The diagnosis of Neurofibromatosis type 1 (NF1) is usually delayed in children without a family history. We aimed to define the prevalence and characteristics of prevalent skin manifestations in NF1 compared to the general population, which continue to be excluded from the diagnostic criteria for NF1.
Patients and methodsCase–control study, matched by age groups, in which 108 patients with a diagnosis of NF1 and 137 healthy controls were included.
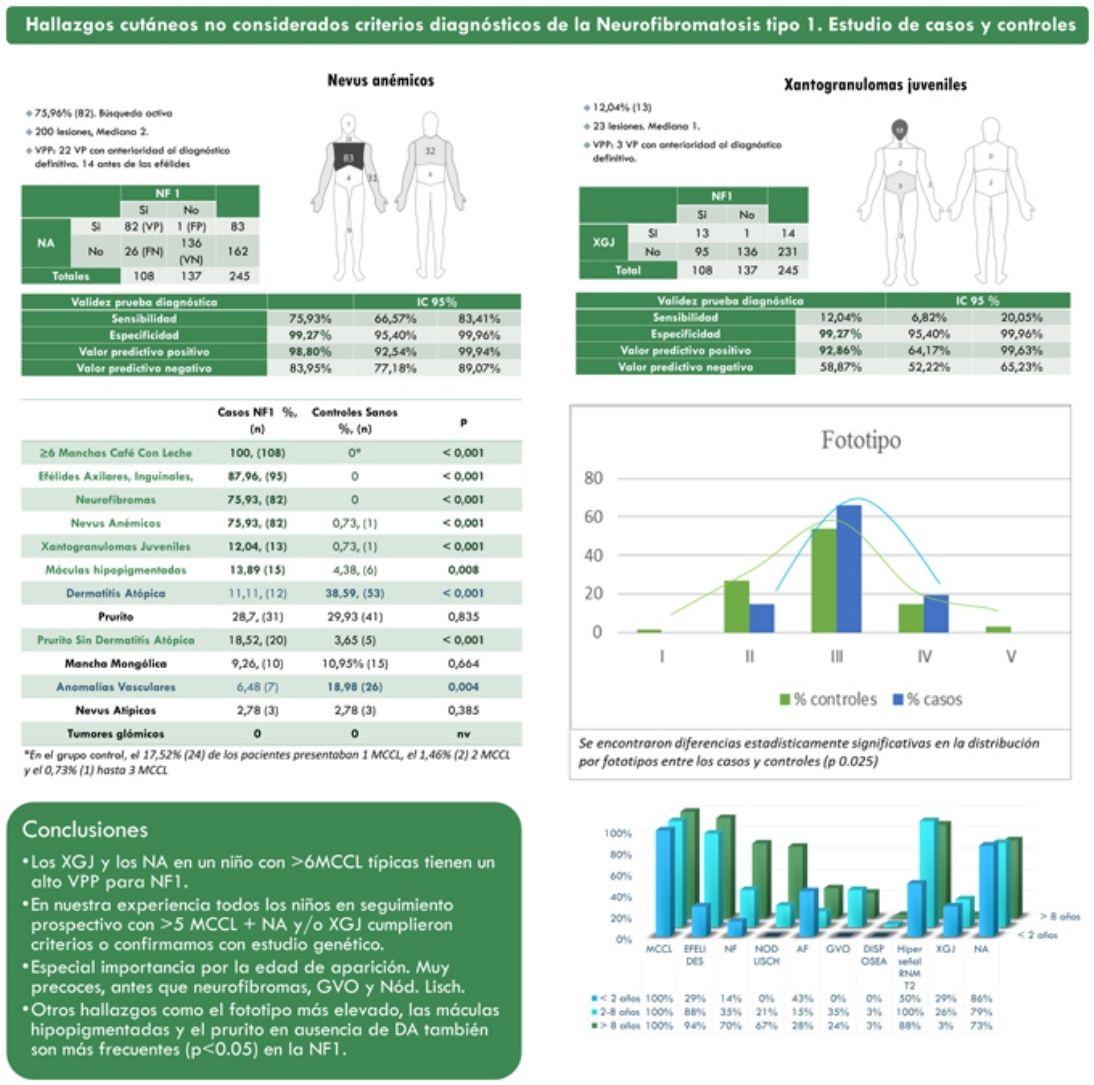
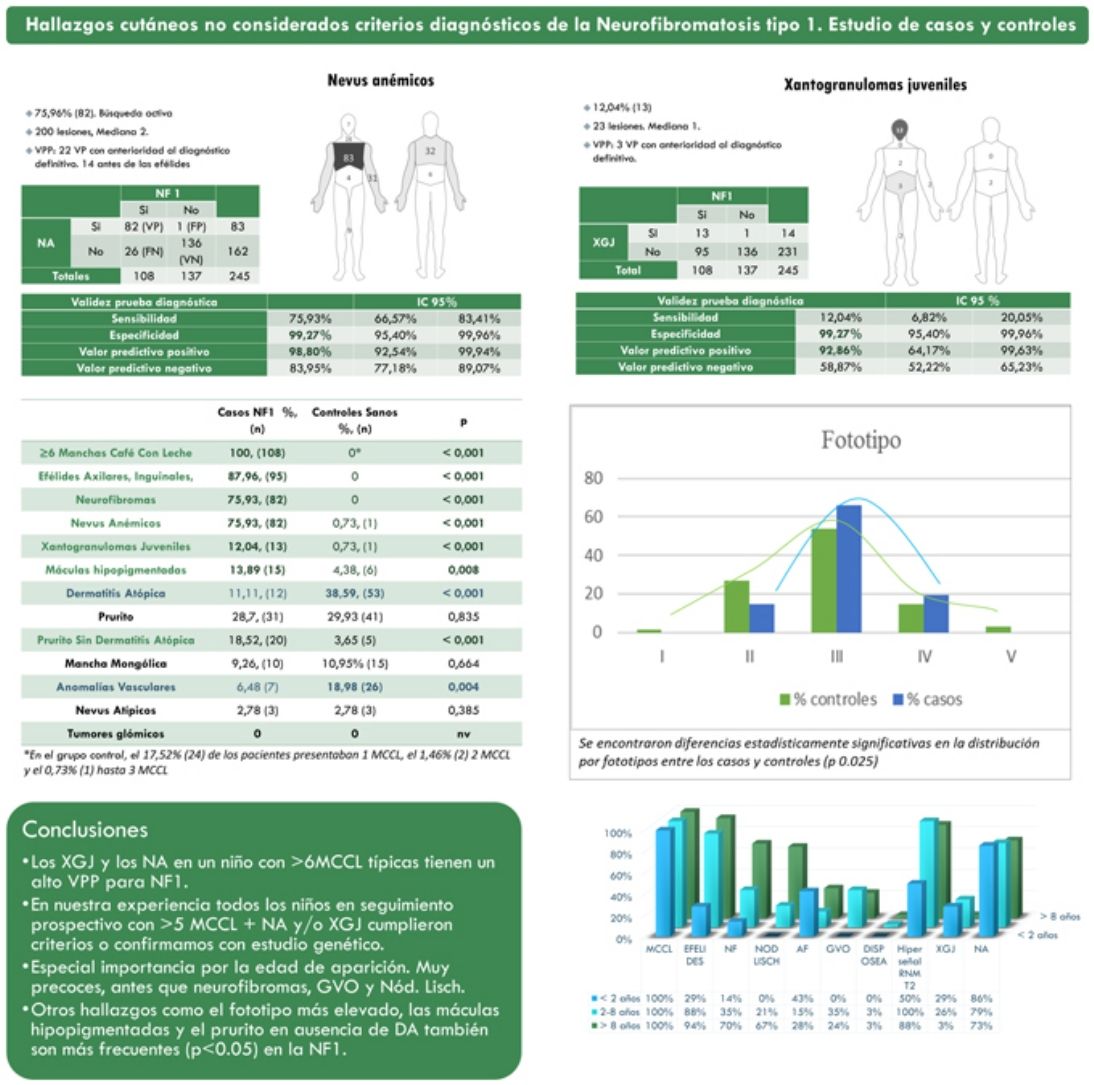
ResultsThe prevalence of nevus anemicus (NA) (p<0.001) and juvenile xanthogranulomas (JXG) (p<0.001) was significantly higher in the population affected by NF1 than in the control population. A specificity of 99.27% [confidence interval (CI): 95.4–99.96%] and a positive predictive value (PPV) of 98.80% [92.54–99.94%] were estimated for NA and a specificity of 99.27% [95.4–99.96%] and a PPV of 92.86% [64.17–99.63%] for JXG in the diagnosis of NF1 in children who present 6 or more Café-au-lait macules. Statistically significant differences were also evidenced in the distribution by phototypes (p 0.025) and in relation to generalized itching with no other cause (p<0.001).
ConclusionsNA and JXG are relevant clinical findings for the diagnosis of NF1, especially during the first years of life. We consider that its inclusion among the diagnostic criteria of the disease should be evaluated.
El diagnóstico de la neurofibromatosis tipo 1 (NF1) se demora normalmente en niños sin antecedentes familiares. Nuestro objetivo fue definir la prevalencia y características de las manifestaciones cutáneas prevalentes en NF1, en comparación con la población general, que siguen siendo excluidas de los criterios diagnósticos para NF1.
