis (Sister Mary Joseph's nodule); multiple, confluent, ulcerated papules and nodules in the umbilical and periumbilical regions. C, Alopecia neoplastica. Plaque of alopecia over a metastasis from a ductal adenocarcinoma of the breast in a woman. D. Subungual metastasis. Metastasis from a squamous cell carcinoma of the lung presenting as a painful inflammatory nodule that deformed the distal phalanx of the second finger of the left hand.
Clown nose is considered to be the result of an SM arising on the tip of the nose, typically from a carcinoma of the lung or breast.64
Alopecia neoplastica is defined as hair loss secondary to invasion of the scalp by malignant cells. It can present as 1 or more plaques of scarring alopecia that are often indurated and have a bluish or violaceous color. These plaques must be differentiated from alopecia areata65 (Fig. 4). The neoplastic cells can destroy the hair follicles by producing fibroplasia induced by the release of inflammatory mediators that bring inflammatory cells into the area, and/or by substitution of the normal cells.66 Breast cancer is the underlying primary malignant tumor in 84% of patients with alopecia neoplastica.67
Paget's disease of the nipple and areola is a skin sign associated with an underlying adenocarcinoma of the breast in 100% of cases.68 This skin condition is produced by epidermotropic SMs due to the spread of an intraductal tumor along the lactiferous ducts to reach the overlying skin.
Subungual metastases merit special consideration. These metastatic lesions are usually painful and often need to be differentiated from infectious disorders, in particular acute paronychia, or from glomus tumors69 (Fig. 4). Presentation as painless dactylitis has been reported.70
Sister Mary Joseph's nodule or umbilical metastasis is widely described in the literature. This form of metastasis presents as single or multiple, indurated umbilical or periumbilical nodules that can sometimes ulcerate or have a friable appearance (Fig. 4). Although the site of the primary tumor is unknown in approximately 29% of cases,71 the majority of umbilical metastases come from tumors that arise in the stomach, ovary, colon, rectum, or pancreas.72 The proposed pathogenesis of metastases in the umbilical region includes both contiguous infiltration and spread via the blood or lymph.73
Finally, clinically occult metastases are those with no detectable clinical findings but which are discovered as incidental findings on a histopathological examination performed for some other reason.74
Differential DiagnosisThe differential diagnosis of SMs is very broad and includes many more disorders in addition to those mentioned above. First we must consider primary skin tumors,75 both benign (dermatofibroma, pyogenic granuloma,76 epidermal cyst,77 adnexal tumors78) and malignant (basal or squamous cell carcinoma, melanoma, Merkel cell tumor, angiosarcoma79). Other skin diseases to be considered are eczema, erythema annulare centrifugum,80 erythema multiforme [target-shaped SMs], and vasculitis.81
DiagnosisA detailed medical history and complete physical examination are essential to establish the initial diagnostic suspicion.
