Psoriasis is an autoimmune chronic inflammatory skin disease that is common in Spain. Connective tissue diseases are a heterogeneous group of conditions characterized by the abnormal function or structure of one or more of the elements that make up connective tissue. These diseases are also autoimmune in origin. In spite of the high prevalence of psoriasis in the general population, its association with a connective tissue disease such as systemic lupus erythematosus, dermatomyositis, scleroderma, or rheumatoid arthritis has only occasionally been reported. It is nevertheless important to have an understanding of such associations, given their significant clinical and therapeutic implications. The association between psoriasis and systemic lupus erythematosus is the one most often described, although the few reports available in the literature have mostly involved single cases. This review will also look at the characteristics of patients with psoriasis and dermatomyositis, mainly focusing on clinical features. The associations between psoriasis and either rheumatoid arthritis or systemic sclerosis will be examined more briefly. The review therefore aims to reflect the literature on psoriasis in association with rheumatic diseases, including coverage of etiologic, pathogenic, clinical, and therapeutic aspects. We emphasize that such cases should be managed by a multidisciplinary team in which care will usually be shared by a rheumatologist and a dermatologist.
La psoriasis es una enfermedad cutánea inflamatoria crónica de etiología autoinmune muy frecuente en nuestro medio. Las enfermedades del tejido conectivo constituyen un grupo heterogéneo de enfermedades que se caracterizan por la anormal función o estructura de uno o varios elementos del tejido conectivo, de origen autoinmune. Pese a la alta frecuencia de la psoriasis en la población general, son pocos los casos publicados en los que coexistan psoriasis y enfermedades del tejido conectivo, como lupus eritematoso sistémico, dermatomiositis, esclerodermia o artritis reumatoide. Sin embargo, dadas las implicaciones clínicas y, principalmente, terapéuticas que presentan estos pacientes, resulta importante conocer estas asociaciones. La más frecuentemente descrita es la de psoriasis y lupus eritematoso sistémico, aunque son pocos los estudios publicados y la mayoría se basan en casos únicos. También trataremos las características específicas de los pacientes con dermatomiositis y psoriasis, principalmente de tipo clínico. Abordaremos de forma más breve la coexistencia de esclerosis sistémica y psoriasis, y de artritis reumatoide y psoriasis. Se pretende, por tanto, realizar una revisión de la literatura sobre psoriasis y su coexistencia con enfermedades reumatológicas, que comprende aspectos etiopatogénicos, clínicos y terapéuticos. Además queremos resaltar el manejo multidisciplinar que requieren estos pacientes, generalmente entre el reumatólogo y el dermatólogo.
In practice, we often encounter patients with connective tissue diseases or psoriasis. However, very few patients present with both conditions and in such cases the signs and symptoms differ from those seen in patients with either disease alone. Most reports of this combination in the literature refer to single cases or small series and no controlled trials or review articles exist. Consequently, we considered that it would be interesting to review the association from the point of view of clinical features, therapeutic management, and their shared common pathogenesis.
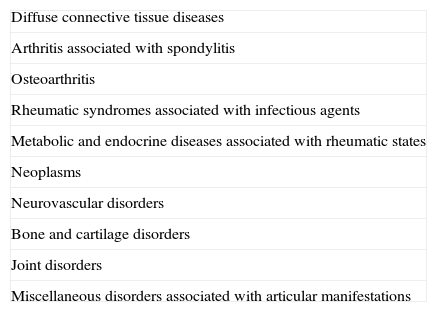
Connective tissue diseases are a heterogeneous group of acquired and hereditary disorders characterized by abnormal function or structure of one or more of the elements of connective tissue, such as collagen, elastin, or the mucopolysaccharides. The group is one of the 10 categories of rheumatic diseases in the American College of Rheumatology classification (Table 1) and it includes entities such as lupus erythematosus, rheumatoid arthritis, Felty syndrome, juvenile chronic arthritis, adult Still disease, scleroderma, fasciitis, polymyositis, vasculitis, Sjögren syndrome, and polymyalgia rheumatica.1 This review deals with the coexistence of psoriasis with systemic lupus erythematosus (SLE), dermatomyositis (DM), scleroderma, and rheumatoid arthritis (RA).