Pacientes y métodosEstudio de casos y controles, pareado por grupos de edad, en el que se incluyeron 108 pacientes diagnosticados de NF1 y 137 controles sanos.
ResultadosLa prevalencia de nevus anemicus (NA) (p < 0,001) y xantogranuloma juvenil (XJ) (p < 0,001) fue significativamente superior en la población afectada de NF1, en comparación con el grupo control. Se estimaron una especificidad del 99,27% [Intervalo de confianza (IC): 95,4-99,96%] y un valor predictivo positivo (VPP) del 98,80% [92,54-99,94%] para NA, y una especificidad del 99,27% [95,4-99,96%] y VPP del 92,86% [64,17-99,63%] para XJ en el diagnóstico de NF1 en niños que presentan 6 o más manchas café con leche. También se evidenciaron diferencias estadísticamente significativas en la distribución por fototipos (p 0,025), y con relación al prurito generalizado sin ninguna otra causa (p <,001).
ConclusionesLos NA y los XJ son hallazgos clínicos relevantes para el diagnóstico de NF1, especialmente durante los primeros años de vida. Consideramos que debería evaluarse su inclusión en los criterios diagnósticos de la enfermedad.
Neurofibromatosis type 1 (NF1; OMIM 613113) is the most common inherited neurocutaneous disorder. The diagnosis of NF1 does not usually pose difficulties in adults. However, in young children, especially those without a family history, the diagnosis usually takes several years.1 The inclusion of nevus anemicus (NA) and juvenile xanthogranulomas (JXG) has been repeatedly proposed among the skin manifestations considered diagnostic criteria for NF1 in childhood based on several retrospectives and prospective observational studies.2–5 Despite this, a recent review of the NIH diagnostic criteria based on a modified Delphi process did not reach a consensus (median score 4–6/10) for the inclusion of NA among the new diagnostic criteria.6 The authors allude to a lack of sensitivity and specificity and the difficulty of their identification by non-dermatologist physicians. The JXGs were not included either due to their transitory nature despite the existence of a consensus. The authors did consider the inclusion of the choroidal abnormalities observed by optical coherence tomography (OCT)/near-infrared reflectance (NIR) imaging.
In this work, we aimed to define the positive predictive value and the strength of the association between the most frequent cutaneous manifestations in NF1 that continue not to be considered as diagnostic criteria (Fig. 1), through a case–control study. Likewise, we intended to identify associations with other skin manifestations that we frequently observe among children with NF1 and that have received less attention in the literature, such as generalized itching, hyperpigmentation, or hypopigmented macules.7
Material and methodsA cross-sectional case–control study design matched by age groups was used, exclusively including patients with a confirmed diagnosis of NF1. The age groups were established according to the age of onset of the main diagnostic events (younger than 2 years, between 2 and 8 years and older than 8 years). For the control group, it was estimated that the sample should be composed of at least 137 healthy children, with a confidence level of 95% and a statistical power of 80%, expecting to find a difference of ±8 percentage points.
All patients under 18 years of age with a definitive diagnosis of NF1 were included as cases (meeting more than 2 criteria or 2 criteria and the detection of mutations in the NF1 gene for those who presented only café-au-lait macules (CALMs) and skinfold freckling), attended from May 1, 2012, to February 10, 2017, in our NF1 multidisciplinary unit, after having obtained the informed consent of the caregivers. For the healthy controls, children under 18 years of age, healthy companions, or patients seen between October 1, 2016, and February 10, 2017, whose reason for consultation did not coincide with any main variable of the study, were included.
During the visits and after reviewing their medical records, data regarding family history, personal dermatological history, the phototype, and the clinical findings of the examination related or not to NF1 were collected.
The variables referring to the most relevant cutaneous manifestations in NF1 were also contrasted with the rest of the dermatological findings appreciated during the study, with the main comorbidities associated with NF1 and with the diagnostic criteria of NF1. Pearson's chi2 test or Fisher's exact test was used in contrasting the variables. All contrasts were performed with two tails and p-values less than 5% were considered significant. The statistical program used was STATA/SE version 10.0 and Excel. For the variables that showed statistically significant associations, the direction of the association was calculated using the Odds Ratio (Supplementary material).
The study protocol has been reviewed and approved by the ethics and research committee of the Hospital Niño Jesús de Madrid, R-0024/16 Act No. 11/16, September 27, 2016.