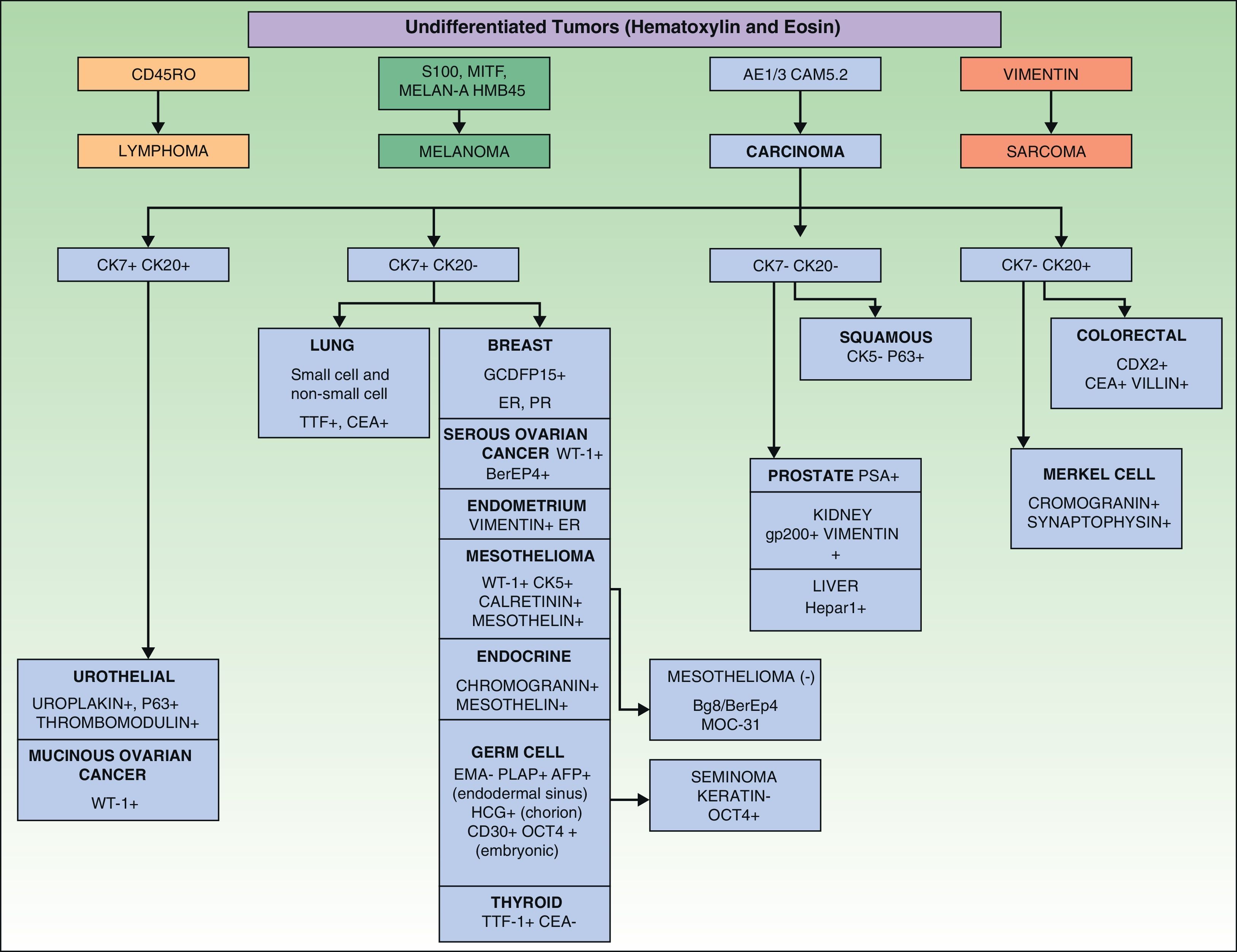
The histopathology of SMs may reveal the same characteristics as the tumor of origin, or there may be a more anaplastic appearance. Immunohistochemistry can help to determine the possible origin in the case of undifferentiated tumors. Incisional or excisional skin biopsy is essential to reach a diagnosis. Cytology study by fine-needle aspiration biopsy can be useful in certain circumstances.82,83 The pattern observed and the microscopic appearance of the tissue often suggest its origin.84
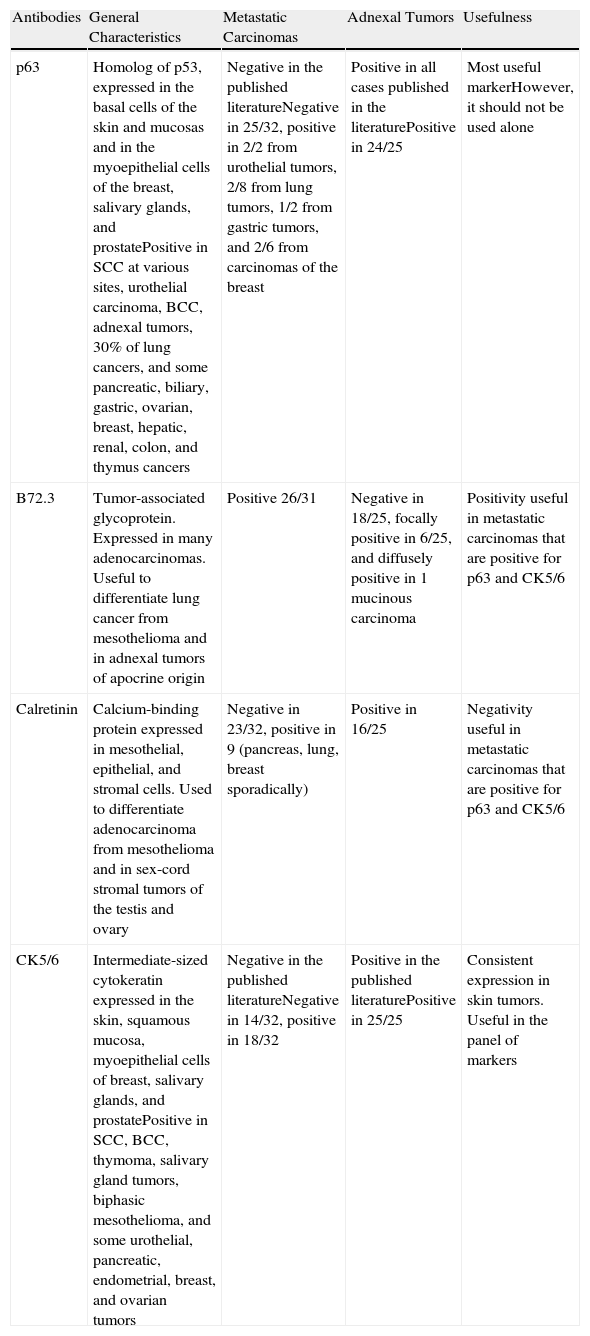
In some cases, such as renal cell carcinoma, characteristic histological findings will identify the primary tumor, but the majority of SMs can only be classified in general terms as adenocarcinoma, squamous cell carcinoma, or undifferentiated carcinoma. It is also necessary to differentiate between metastatic skin lesions and primary skin tumors; as these 2 types of lesion frequently have very similar morphological patterns, immunohistochemical markers can be very useful for their differentiation. Table 3 summarizes the results of the literature review published by Sarya et al.85 in 2007.
Summary of the Immunohistochemical Markers Useful for the Differentiation Between Skin Metastases and Primary Skin Tumors, and Synopsis of the Data Obtained in the Series by Sarya et al.
| Antibodies | General Characteristics | Metastatic Carcinomas | Adnexal Tumors | Usefulness |
| p63 | Homolog of p53, expressed in the basal cells of the skin and mucosas and in the myoepithelial cells of the breast, salivary glands, and prostatePositive in SCC at various sites, urothelial carcinoma, BCC, adnexal tumors, 30% of lung cancers, and some