The American College of Rheumatology 1983 Classification of Rheumatic Diseases (Adapted).1
| Diffuse connective tissue diseases |
| Arthritis associated with spondylitis |
| Osteoarthritis |
| Rheumatic syndromes associated with infectious agents |
| Metabolic and endocrine diseases associated with rheumatic states |
| Neoplasms |
| Neurovascular disorders |
| Bone and cartilage disorders |
| Joint disorders |
| Miscellaneous disorders associated with articular manifestations |
Psoriasis is a common inflammatory disease with an estimated mean prevalence worldwide of around 2%, although according to the Epiderma study the prevalence in Spain is slightly lower (1.4%).2 Despite its prevalence in the general population, there are only a few published cases of concomitant psoriasis and connective tissue disease. Although the pathogenesis of psoriasis has not been clearly established, in patients who present with this combination it is believed that there may be a shared autoimmune base, which we will discuss below3,4; most connective tissue diseases are autoimmune in nature.
While the prevalence of rheumatic diseases is high in Spain (23% according to the EPISER study5), the prevalences of RA and SLE are much lower. That study estimated that there are around 500 cases of RA per 100000 population in Spain (worldwide prevalence ranges from 300 to 1200 cases per 100 000 population1) and around 9 cases of SLE per 100 000 population. Although the epidemiologic data available on connective tissue diseases is scant, we indicate below the data obtained from several Spanish epidemiologic studies. In 2003, López et al6 reported a prevalence of 34.1 cases and an incidence of 2.2 cases per 100 000 population for SLE. In a study published in 2007, Vargas-Leguás et al7 reported an incidence in Spain of 4.9 cases per million population per year for DM. Although the data on the prevalence of scleroderma in Spain is scant, a study published by Villaverde-Hueso et al8 in 2007 reported a prevalence of 0.23-2.58 cases per 10 000 population.
These findings indicate a low prevalence of connective tissue diseases in the general population, explaining why there are few series in the literature and why most published reports refer to single cases. We will describe each of these 4 entities separately, covering both pathogenesis and etiology.
Immunopathogenesis of Psoriasis and Autoimmune Connective Tissue DiseasesThe etiology and pathogenesis of psoriasis are not yet clearly understood, but the condition is thought to be related to a polygenic predisposition and a number of environmental trigger factors, such as stress, trauma, infection, and drugs.9,10 While the model of inheritance for psoriasis is quite complex,11 a genetic component has been established by studies that have demonstrated a risk of both twins having psoriasis that is 2 or 3 times