ResultsFrom a cohort of 135 children under follow-up for NF1 by the Dermatology Service of our center, 108 children with a confirmed diagnosis of NF1 and 137 healthy controls between 2 months and 18 years were included. The prevalence of NA (p<0.001) and JXG (p<0.001) was significantly higher in the population affected by NF1 than in the control population. In 82 of the cases, NA was observed, while in the controls a single NA was evidenced. Therefore, we estimate that NA had a sensitivity of 75.9% [66.6–83.4%], a specificity of 99.3% [95.4–99.9%], a positive predictive value (PPV) of 98.8% [92.5–99.9%] and a negative predictive value (NPV) of 84.0% [92.5–99.9%] for the diagnosis of NF1. In the case of JXG, the sensitivity was 12.0% [6.8–20.1%], the specificity was 99.3% [95.4–99.9%], the PPV was 92.9% [64.2–99.6%] and the NPV of 58.9% [52.2–65.2%]. Table 1 summarizes the clinical characteristics of the NA and the JXG.
Clinical characteristics of nevus anemicus and juvenile xanthogranulomas.
| Clinical signs | Nevus anemicusn (%) | Juvenile xanthogranulomasn (%) |
|---|---|---|
| 108 patients | 82 (76) | 13 (12) |
| Sex | p=0.124 | p=0.026 |
| Male | 45 (54.9) | 4 (30.8) |
| Female | 37 (45.1) | 9 (69.2) |
| Frequencies | ||
| Lesion count | 200 | 23 |
| Mean | 2.4 (SD±1.8) | 1.8 (SD±1.2) |
| Median [range] | 2 [1–12] | 1 [1–4] |