pancreatic, biliary, gastric, ovarian, breast, hepatic, renal, colon, and thymus cancers | Negative in the published literatureNegative in 25/32, positive in 2/2 from urothelial tumors, 2/8 from lung tumors, 1/2 from gastric tumors, and 2/6 from carcinomas of the breast | Positive in all cases published in the literaturePositive in 24/25 | Most useful markerHowever, it should not be used alone |
| B72.3 | Tumor-associated glycoprotein. Expressed in many adenocarcinomas. Useful to differentiate lung cancer from mesothelioma and in adnexal tumors of apocrine origin | Positive 26/31 | Negative in 18/25, focally positive in 6/25, and diffusely positive in 1 mucinous carcinoma | Positivity useful in metastatic carcinomas that are positive for p63 and CK5/6 |
| Calretinin | Calcium-binding protein expressed in mesothelial, epithelial, and stromal cells. Used to differentiate adenocarcinoma from mesothelioma and in sex-cord stromal tumors of the testis and ovary | Negative in 23/32, positive in 9 (pancreas, lung, breast sporadically) | Positive in 16/25 | Negativity useful in metastatic carcinomas that are positive for p63 and CK5/6 |
| CK5/6 | Intermediate-sized cytokeratin expressed in the skin, squamous mucosa, myoepithelial cells of breast, salivary glands, and prostatePositive in SCC, BCC, thymoma, salivary gland tumors, biphasic mesothelioma, and some urothelial, pancreatic, endometrial, breast, and ovarian tumors | Negative in the published literatureNegative in 14/32, positive in 18/32 | Positive in the published literaturePositive in 25/25 | Consistent expression in skin tumors. Useful in the panel of markers |
Source: Sarya et al.85 Abbreviations: BCC, basal cell carcinoma; SCC, squamous cell carcinoma.