greater in monozygotic than in dizygotic twins.12 Several genes that confer increased susceptibility to psoriasis have recently been identified, among which the PSORS gene complex (1 to 9) is of particular interest.13 The principal genetic determinant is PSORS1, a susceptibility locus that lies within the major histocompatibility complex on chromosome 6p and is thought to account for up to 50% of the genetic transmission of psoriasis.14 The various clinical forms of psoriasis are related to different genetic expressions, at least in the case of PSORS1.15,16 Alterations in interleukin (IL) 12B and in the IL-23 receptor that appear to be risk indicators in psoriasis have also been identified.17 The following are other genes that appear to play a role: CDKAL1 located on chromosome 6p, which is also implicated in Crohn disease and type 2 diabetes mellitus18; the zinc-finger 313 gene (ZNF313); and the PTPN22 gene present in type 1 diabetes mellitus, idiopathic juvenile arthritis, SLE, and RA.11,19
Psoriasis shares both immunologic and genetic risk factors with other autoimmune diseases such as the connective tissue diseases RA and SLE. Previously, CD4 helper T (TH) 1 cells were considered to be the most important cells in the pathogenesis of these diseases, but greater importance is now given to the role of TH17 cells,20 a novel CD4 T effector cell that plays an important role in many autoimmune diseases.21,22
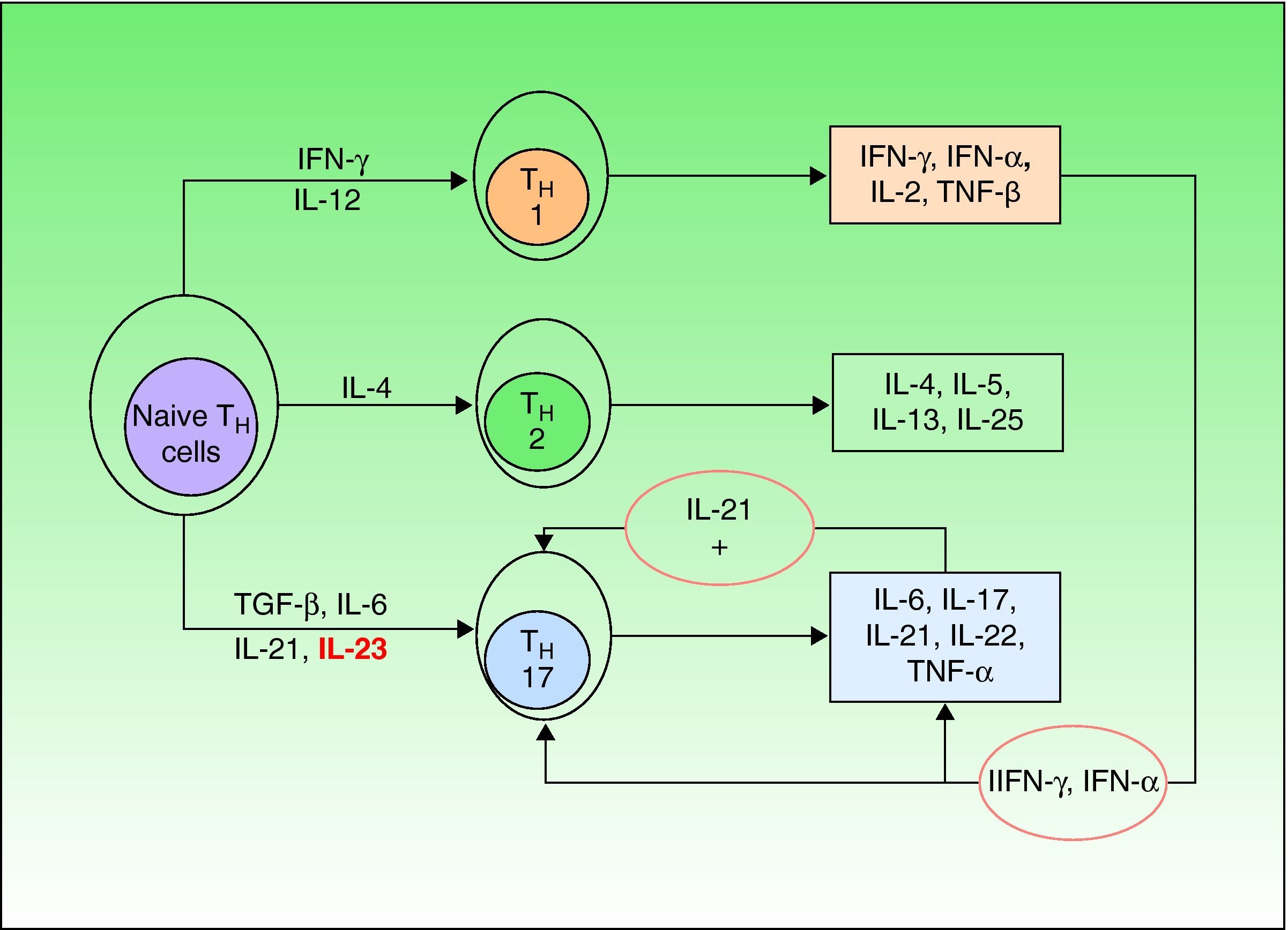
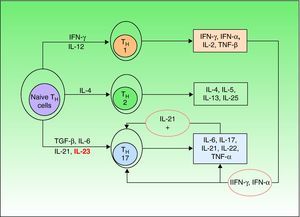
Until a few years ago, only 2 types of such cells were known (TH1 and TH2), but a third type—TH17—has now been identified. TH1 cells intervene in the development of CD8 cells and TH2 cells are responsible for the antibody-mediated immune response, while TH17 cells are implicated in the autoimmune inflammatory response.23 Naive TH cells differentiate into specific types depending on the stimulus they receive (Fig. 1).21 Differentiation into TH17 cells is mediated primarily by transforming growth factor-β, while IL-23 contributes to the survival and proliferation of these cells. IL-23 concentration is elevated in psoriasis lesions and decreases when the lesions respond to treatment, revealing a direct correlation between overproduction of IL-23 and active psoriasis. It has been suggested that the IL-23/TH17 axis may play a fundamental role in the pathogenesis of psoriasis.24
Naive T helper (TH) cells differentiate into 3 different subsets (TH1,TH2,and TH17) depending on the cytokine stimuli they receive. Transforming growth factor (TGF) β and interleukin (IL) 6 are the primary mediators of differentiation into TH17 cells, while IL-23 contributes to the survival and proliferation of these cells. Among the many cytokines secreted by TH17 cells, IL-21 intervenes in a positive feedback loop. IFN indicates interferon; TNF, tumor necrosis factor.