| Location | ||
| Head and neck | 35 (17.5) | 12 (52.2) |
| Thoracic | 115 (57.5) | 2 (8.7) |
| Abdominal | 4 (2) | 3 (13) |
| Lumbar | 6 (3) | 2 (8.7) |
| Arms | 31 (15.5) | 2 (8.7) |
| Lower limbs | 9 (4.5) | 2 (8.7) |
| Size | ||
| <1cm | 91 (45) | 22 (95.6) |
| 1–5cm | 81 (41) | 1 (4.4) |
| >5cm | 28 (14) | 0 |
| Morphology | ||
| Rounded or oval | 94 (47) | 23 (100) |
| Lobulated | 53 (26) | 0 |
| Mottled | 50 (25) | 0 |
| Clustered | 3 (2) | 0 |
SD: Standard Deviation.
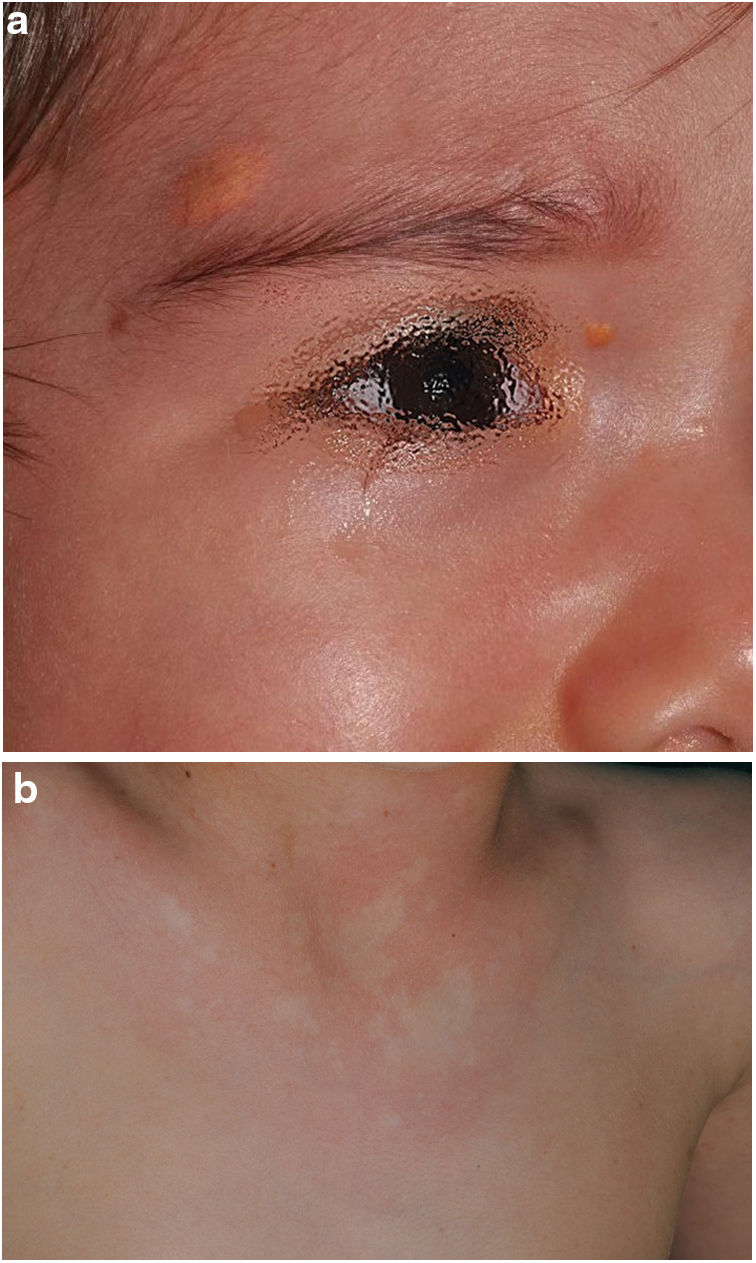
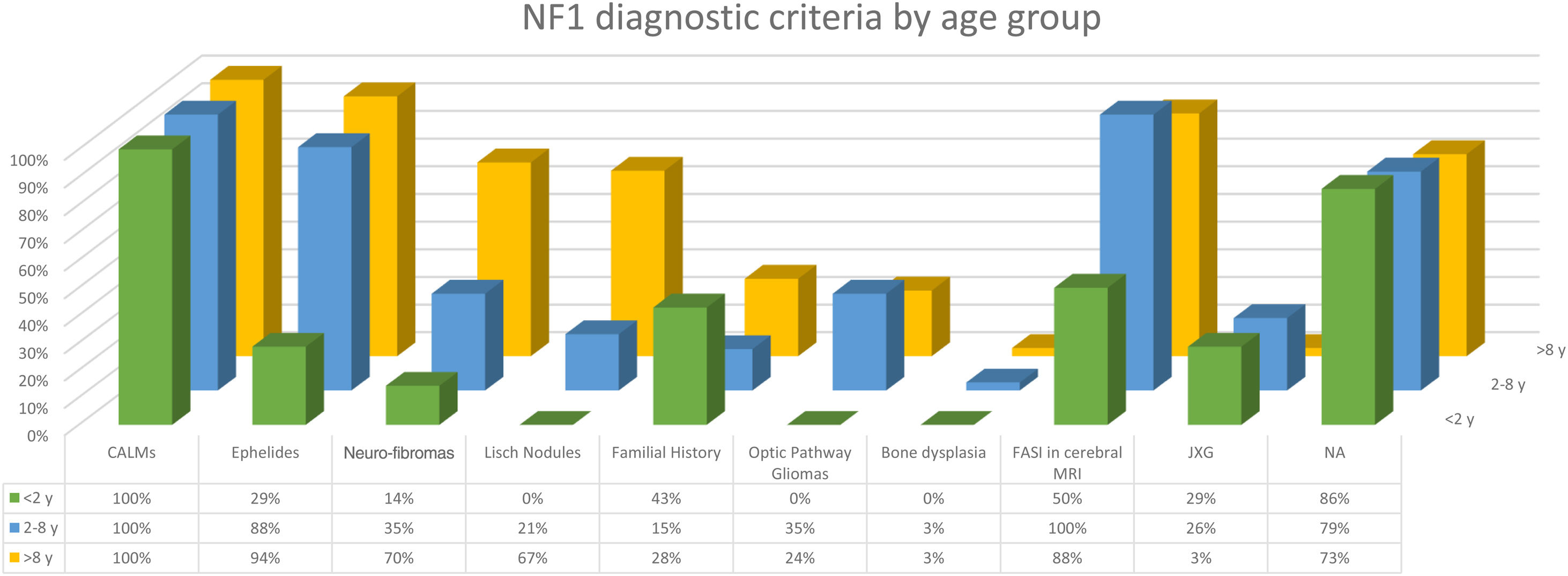
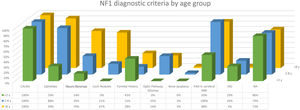
The distribution by age group of the diagnostic criteria and the most relevant findings are shown in Fig. 2. During the study period, we included 22 patients in whom we observed the presence of NA before the establishment of the definitive diagnosis. In 63.6% of them (14), NA were observed before observing the axillary or inguinal ephelides. In 7 patients, the subsequent demonstration of Lisch nodules (3) or neurofibromas (4) allowed the diagnosis to be verified. In the remaining 15 cases, the diagnosis was confirmed by genetic testing before the presence of other diagnostic criteria was demonstrated. As in the case of NA, we were able to observe 3 patients with JXG before they presented the diagnostic criteria for NF1.