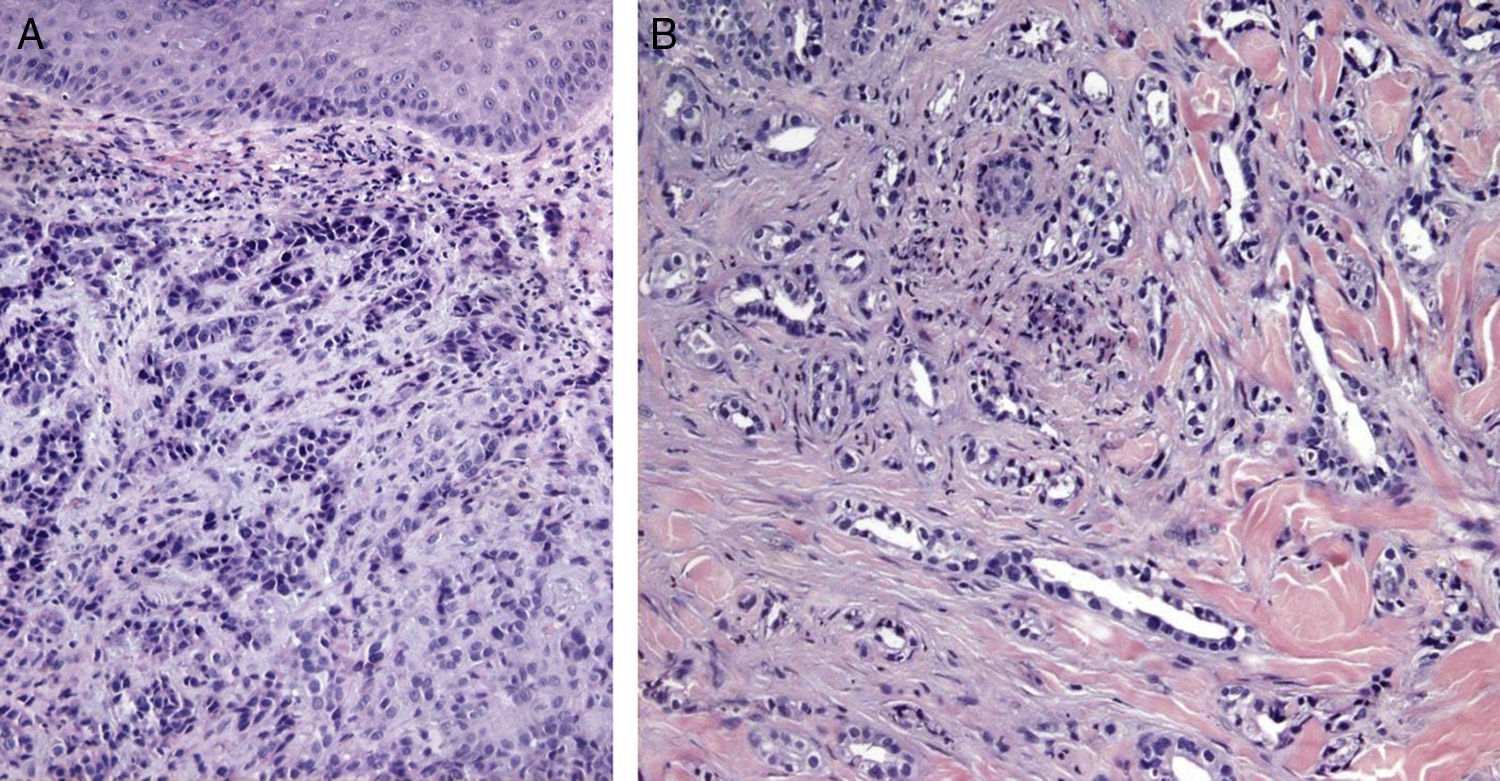
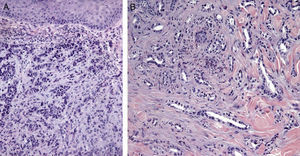
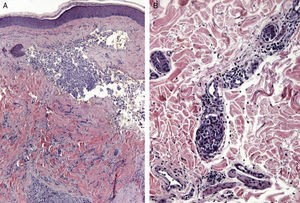
Certain histological features differentiate metastases from primary tumors. Characteristics suggestive of SMs include the presence of neoplastic cells within the lymph and blood vessels, localization of the lesion in the deep reticular dermis and hypodermis, and the presence of neoplastic cells running along the bundles of collagen.86 Metastatic tumors typically develop as round nodules located in the dermis or hypodermis, and are not usually in contact with the epidermis. This Grenz zone is much more common in metastatic lesions. Fibrosis and inflammation may be evident (Figs. 5 and 6).
Histological images of skin metastases. A, Dermal metastasis from a poorly differentiated carcinoma of unknown origin. Infiltration of the dermis by cords and nests of epithelioid cells (hematoxylin-eosin, original magnification x10). B, Dermal metastasis from a moderately differentiated adenocarcinoma of the pancreas. Glandular lumens lined by a layer of epithelial cells can be seen between the dermal collagen bundles (hematoxylin-eosin, original magnification x10).