The main cytokines secreted by TH17 are IL-6, IL-17, IL-21, and IL-22; the last 2 induce keratinocyte hyperproliferation.23,25 Moreover, it is thought that IL-21 may also act as a feedback mechanism stimulating production of TH17 cells. The most recent theories posit that therapies targeting IL-23 alone would be sufficient to improve psoriasis, but some authors have suggested that neutralizing IL-21 in vivo by means of soluble receptors or monoclonal antibodies could be a useful tool in the treatment of psoriasis.26
Thus, current theories postulate that in a genetically predisposed individual the pathogenesis of psoriasis would require an unknown stimulus to act on epidermal keratinocytes to produce a number of chemical mediators, such as tumor necrosis factor (TNF) α, interferon(IFN) γ, and IFN-α (the key cytokines in psoriasis); these mediators would in turn activate plasmacytoid and myeloid dendritic cells. Once activated, these cells would induce the differentiation of naive T cells into TH1 and TH17 cells, which would then migrate to the skin, acting as antigen-presenting cells and releasing cytokines, such as TNF-α, IFN-γ, IL-23, IL-22, and IL-17. These cytokines would induce the proliferation of keratinocytes and alter their maturation, giving rise to the epidermal hyperproliferation which, together with inflammatory T cell infiltration, constitutes the fundamental basis of psoriasis lesions.11
This etiologic and pathogenic complexity is not exclusive to psoriasis and, in fact, it would appear that TH17 cells, as well as IL-17 and IL-23, are also implicated in SLE, although the role of these cytokines in humans is poorly understood because most studies to date have been carried out in mice. In fact, elevated TH17-cell serum concentrations have been observed in some patients with SLE; IL-17 and IL-23, which may contribute to the renal damage, have also been detected in the kidneys of patients with lupus nephritis.20 Since reductions in the production of IL-17 in mice have been correlated with clinical improvement, it has been proposed that anti-IL-17 therapy could be useful in the treatment of patients with elevated serum levels of this cytokine.27 IL-21 levels are also elevated in the serum of these patients, although in this case there is no apparent correlation with disease severity.23 Polymorphisms have recently been identified within the chromosome 4q27 region, which harbors the genes that code for IL-2 and IL-21. These variants are associated with SLE, RA, psoriasis, ulcerative colitis, diabetes, and asthma,28–32 an indication that these diseases may share a common genetic factor.
TH17 cells also appear to play an important role in the development of RA. In both mice and humans, elevated levels of IL-1733 and IL-2134 in serum and synovial fluid have been seen to correlate with joint damage, and in fact, mice with an IL-17 deficit do not develop RA. In RA, as in psoriasis, IL-23 has been identified as the main target for blockade. Moreover, blockade of Il-21 has also been proposed as a way to improve disease in RA, and the effectiveness of this mechanism has been demonstrated in animal models.35
What is clear is that the immune response in these diseases is very complex and involves a large number of regulating mechanisms, including the key role played by TH1 cells and their cytokines IFN-γ and IFN-α, which act by inhibiting or downregulating the expression of IL-17 and TH17 differentiation in vitro and in vivo.36 An understanding of these mechanisms allows us to understand in greater detail the complexity of these diseases and the relationship between them. Moreover, as we have briefly noted above, future therapies for many of these diseases, whether they occur in isolation or concomitantly, will be based on the control of these regulation mechanisms.
Systemic Lupus Erythematosus and PsoriasisSLE is a rheumatic autoimmune disease of unknown etiology characterized by a set of clinical signs and symptoms associated with the presence of autoantibodies. Although its pathogenesis is poorly understood, a number of contributing factors have been identified, the most important of which is the production of autoantibodies. These autoantibodies are thought to be the key in the pathogenesis of SLE because they can interfere directly with cell function or act through immune complexes. They are produced through polyclonal B cell activation or autoantigen-directed immune stimulation.37 Genetic, environmental, and hormonal factors have also been described.
Diagnosis of SLE is based on clinical and analytic criteria that were first described in 1982 by the American College of Rheumatology and later revised in 1997 (Table 2).5
| Malar rash |
| Discoid rash |
| Photosensitivity |
| Oral ulcers |
| Arthritis |
| Serositis: pleuritis or pericarditis |
| Renal disorder: proteinuria (<0.5 g/d) or cellular casts |
| Neurologic disorder: seizures or psychosis |
| Hematologic disorder: hemolytic anemia, leukopenia, lymphocytopenia or thrombocytopenia |
| Immunologic disorder: anti-DNA, anti-smooth muscle, or antiphospholipid antibodies |
| Antinuclear antibodies |
SLE is associated with various autoimmune diseases, such as RA, Sjögren disease, Hodgkin disease, and Crohn disease.3,38 However, the association of SLE and psoriasis is very rare. The mechanisms involved in this association are poorly understood, but it is thought that there must be a common immunologic basis.4 T cells play a primary role in the pathogenesis of psoriasis while B cells appear to be central in SLE, giving rise to the view that superantigens might be the common mediator.39–41 However, it is now known that alterations in the TH17 cell pathway occur in both diseases.
