The clinical concept of urticaria embraces a heterogeneous group of conditions classified according to their clinical course as acute (lasting less than 6 weeks) or chronic (lasting 6 weeks or more). Chronic urticaria may be either spontaneous or induced. Few tools are available for monitoring the various clinical forms of this disease or for evaluating its impact on quality of life. The recently developed Urticaria Control Test to evaluate disease control is available in German, the original language, and American English.
ObjectiveTo culturally adapt the long and short versions of the Urticaria Control Test to Castilian Spanish to ensure equivalence between the translated items and those of the original version.
Material and methodsTo translate the Urticaria Control Test we followed the International Society for Pharmacoeconomics and Outcomes Research good practice guidelines, starting with forward translation and moving through back translation and cognitive debriefing steps.
ResultsThree items were modified when the first Spanish version, translated from German, was discussed (cognitive debriefing). The revised translation was then translated back to German and sent to the Urticaria Control Test authors, who modified one item they considered had acquired a different focus through translation. A third Spanish version was then prepared and after minor proofreading changes was considered definitive.
ConclusionsThis study was the first step in making it possible to use the Urticaria Control Test questionnaire in Castilian Spanish. The next step will be to validate the translated questionnaire.
Bajo el concepto de urticaria se incluye un grupo heterogéneo de entidades clasificadas según su evolución en urticaria aguda (duración inferior a 6 semanas) y urticaria crónica (duración igual o superior a 6 semanas). Las formas crónicas incluyen la urticaria crónica espontánea y las urticarias inducibles. Disponemos de un número limitado de herramientas para la monitorización de las distintas formas clínicas de urticaria y para la valoración de su impacto en la calidad de vida. Recientemente se ha creado el cuestionario Urticaria Control Test como herramienta para valorar el control de la urticaria, disponible tanto en alemán, el idioma original, como en inglés americano.
ObjetivoRealizar la adaptación transcultural al castellano de las versiones larga y corta del Urticaria Control Test garantizando su equivalencia con la versión original.
Material y métodosMetodología de traducción directa, traducción inversa y entrevistas cognitivas siguiendo los principios de buena práctica de la International Society for Pharmacoeconomics and Outcomes Research.
ResultadosLa primera versión del cuestionario en español, obtenida por traducción directa de la versión original, fue sometida a entrevistas cognitivas, realizándose modificaciones en 3 preguntas. Al valorar los autores originales la nueva versión obtenida por retrotraducción, se modificó únicamente una pregunta, ya que consideraron que con el cambio había perdido el enfoque global de la cuestión original. Se realizó una tercera versión, que fue sometida a mínimas modificaciones, obteniéndose la versión definitiva.
ConclusionesEste trabajo facilita la utilización en castellano del cuestionario Urticaria Control Test en un paso previo a su validación.
Urticaria is an inflammatory skin disease characterized by the appearance of wheals and/or angioedema. Wheals are defined as papules or plaques that are erythematous, edematous, intensely pruritic, and evanescent—lasting less than 24hours and disappearing without leaving residual signs. Angioedema, on the other hand, is characterized by intense swelling secondary to edema in the deep dermis and subcutaneous cellular tissues; these lesions are more painful and may be found alone or concurrent with wheals.1,2 An estimated 1 in 5 individuals will experience at least one episode of urticaria in the course of a lifetime.3,4 When urticarial lesions last more than 6 weeks the condition is classified as chronic.1,2 The prevalence of chronic urticaria in the general population is 0.5% to 1%,3,4 and its impact on quality of life (QOL) is considerable.1–3,5
The Urticaria Activity Score (UAS) is the main tool currently used to assess activity and intensity; in fact, the UAS is practically the only tool at our disposal. It consists of a questionnaire that collects the patient's record of symptoms (pruritus and wheals) prospectively on consecutive days.1,6 Designed for patients with spontaneous chronic urticaria, this questionnaire is difficult to use with patients with angioedema or inducible urticaria. It was also not designed to assess disease control over time.6,7
The Urticaria Control Test (UCT) was designed by Weller et al.7 to evaluate the control of the various types of urticaria retrospectively. Short and long versions are available. The short UCT is used in routine clinical practice to calculate a score that reflects the degree of disease control in follow-up. The long UCT gathers clinical information relevant to the impact of urticaria on various aspects of a patient's life. The UCT was developed and validated first in German; later a cross-cultural adaptation to American English was prepared.7
The aim of this study was to culturally adapt the long and short forms of the UCT to Castilian Spanish so that we could then validate this instrument for use in Spain.
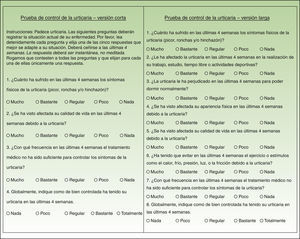
Material and MethodsThe short form of the UCT occupies a single sheet and has 4 items answered on 5-option scales scored from 0 to 4 points. The minimum and maximum scores, therefore, are 0 and 16. A score of 16 indicates complete control of the disease.