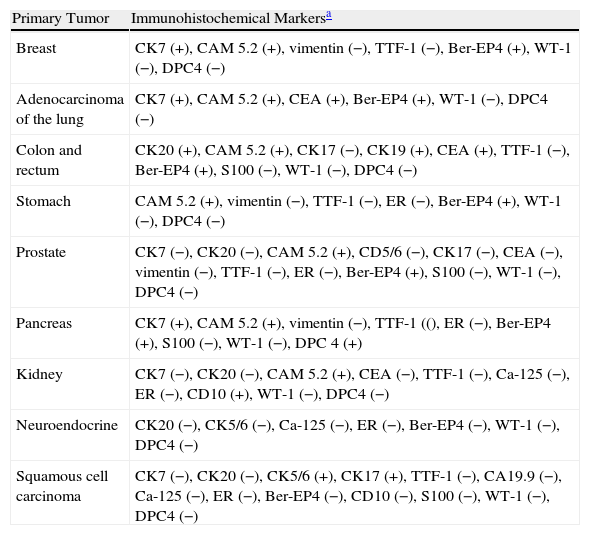
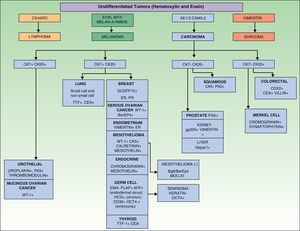
Immunohistochemical markers and, sometimes, ultrastructural studies are valuable tools for establishing the origin of SMs.87Fig. 7 shows the diagnostic algorithm that should be applied for undifferentiated SMs. The basic recommended battery of markers includes CD45 (for lymphoid tumors), pancytokeratin AE1/AE3 (for the majority of carcinomas), S100 (for melanoma) and CD34 (for vascular tumors and leukemia). The second recommended battery of markers includes lymphoid markers (CD3 and CD20), epithelial markers such as epithelial membrane antigen and carcinoembryonic antigen, chromogranin (neuroendocrine tumors), prostate specific antigen and acid phosphatase (carcinoma of the prostate), thyroid transcription factor (carcinoma of the lung), Wilms’ tumor protein (carcinoma of the ovary), CDX288 (intestinal carcinomas), and Hep Par189 (hepatocellular carcinoma).90 The immunophenotypes of the main tumors that metastasize to the skin are summarized in Table 4.