In 1927, O’Leary42 published one of the first case reports of concomitant psoriasis and SLE. Since then, most publications have referred to isolated cases or small series.39,43–45 The largest series was published in 1996 by Zalla and Muller,46 who studied 9420 patients with psoriasis. They found that psoriasis and SLE occurred together in 0.69% of patients with psoriasis and in 1.1% of patients with SLE. Their findings differ only slightly from those of Dubois et al,47 who reported concomitant psoriasis in 0.6% of 520 patients with discoid SLE. There is no established pattern of onset when both are present: psoriasis develops first in some cases and lupus erythematosus (LE) in others. Onset can even be simultaneous, although this occurs less frequently.43,46 As can be expected in view of the higher incidence of LE in women, the coexistence of psoriasis and LE is also more common in women, while the association of drug-induced lupus and psoriasis affects both sexes equally.43,46 The characteristics of psoriasis in Zalla and Muller's patients with LE were similar to those in patients with psoriasis alone, and the most common clinical picture included plaques and limb involvement. These authors did, however, report a higher risk of erythroderma in patients with LE. A case of linear psoriasis—a rare form of the disease— was reported in a patient with SLE following a flare episode with neurologic manifestations.39 The distribution of the different clinical forms of LE in patients who also have psoriasis has been reported to be first SLE, then discoid LE, and finally drug-induced lupus.43,46 The same sources also noted that the manifestations of LE occurred later in patients with psoriasis and that photosensitivity was more frequent.
A controversial aspect of the SLE-psoriasis association is whether or not specific laboratory markers are present. In 1993, Kulick et al48 studied a series of 4 patients with coexistent LE and psoriasis and 24 with psoriasis alone. The 4 patients with the combination all presented the anti-Ro antibody while the 24 controls did not. These authors also described the potential severity of photosensitive reactions in patients with this combination, which are probably linked to the anti-Ro antibody. This would suggest that that the anti-Ro antibody may be a marker for the association between psoriasis and LE. However, Hays et al49 later reported on 4 patients with LE and psoriasis in whom the anti-Ro antibody was not found. The current view is that no specific serologic marker exists for the psoriasis-SLE overlap. Most authors recommend that a detailed medical history include any history of photosensitivity, a symptom that occurs in almost 50% of these patients, and that patients be tested for antinuclear antibodies, anti-double stranded DNA antibodies, and extractable nuclear antigen antibodies.4,50
In their typical forms, psoriasis and SLE are easily differentiated. However, the differential diagnosis can sometimes be difficult because the clinical spectrum, mainly of SLE, is very broad.40,51 The LE lesions most often confused with psoriasis lesions are those caused by subacute lupus,4,50–52 a disorder in which psoriasiform lesions have been reported in 15% to 50% of patients.53 Cases of SLE associated not only with classic psoriasis skin lesions but also with psoriatic arthritis have recently been reported54; this association has implications for differential diagnosis, treatment, and prognosis.
Choice of treatment is the main problem in patients with concurrent SLE and psoriasis. UV radiation, one of the principal treatments for psoriasis, can trigger and worsen SLE.55,56 Although the outcome is favorable in most cases, toxic epidermal necrolysis has been reported in a patient treated with UV-B for psoriasis who had a history of SLE which he omitted to mention.48
Antimalarial agents (hydroxychloroquine, chloroquine) are among the drugs most often used to manage both cutaneous and systemic LE, and it is well known that these drugs can trigger or aggravate psoriasis.57 In vitro studies have shown that hydroxychloroquine produces hyperproliferation and irregular keratinization in skin taken from patients with psoriasis.58 However, cases have been reported in which the course of psoriasis was not altered when hydroxychloroquine was prescribed to patients with concomitant SLE.51,59 Another treatment-related problem is the risk of triggering a severe psoriasis flare when systemic corticosteroids are prescribed to manage SLE. In theory, the systemic use of corticosteroids would be contraindicated in patients with psoriasis owing to the possibility of psoriatic erythroderma.57
Drugs that inhibit TNF-α (anti-TNF-α agents) are currently one of the main treatments for severe psoriasis.60 These drugs have been somewhat effective when occasionally used to treat patients with SLE,51 but there have been reports of exacerbation of SLE following their use, most often in the case of infliximab51,61 and etanercept.62,63 These reports raise the question of whether SLE is a contraindication to the use of anti-TNF agents.64 Drug-induced lupus, sometimes involving anti-TNF-α agents, has also been described. However, some authors believe that TNF inhibitors give rise to a very specific clinical picture—known as anti-TNF-α-induced lupus (ATIL)—a much less common entity than drug-induced lupus and one with different characteristics.65,66 ATIL is characterized by a higher incidences of hypocomplementemia and high titers of anti-DNA antibodies in comparison with drug-induced lupus, while in the latter there will be higher titers of anti-histone antibodies. Renal and central nervous system involvement is more common in ATIL than in drug-induced lupus. ATIL is a self-limiting condition that usually disappears upon withdrawal of the anti-TNF-α therapy, but it may occasionally require treatment with corticosteroids or immunosuppressants.