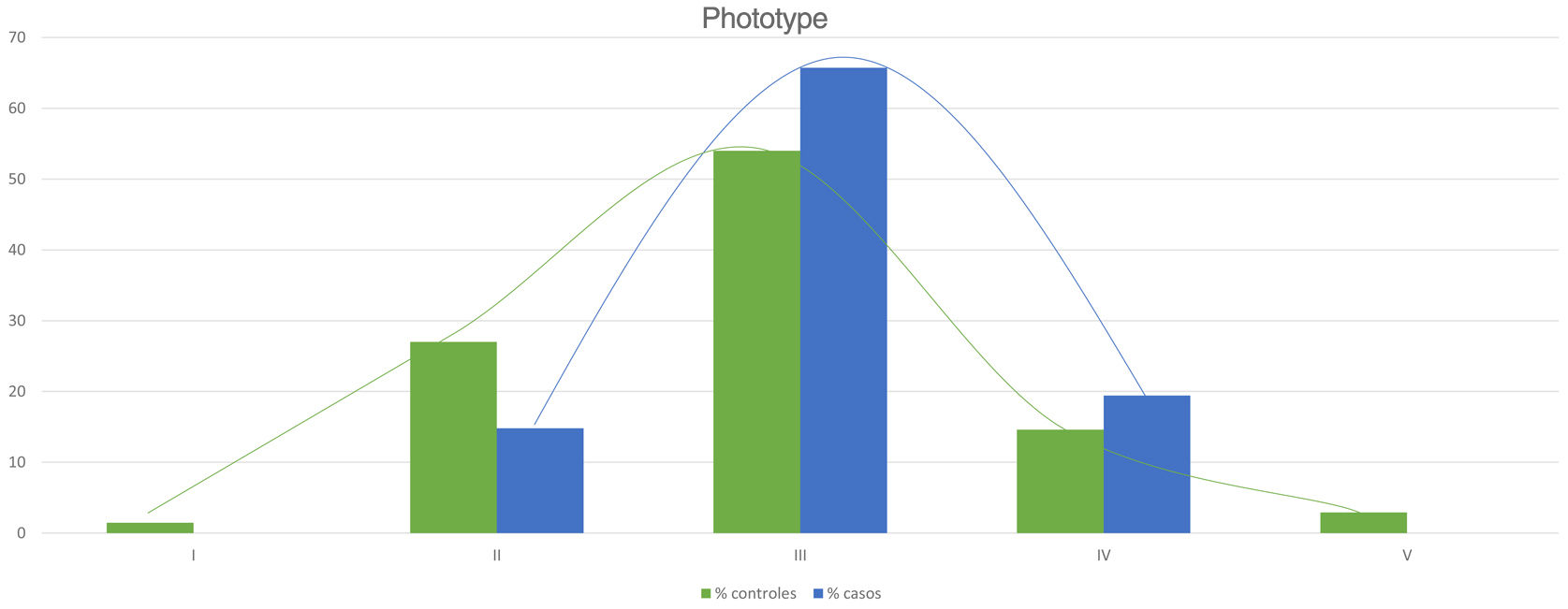
Table 2 shows the results of the case–control study regarding other dermatological manifestations. Statistically significant differences were observed in the distribution by phototypes (p=0.025), by patients with NF1 presenting a proportionally higher phototype (III–IV) than the control population (Fig. 3). We consider it relevant to highlight those focal areas of signal intensity (FASI) were observed in the cerebral magnetic resonance image (MRI) in 91.1% of the patients included in this study. The most relevant results of the analysis of variables are collected in the Supplementary material.
Results of the case-control study and statistical significance.
| NF1 casesn (%) | Controls healthyn (%) | p | |
|---|---|---|---|
| Cutaneous manifestations considered NIH diagnostic criteria | |||
| ≥6 CALMs | 108 (100) | 0* | <0.001 |
| Axillary, Inguinal Ephelides, | 95 (88) | 0 | <0.001 |
| Neurofibromas | 82 (75.9) | 0 | <0.001 |
| Manifestations not considered NIH diagnostic criteria | |||
| Nevus anemicus | 82 (75.9) | 1 (0.7) | <0.001 |
| Juvenile xanthogranulomas | 13 (12)) | 1 (0.7) | <0.001 |
| Hypopigmented macules7 | 15 (13.9) | 6 (4.4) | 0.008 |
| Atopic dermatitis | 12 (11.1) | 53 (38.6) | <0.001 |
| Pruritus | 31 (28.7) | 41 (29.9) | 0.835 |
| Pruritus without Atopic Dermatitis | 20 (18.5) | 5 (3.7) | <0.001 |
| Dermal melanocytosis | 10 (9.3) | 15 (11) | 0.664 |
| Vascular abnormalities | 7 (6.5) | 26 (19) | 0.004 |
| Atypical nevus | 3 (2.8) | 3 (2.8) | 0.385 |
The observation of 6 or more rounded to oval CALMs greater than 5mm in an infant is highly suggestive and forces NF1 to be ruled out first,8 but these findings are not pathognomonic.9–12 The description of other syndromes that can also manifest CALMs and ephelides during the first years of life, such as Legius syndrome, Noonan syndrome, or constitutional mismatch repair deficiency, has shown that these criteria alone are insufficient to consider the NF1 diagnosis proven. This circumstance forces us to decide between confirming our diagnostic suspicion using the genetic testing or waiting for the development of other criteria, maintaining indefinitely the anxiety that this diagnosis causes among the parents.9 For this reason, before the publication of the revised criteria by Legius et al.,6 exclusively patients with a confirmed diagnosis of NF1 were included in our sample to obtain conclusions of greater statistical significancy. Therefore, 27 patients who only presented CALMs and ephelides were excluded, as well as those patients with a suspected diagnosis of mosaic NF1. Thus, the percentage of patients in our series who presented at least 3 of the diagnostic criteria exceeded 81%. In the rest of the patients who exclusively presented pigmentary diagnostic criteria, that is, CALMs and ephelides, the diagnosis was confirmed by the genetic study, therefore the existence of misdiagnosis in our series was highly unlikely.