Immunophenotypes of the Metastases of the Principal Carcinomas.
| Primary Tumor | Immunohistochemical Markersa |
| Breast | CK7 (+), CAM 5.2 (+), vimentin (−), TTF-1 (−), Ber-EP4 (+), WT-1 (−), DPC4 (−) |
| Adenocarcinoma of the lung | CK7 (+), CAM 5.2 (+), CEA (+), Ber-EP4 (+), WT-1 (−), DPC4 (−) |
| Colon and rectum | CK20 (+), CAM 5.2 (+), CK17 (−), CK19 (+), CEA (+), TTF-1 (−), Ber-EP4 (+), S100 (−), WT-1 (−), DPC4 (−) |
| Stomach | CAM 5.2 (+), vimentin (−), TTF-1 (−), ER (−), Ber-EP4 (+), WT-1 (−), DPC4 (−) |
| Prostate | CK7 (−), CK20 (−), CAM 5.2 (+), CD5/6 (−), CK17 (−), CEA (−), vimentin (−), TTF-1 (−), ER (−), Ber-EP4 (+), S100 (−), WT-1 (−), DPC4 (−) |
| Pancreas | CK7 (+), CAM 5.2 (+), vimentin (−), TTF-1 ((), ER (−), Ber-EP4 (+), S100 (−), WT-1 (−), DPC 4 (+) |
| Kidney | CK7 (−), CK20 (−), CAM 5.2 (+), CEA (−), TTF-1 (−), Ca-125 (−), ER (−), CD10 (+), WT-1 (−), DPC4 (−) |
| Neuroendocrine | CK20 (−), CK5/6 (−), Ca-125 (−), ER (−), Ber-EP4 (−), WT-1 (−), DPC4 (−) |
| Squamous cell carcinoma | CK7 (−), CK20 (−), CK5/6 (+), CK17 (+), TTF-1 (−), CA19.9 (−), Ca-125 (−), ER (−), Ber-EP4 (−), CD10 (−), S100 (−), WT-1 (−), DPC4 (−) |
Ultrastructural studies can be useful in the identification of certain undifferentiated tumors. Desmosomes may be observed in carcinomas, cytoplasmic vacuoles in adenocarcinomas, melanosomes in melanomas, and neurosecretory granules in neuroendocrine tumors. However, ultrastructural studies are long and costly techniques that are not available in the majority of centers and require highly specialized staff; immunohistochemical studies therefore tend to be more useful in clinical practice.
Positron emission tomography (PET) gives a large number of false positives.91 PET-computed tomography is more useful.92 However, PET may understage tumors in some cases, and small metastases or cerebral lesions may be missed.93
PrognosisThe presence of metastatic disease in the skin usually implies widespread systemic disease with a high mortality, although the prognosis varies considerably depending on the type of primary tumor.94,95 SMs in the absence of other distant metastases only occur in 6.4% to 7.8% of cases.96 However, it would appear that recent advances in chemotherapy have considerably increased survival.97
It is estimated that mean survival after the diagnosis of SMs is 50% at 6 months. A number of survival analyses of patients with SMs have been published, those of Benmously et al.98 and Schoenlaub et al.97 being particularly relevant All the analyses report a better survival in cases of breast cancer than in other types of cancer.99,100
The mean interval between the diagnosis of a primary tumor and the appearance of SMs varies between 2 and 3 years, but periods of up to 22 years have been reported. Recent studies indicate that this interval varies depending on the type of primary tumor.101,102 In 141 patients analyzed by Hu et al.,103 the mean interval between the excision or treatment of the primary tumor and the appearance of SMs was longer in patients with breast cancer (47.2 months) than in those with other tumors, such as lung cancer (15.7 months), colorectal cancer (16.5 months), or stomach cancer (19.8 months). According to some studies, lung cancer is the tumor that most rapidly metastasizes to the skin.104,105
TreatmentThe therapeutic approach to SMs is based on appropriate management of the primary tumor, if it has been identified.106 As the presence of SMs implies the coexistence of other metastatic lesions in most cases, chemotherapy directed against the tumor of origin is usually the only option that might achieve complete remission.107 Surgery and radiation therapy are often used to treat SMs due to the ease of access to the majority of these lesions; however, no clear increase in survival has been demonstrated, and the aim of such treatments is often only palliative.108 It has been suggested that surgery might improve survival in cases of SM from lung cancer109 or gastric cancer.110,111 Radiation therapy has achieved complete responses and lasting palliation in some cases of metastases from renal cell carcinoma.112 In 1 study, pulsed brachytherapy achieved local control in 41 (89%) of 46 cases of SMs from breast cancer.113 The topical application of 6% miltefosine solution to SMs from carcinoma of the breast achieved good control of the SM in comparison with placebo in a randomized study.114 In another study, 10 patients with SM from breast or colon cancer were treated with intratumoral injections of recombinant single-chain antibodies targeted to ErbB2/HER2, with complete remission in 4 cases.115