The long form of the UCT also occupies a single sheet but has 8 items, also answered on 5-option scales. Since the 4 items on the short form are also included in the long one, a patient's UCT score can be calculated with either instrument.
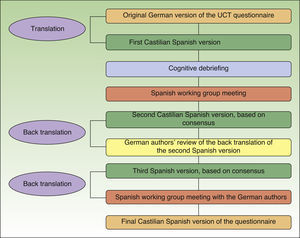
We followed published procedures8–11 for cross-culturally adapting the UCT to Castilian Spanish, paying particular attention to the recommendations of the International Society for Pharmacoeconomics and Outcomes Research.9 The method was a process of forward and back translation (Fig. 1). A professional translator produced a first draft of the Spanish version using the original German questionnaire as the source text. It was then reviewed and the concepts were discussed by the group of Spanish investigators. Patients also read this version and underwent cognitive debriefing. The revised Spanish text was then translated back to German and reviewed by the original developers of the tool.
A total of 4 versions of each tool (short and long forms) underwent this process. The fourth Spanish version of each was considered definitive.
The cognitive debriefing interviews of patients were held in order to ascertain how they understood the items and to adapt the wording to colloquial language. The patients recruited were diagnosed with chronic urticaria and were being followed by our hospital's specialist unit for treating this disease. This patient sample consisted of 10 individuals of both sexes and varying ages and levels of education (Table 1). The objectives of the study and nature of the questionnaire were explained to them in detail before the interviews started and they gave their signed, informed consent to participation.
Demographic and Clinical Characteristics of the Patients Who Agreed to Cognitive Debriefing for the Cross-Cultural Adaptation of the Urticaria Control Test.
| Patient No. | Type of Urticaria | Age, y | Sex |
|---|---|---|---|
| 1 | Spontaneous chronic urticaria | 68 | Male |
| 2 | Spontaneous chronic urticaria | 60 | Female |
| 3 | Spontaneous chronic urticaria | 65 | Female |
| 4 | Spontaneous chronic urticaria | 68 | Female |
| 5 | Spontaneous chronic urticaria | 49 | Female |
| 6 | Spontaneous chronic urticaria | 65 | Female |
| 7 | Spontaneous chronic urticaria | 66 | Female |
| 8 | Symptomatic Dermographism | 19 | Male |
| 9 | Spontaneous chronic urticaria | 40 | Female |
| 10 | Cold urticaria | 17 | Male |
The interviews were carried out by a single independent person who asked probing questions11–13 about each patient's perceptions. According to the debriefing protocol, participants first read the instructions and then read and responded to each of the items one by one. During the process they were asked to try to explain the meaning of each item and each answer in their own words and to identify words or phrases that were difficult to understand or that they found confusing. Finally, they were asked if they would change the wording of any of the items in order to make them easier to understand. The patients’ body language and comments that might suggest difficulty (with reading or understanding the test's format) were also noted during the interview. Next, the participants were asked if they would make any improvements in the questionnaire. The interviewer took detailed, literal notes on all these aspects for later evaluation. Each interview took 15to 20minutes on average.
The version that was then translated back to German by a professional translator was the second draft, obtained after the cognitive debriefing sessions. After the original authors reviewed that translation, a third Spanish version was prepared on the basis of their comments and suggestions. The third version was again back translated and reviewed with the German authors of the tool. At that point, minimal changes were made to produce the fourth (definitive) versions of both the short and long forms (Fig. 2).
ResultsTwo of the 4 items in the short version and 5 of the 8 in the long version required no changes. The instructions for both versions, the response choices, and the overall format of the questionnaires also required no modifications. Changes were required in 3 items in total: 2 that were present in both the short and long versions plus 1 that appeared only in the long version.
A single word was changed in question 3 of the short version (S3) and in equivalent question L7 in the long version. Similarly, a single word was changed in question L6 of the long version (Fig. 3). The problematic word had been translated too literally from German to Spanish, such that the semantic scope of the Spanish term was not equivalent to the German original, leading to misunderstanding.
In contrast, item 4 of the short form (S4), and the equivalent item L8 of the long form required more extensive modification of the sentence. The reason was that the expression used in the original translation was vague, making it difficult for nearly all the patients to understand. A more direct, specific sentence was therefore drafted.
After the items were back translated to German, the original authors considered that the sense of questions S3 and L6 were accurate. However, they noted that on shifting item S4 (and L8) toward greater specificity, the overall meaning of the original item had been lost. Therefore, this item was changed again and a back translation was sent to the German authors. Following discussion by all parties involved, the Spanish wording was changed slightly and the new version was considered the definitive one (Fig. 4).