Methotrexate has been used successfully to treat patients with coexistent SLE and psoriasis67 and is emerging as one of the best therapeutic options in this setting.68
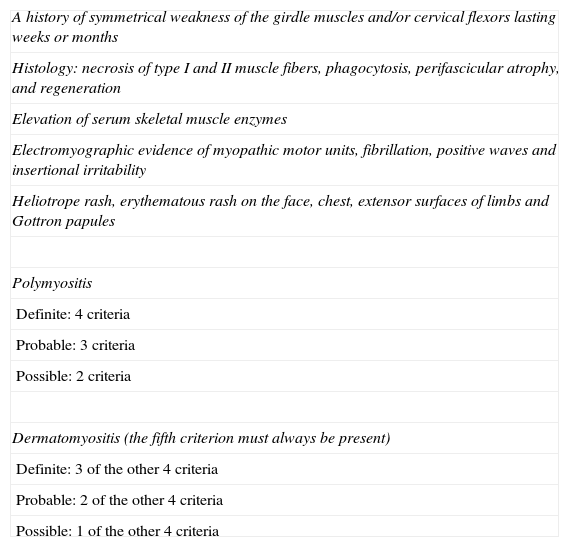
Dermatomyositis and PsoriasisDM is an autoimmune connective tissue disease with characteristic skin involvement and muscle inflammation.37 It belongs to a group of idiopathic inflammatory myopathies that includes polymyositis and inclusion body myositis. DM is suspected based on clinical criteria, elevated serum muscle enzyme (creatine-kinase), and electromyographic abnormalities. Diagnosis is confirmed by muscle biopsy. Several diagnostic criteria have been proposed to establish whether a diagnosis of DM is probable or possible in cases in which the decision is unclear (Table 3).1
Criteria for the Diagnosis of Dermatomyositis-Polymyositis.1
| A history of symmetrical weakness of the girdle muscles and/or cervical flexors lasting weeks or months |
| Histology: necrosis of type I and II muscle fibers, phagocytosis, perifascicular atrophy, and regeneration |
| Elevation of serum skeletal muscle enzymes |
| Electromyographic evidence of myopathic motor units, fibrillation, positive waves and insertional irritability |
| Heliotrope rash, erythematous rash on the face, chest, extensor surfaces of limbs and Gottron papules |
| Polymyositis |
| Definite: 4 criteria |
| Probable: 3 criteria |
| Possible: 2 criteria |
| Dermatomyositis (the fifth criterion must always be present) |
| Definite: 3 of the other 4 criteria |
| Probable: 2 of the other 4 criteria |
| Possible: 1 of the other 4 criteria |
Few cases of patients with coexistent DM and psoriasis have been reported. In some, DM developed in patients with a long-standing history of psoriasis.69 The opposite situation can also occur, with psoriasis developing in a patient with DM.70 In all published cases, the course of each disease is independent.