The results after intralesional immunotherapy with interferon alfa or interleukin 2116 have been variable.117,118 Reports of metastatic melanoma treated with imiquimod have also been published.119
Other locally ablative procedures, such as electrochemotherapy,120 electrocoagulation, electroporation, and electrovaporization,121 have also been used successfully. In particular, electrochemotherapy with bleomycin is an option in multiple skin and subcutaneous metastases. One study of electrochemotherapy with bleomycin included 174 tumor nodules in 52 patients with breast cancer122; complete responses were observed in 80% and partial remission in 20% after repeated application. Electrochemotherapy with cisplatin has also been evaluated in the treatment of SMs from breast cancer, but was less successful.123
No specific chemotherapy has been shown to be the most effective for the systemic treatment of SMs. Observations of SMs that have regressed after systemic chemotherapy are limited to case reports, such as a patient with SMs from cancer of the pancreas treated with gemcitabine,124 and a patient with urothelial carcinoma of the bladder treated with cyclophosphamide, methotrexate, and 5-fluorouracil.125 Case reports of SMs from carcinomas of unknown origin treated with cisplatin, gemcitabine, vinorelbine, and paclitaxel have also been published.126,127 Determination of the expression of certain molecules such as EGF, Her-2/neu kinase, and c-kit tyrosine kinase is very important as it will provide possible targets for systemic therapy. In addition, drugs targeted against stromal function and angiogenesis could prove useful.128The palliative treatment of SMs includes adequate management of the pain, pruritus, possible bacterial superinfection, and, in some cases, of the unpleasant smell that these lesions can produce.129–132
ConclusionsThe majority of tumor recurrences are diagnosed on the basis of a detailed medical history and complete physical examination, combined with the relevant imaging studies. As SMs are usually asymptomatic, active screening is essential.
SMs can play a crucial role in alerting the physician to a possible tumor recurrence or may even be the first sign of previously unknown tumor. The site and distribution of the skin lesions can suggest the organ of origin.
Histological and immunohistochemical study of SMs is required in order to identify the primary tumor.
Early detection of SMs is essential in order to start appropriate treatment. Although strong scientific evidence of a survival benefit is lacking, case reports and certain preliminary publications would suggest that early diagnosis improves life expectancy.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We would like to thank Dr Concepción Román Curto, author of the doctoral thesis “Tumores cutaneous metastásicos. Estudio clinic, histológico y ultraestructural” for her help and Dr Jesús Millán Núñez-Cortés, Professor of Medicine, Hospital General Universitario Gregorio Marañón, Facultad de Medicina, Universidad Complutense, in Madrid, Spain, for his inestimable collaboration.
Please cite this article as: Fernández-Antón Martínez MC, Parra-Blanco V, Avilés Izquierdo JA, Suárez Fernández RM. Metástasis cutáneas de origen visceral. Actas Dermosifiliogr. 2013;104:841–853.
This review is part of the introduction to the doctoral thesis entitled Metástasis cutáneas: estudio descriptivo clínico-histopatológico de las metástasis cutáneas de neoplasias viscerales.