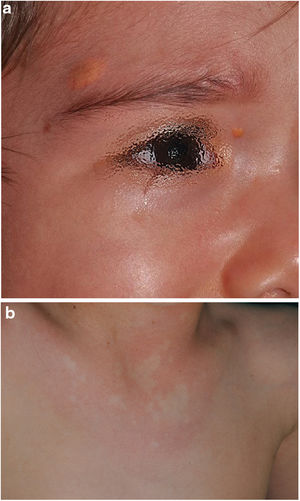
In the last decade, it has become clear that NA represent a characteristic finding of NF1. As it is a prospective study and the result of accumulated experience in the search and recognition of these lesions, the prevalence of NA in our current series amounts to 76%, even surpassing our results published in 2015.3 It, therefore, demonstrate the importance of actively searching for NA, since they may go unnoticed in the routine physical examination (Fig. 1b). To detect them, we recommend routinely asking parents if they have seen transient whitish lesions during bathing, feverish processes, crying, or exercise, and vigorous gently rubbing the preesternal area and back of infants and young children to highlight the lesions. Although it has been considered a congenital lesion, the age of appearance of these is not known exactly. In our series, numerous NA were identified in children under 2 years of age. The main publications on NA in NF1 refer to the pediatric population,3–5 so we do not know the prevalence of NA in adult patients. Some authors have suggested that NA can be camouflaged by the development of neurofibromas.13 Regression of NA has also been described,7 although in our series we have only found one case of regression of NA. Our data showed that the prevalence of NA is proportionally higher in patients included in the age group under 2 years (86%) compared to those over 8 years (73%). This circumstance is a fact contrasted in other clinical features of NF1 such as the optic pathway gliomas (OPG) and FASI in MRI.14 The pathophysiology of NA has not been confirmed at this time. The location, morphology, and size will not be discussed since the results do not differ from those published in 2015.3
To our knowledge, currently, only one case of NA has been described in relation to Legius syndrome15 and another in tuberous sclerosis complex (TSC).16 Therefore, new prospective studies are required in others Rasopathies to clarify whether the NA are a specific finding of NF1 as we suspect. Some authors maintain that the detection of NA in patients with CALMs and/or ephelides would confirm the diagnosis of NF1.17 Our results showed that the 22 children affected by the skin pigmentary criteria and NA, were subsequently diagnosed with NF1 (theoretical PPV of 100%) and that the presence of NA could have anticipated the diagnosis if they were considered as a criterion, even before the appearance of axillary or inguinal ephelides. In addition, the early age of onset of NA would facilitate the early diagnosis of the disease. Likewise, when observing one or multiple NAs in any infant, we recommend inspecting the child for other signs of NF1.
Although less prevalent than NA, JXG are also considered frequent lesions in children affected by NF1. The frequency of JXG in pediatric patients varies significantly in the different publications on NF1, ranging from 3.9% to 37.5% of children.18,19 Our results showed a prevalence of 12%, however, at the end of the study this prevalence would fall to 5.6% due to the transitory nature of the JXG. In our series, 80% of the JXG were registered during the first 2 years of life and disappeared after 1 or 2 years, being uncommon in patients older than 5 years (Fig. 1a). Our results regarding location, morphology, and size will not be discussed either, since they coincide with those previously published.20 We would highlight the existence of a statistically significant difference in the distribution by sex, with the presence of JXG being predominant in girls. This finding has not been previously reflected in the literature.