DiscussionThe various tools and scoring systems for evaluating urticaria currently available include the UAS, the Chronic Urticaria QOL questionnaire (CU-Q2OL), the Urticaria Severity Score, the Angioedema Activity Score, the Angioedema QOL questionnaire, and the UCT.
The UAS is reliable for determining urticaria activity.1,6 The patient keeps a daily record of the number of wheals and the intensity of pruritus on a simple scoring system over a 7-day period, providing a clear picture of disease activity. This instrument is the one most often chosen for use in routine clinical practice. Validated and easy to answer, it is used in different research centers thus, unified scores can be compared.1,6 However, this system has certain limitations: since information is gathered prospectively it is not useful for a baseline urticaria evaluation, it does not assess angioedema or induced urticaria, and it relies on patient compliance.7
The CU-Q2OL is designed to evaluate QOL in patients with spontaneous chronic urticaria.14 It is also useful for monitoring the impact of treatment in these patients because it detects changes in the severity of symptoms.1,14 It includes 23 items in 6 dimensions. Although this tool is not particularly difficult for the patient to complete, doing so is laborious; as it takes about 5minutes on average, it is difficult to use in routine clinical practice.1,14
The validated Urticaria Severity Score was created specifically to evaluate and follow patients with chronic urticaria. It combines assessments of symptom severity, impact on QOL, and the type and amount of medication required to control symptoms. The tool has 12 questions, each with 7 or 8 possible answers to choose from. An advantage of the Urticaria Severity Score over the UAS or the CU-Q2OL is that the amount of medication required and symptom severity are scored simultaneously.15 On the other hand, the number of questions and possible answers the patient must evaluate are not negligible and this property may be problematic for the patient.
The Angioedema Activity Score was the first validated, reliable way to evaluate recurring angioedema. It collects information prospectively and contains 5 items scored from 0 to 3. The maximum daily score of 15 indicates the highest level of disease activity. This tool is used to evaluate symptoms over periods of 7 days, 4 weeks, and 12 weeks. It is easy to use, usually taking less than a minute to complete.16
The Angioedema QOL questionnaire was the first validated instrument to measure the impact of recurring angioedema on daily living. There are 17 questions, each with 5 possible answers. This tool is simple and takes less than 5minutes to complete. Its main limitation is that it was validated in a relatively small patient sample at highly specialized clinics, possibly limiting its use to populations with the same characteristics as the validation sample.17
Until now, the CU-Q2OL was the only instrument for which an adapted translation to Castilian Spanish has been prepared and validated.18
Finally, the UCT has been available for only a short time. This new questionnaire was specifically designed to be a simple tool for evaluating chronic urticaria to aid therapeutic decision-making, to make up for the deficiencies in the UAS. It collects retrospective information about the patient's view of urticaria control over the last 4 weeks. With only 4 items, each with 5 possible responses, it is easy to complete.7 These properties make the UCT useful for evaluating control of disease in clinical practice and potential clinical trials.
An advantage of the easy-to-use UCT over the UAS is that it can be applied to both spontaneous chronic urticaria and induced urticaria; moreover, it includes angioedema among the symptoms named.7
When more information is needed, the long version of the UCT gathers responses about how the disease affects the patient with regard to work, school, free time, sports, sleep, and personal appearance. The UCT also asks about physical stimuli the patient must avoid to prevent symptoms from worsening.7
The present study makes Castilian Spanish translations of the UCT available. A rigorous, systematic translation process was followed in the interest of ensuring that the measurement properties of the Spanish and German questionnaires will be equivalent. In Spain and in the rest of Europe, the processes of cognitive debriefing and forward and back translation have been used often to adapt instruments for use in clinical practice and both clinical and epidemiologic research.8–13 The next step is to study the Spanish version's sensitivity, specificity, validity, and reliability in patients in clinical practice settings.13,19–21
In conclusion, we stress that the UCT is the first validated tool available at present for reliably measuring the control of chronic urticaria, whether spontaneous or induced, in a simple manner. We think that the Spanish translation of the UCT will help physicians with routine clinical management of this disease.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that the procedures followed adhered to the ethical guidelines of the responsible committee on human experimentation and comply with the Declaration of Helsinki of the World Medical Association.
Data confidentialityThe authors declare that they followed their hospitals’ regulations regarding the publication of patient information and that written informed consent for voluntary participation was obtained for all patients.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects referred to in this article. The signed forms are in the possession of the corresponding author.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: García-Díez I, Curto-Barredo L, Weller K, Pujol RM, Maurer M, Giménez-Arnau AM. Adaptación transcultural del cuestionario Urticaria Control Test del alemán al castellano. Actas Dermosifiliogr. 2015;106:746–752.