DM sometimes presents without muscle involvement, and in such cases is called amyopathic DM.71 When this occurs, cutaneous manifestations may initially be the only diagnostic criteria, making it important to stress that, in such cases, the clinical picture is often atypical and can mimic other skin diseases, such as psoriasis. One atypical manifestation of DM is scalp involvement presenting as erythema, desquamation and atrophy; in the absence of other manifestations, these symptoms may be confused with psoriasis of the scalp.72 Cutaneous symptoms affecting the extensor surfaces of the hands (Gottron papules) have been reported as well as rashes that have initially been erroneously diagnosed as psoriasis.73
Physicians should be aware of the problems involved in the treatment of patients who present with both these diseases. Although hydroxyurea is rarely prescribed in the management of psoriasis, its safety and efficacy in this setting has been demonstrated.74 The side effects reported in patients receiving prolonged treatment with hydroxyurea for psoriasis include clinical signs similar to those of dermatomyositis.75 Psoralen UV-A (PUVA) treatment, unlike hydroxyurea, is frequently used to manage psoriasis, and a case in which DM developed during such treatment has been reported.76
In patients with DM, prognosis is determined by the systemic manifestations, which primarily affect the muscles, lungs, and heart. Muscle damage and interstitial lung disease require treatment with high doses of oral corticosteroids. Since these drugs are contraindicated in patients with psoriasis, other immunomodulatory and immunosuppressive therapies, such as methotrexate or azathioprine may be prescribed.57 Tacrolimus and mycophenolate mofetil have also been shown to be useful in interstitial lung disease.77,78
Instances have been reported of improvement in both skin and muscle symptoms in patients with DM or polymyositis treated with anti-TNF-α following poor response to other classic treatments, such as methotrexate.79 Such an outcome would be of great interest in a patient with concomitant psoriasis.Finally, we note that ciclosporin A, a drug widely used in patients with psoriasis, has been used successfully to treat cases of refractory DM and polymyositis.80 Because ciclosporin can cause myolysis, some authors recommend that this drug be prescribed on a case by case basis in these patients.81–83
Scleroderma and PsoriasisSystemic scleroderma is a multisystem autoimmune connective tissue disease of unknown etiology1,37 characterized by generalized vascular involvement that results in tissue ischemia and secondary fibrosis. There are 2 main types: limited and diffuse. As in other rheumatic diseases, diagnosis is based on clinical criteria (Table 4). While skin involvement is the main clinical feature, prognosis depends on the systemic manifestations.
The American College of Rheumatology 1980 Diagnostic Criteria for Systemic Sclerosis.1
Very few cases of concomitant psoriasis and systemic sclerosis have been reported,84–88 with the largest series in the literature involving only 3 cases.87,88 Of particular interest is the study by Harrison et al,88 in which the prevalence of psoriasis in patients with systemic scleroderma was 5.3%. Some authors have suggested that the 2 entities may share a common genetic and immunologic basis,87,88 and although the HLA DRw52 serotype has been associated with both systemic scleroderma and psoriasis, studies have failed to establish an HLA pattern in patients with both diseases. It has also been suggested that the skin alterations caused by psoriasis may act as a triggering factor for systemic sclerosis because in some patients the development of psoriasis was followed by the onset of scleroderma.88
Most reported cases deal with patients who had a long history of psoriasis prior to the onset of scleroderma. The most common type of scleroderma developed is the diffuse systemic form. Several authors indicate that the symptoms of systemic sclerosis may be more aggressive in these patients in comparison to the moderate severity of their psoriasis.87,88
The typical clinical features of systemic scleroderma are not usually confused with psoriasis, and although scleroderma is rarely accompanied by joint involvement, this symptom has been reported in a few cases.89,90 Occasionally, joint involvement in a patient with scleroderma may be confused with or mask psoriatic arthritis with minimal skin involvement.91
Several issues must be taken into account in the treatment of patients with these 2 diseases. Raynaud phenomenon, a disorder characterized by vasospasm of the small distal vasculature (usually in response to cold) and manifested as pallor, cyanosis and hyperemia, is one of the most common presentations of systemic scleroderma.10 This condition is treated with a variety of drugs, the most common of which are calcium channel blockers, such as nifedipine, although losartan (an angiotensin II receptor antagonist) has also been used successfully. Both of these drugs reduce the severity of symptoms, but only losartan reduces the frequency of episodes.92 Although no strong association between psoriasis and calcium channel blockers has been established, cases in which psoriasis developed following the use of these drugs have been reported.93,94 The onset of psoriasis has also been associated with angiotensin II inhibitors; in most published cases, the psoriasis symptoms resolved over the following months.95,96 The endothelin receptor antagonist bosentan has proven effective in preventing ulcers caused by Raynaud phenomenon, but has not been shown to aid the resolution of existing lesions.97 We have found no evidence in the literature linking treatment with bosentan to the development of psoriasis.
The systemic manifestations of scleroderma include kidney damage that can result in renal crisis, the leading cause of death in this setting until the introduction of angiotensin-converting enzyme inhibitors.37,98 These drugs have been clearly implicated in triggering outbreaks of psoriasis.94,95,99,100 However, due to the seriousness of the renal complications of scleroderma, these drugs should be used even at the risk of exacerbating psoriasis.