NF1 is a disorder characterized by hyperpigmentation, despite which, we highlight the high frequency of hypopigmented macules (HM) registered in our series (13.9%) (Table 2). We justify this marked prevalence due to the active search for NA, which has allowed us to identify these lesions, sometimes subtle, which are not accentuated by friction, unlike NA. These HMs could be diagnosed as achromic nevus or nevus depigmentosus, but the absence of anatomopathological or molecular studies and its similarity with the lesions described by Riccardi in 1987,21 we decided to preserve the traditional denomination.7 Clinical similarities of some of these oval-shaped HMs with the ash leaf or lanceolate spots of TSC are evident. Although exceptionally, the association of NF1 and TSC in the same patient has been previously reported.22 We emphasize that none of our patients with hypochromic macules, nor their relatives, presented other signs suggestive of TSC. In the control group, we observed the presence of HM in a considerably high percentage (4.4%), despite this, we confirmed the existence of a statistically significant association between the HM and NF1 (p=0.008). Before establishing a causal relationship between both processes, tissue and molecular studies are required to identify the pathogenic mechanism that gives rise to this localized hypopigmentation.
Regarding other frequent dermatological findings that currently lack relevance in the diagnosis of the NF1, we would like to highlight the statistically significant differences related to the phototype and generalized pruritus in patients without a history or other clinical signs of atopic dermatitis (AD) between patients and controls. This results has allowed us to corroborate the observations of the patients themselves and of the authors who asserted that patients with NF1 present darker phototypes23 and that they more frequently suffer from generalized itching.24 On the contrary, we have not been able to confirm the diagnostic repercussion of glomus tumors in NF1 since we have not observed them in either of the two groups. Its low frequency in pediatric patients reduces its role as a diagnostic criterion, although its detection will motivate the search for other NF1 features in adult patients.25
We consider that the prevalence of other skin diseases such as vascular anomalies or AD was significantly higher in the control group due to a selection bias since they are very frequent reasons for consultation in a pediatric dermatology service.
According to Legius et al.6 it is essential to recognize the limitations, reinterpret and adapt the diagnosis based on the diagnostic criteria established in 1987 by the NIH. This case–control study shows that NA and JXG are more prevalent findings in patients with NF1 than in the general population and confirms the high specificity and PPV of NA and JXG in the diagnosis of NF1. Likewise, we considered the diagnostic utility of these cutaneous manifestations proven because they will allow us to confirm this diagnosis at an earlier age, without the economic and healthcare repercussions associated with the molecular study or the recently proposed complementary tests.
Therefore, this study supports with enough evidence the inclusion of these two signs as diagnostic criteria for NF1 or at least indicate genetic testing when we observe NA or JXG in young children with 6 or more CALMs.
Other findings such as the highest phototype, MH, and generalized pruritus are also prevalent findings in children with NF1 that must be considered in the clinical diagnosis.
Once our results have been analyzed, we consider it proven that a thorough clinical examination will allow dermatologists and pediatric neurologist to diagnose NF1 with greater precision and at earlier ages.
FundingThis work is included in the doctoral thesis awarded with the scholarship entitled Accesit to the Best Doctoral Thesis Edition 2017 of the “Sección Centro” of the Spanish Academy of Dermatology and Venereology - SC-AEDV.
Previous presentationsThis study is included in the Doctoral Thesis entitled:
“Ultrasound clinical study of Neurofibromatosis Type 1 in the pediatric age”.
Results and conclusions included in this doctoral thesis have been previously published or accepted for publication:
García-Martínez F.J., Azorín D., Duat-Rodríguez A., Hernández-Martín Á. Congenital cutaneous neurofibromas in neurofibromatosis type 1: Clinicopathological features in early infancy. J Dtsch Dermatol Ges. 2021 Jan;19(1):73-80. doi: 10.1111/ddg.14322. Epub 2021 Jan 14. PMID: 33448128.
García-Martínez F.J., Duat-Rodriguez A., Torrelo A., Noguera-Morel L., Hernández-Martín A. Hypopigmented macules in neurofibromatosis type 1: A case control study. J Am Acad Dermatol. 2021 Apr;84(4):1128-1130. doi: 10.1016/j.jaad.2020.06.071. Epub 2020 Jun 24. PMID: 32592882.
García-Martínez F.J., Alfageme F., Duat-Rodriguez A., Andres Esteban E., Hernández-Martín A. Clinical and sonographic classification of neurofibromas in children with NF1. A cluster analysis. Ultraschall in der Medizin/European Journal of Ultrasound. 2021 Nov 24. doi: 10.1055/a-1640-9621. Online ahead of print.
Presentación titulada: Hallazgos cutáneos NO considerados criterios diagnósticos de la NF1 en la edad infantil. Estudio de casos y controles. Congreso Nacional Virtual de la AEDV, octubre de 2020.
Consent statementAll participants and their caregivers voluntarily agree to participate in this research study.
Conflict of interestThe authors declare that they have no conflict of interest.
To all the resident physicians who collaborated in the data collection.