Pulmonary hypertension is currently the leading cause of death in these patients. Several treatment options are available, including cyclophosphamide, bosentan, sildenafil, prostacyclin derivatives and oral corticosteroids. We have found no evidence in the literature suggesting that these drugs, with the exception of oral corticosteroids, either induce or exacerbate psoriasis. Moreover, cases have been reported in which no exacerbation of psoriasis was observed following treatment with corticosteroids in patients with systemic scleroderma.87
Rheumatoid Arthritis and PsoriasisRA is an autoimmune disease of unknown etiology characterized by chronic and debilitating symmetrical polyarthritis accompanied by systemic manifestations that can occasionally be life threatening. Its exact etiology is unknown. Like the other diseases discussed above, RA is diagnosed on the basis of clinical criteria (Table 5).
| Morning stiffness | Morning stiffness in and around joints lasting for at least one hour |
| Arthritis in 3 or more joint areas | Involvement of at least 3 joint areas simultaneously with soft tissue swelling or accumulation of joint fluid observed by a physician. The possible areas involved are the left and right proximal interphalangeal, metacarpophalangeal, wrist, elbow, knee, and metatarsophalangeal joints. |
| Arthritis of hand joints | Affecting at least 1 area in the wrist, metacarpophalangeal or proximal interphalangeal joint |
| Symmetrical arthritis | Simultaneous involvement of the same joint areas on both sides of the body |
| Rheumatoid nodules | Subcutaneous nodules over bony prominences, extensor surfaces, or juxtaarticular regions observed by a physician |
| Positive serum rheumatoid factor | |
| Radiographic changes | Erosions or unequivocal bone decalcification localized in the regions adjacent to the involved joints is |
Although neither RA nor psoriasis is uncommon, they rarely occur concomitantly. In 1992, Mazzucchelli et al101 estimated the prevalence of this association to be between 0.03 and 0.15 per 10 000 population. More recent data from a German database of rheumatic diseases indicate that 0.2% of patients with RA and 0.3% of RA seropositive patients have concomitant psoriasis.102
The extra-articular manifestations of RA include associated dermatologic manifestations, such as rheumatoid nodules (a diagnostic criterion), rheumatoid vasculitis, pyoderma gangrenosum, and rheumatoid neutrophilic dermatosis.57 Cases of palmoplantar pustulosis associated with neutrophilic dermatosis have also been reported.103
Although the diagnosis of these entities is not difficult when they occur separately, problems do arise when inflammatory arthritis develops in a patient who has psoriasis. The clinical picture in such cases is usually suggestive of psoriatic arthritis, but we must nonetheless also consider the possibility of other types of inflammatory arthritis, such as RA.
Psoriatic arthritis presents as oligoarthritis, which is usually asymmetrical, primarily affects the distal interphalangeal joints, and is often associated with spondyloarthropathy, dactylitis and nail dystrophy. Juxtaarticular new bone formation is a characteristic radiographic finding. By contrast, RA primarily affects metacarpophalangeal and proximal interphalangeal joints, making it possible in most cases to distinguish the disease on the basis of clinical findings.10 The radiograph demonstrates bone erosion and decalcification in the regions adjacent to clinically affected joints.
Nonetheless, it is sometimes difficult to differentiate between the 2 diseases, particularly when psoriatic arthritis has a polyarticular pattern.104 Certain serologic markers, such as rheumatoid factor and the anticyclic citrullinated peptide antibody, can help to establish a diagnosis; these findings must, however, be interpreted with caution. Rheumatoid factor is found in 85% of patients with RA and is considered a diagnostic criterion. Nevertheless, it is also found in conjunction with other diseases and in healthy populations.10 Rheumatoid factor has traditionally been considered indicative of RA, but this antibody has also been found in between 2% and 10% of patients with psoriatic arthritis.104 As noted above, another serologic marker used is the anticyclic citrullinated peptide antibody, which is highly specific for RA105 and has been used to distinguish RA from other diseases. However, this antibody is also found in 8% to 16% of patients with psoriatic arthritis (more frequently in those with erosive polyarticular disease), and it may even be found in psoriasis patients who have no joint involvement.106 Thus, the presence of anticyclic citrullinated peptide antibody or rheumatoid factor does not exclude a diagnosis of psoriatic arthritis.
As noted above, the diagnosis of psoriatic arthritis is difficult because it is based on clinical, radiographic, and immunologic findings; there are no internationally accepted criteria.107 Taylor et al108 recently suggested a set of criteria called CASPAR (Classification of Psoriatic Arthritis), which has a sensitivity of 91.4% and a specificity 98.7% in this disease. Under the CASPAR criteria, the diagnosis of psoriatic arthritis requires at least 3 points according to the following scoring system: current psoriasis (2 points) or history of psoriasis (1 point), family history of psoriasis (1 point unless current psoriasis is present or there was a history of psoriasis), dactylitis (1 point), juxtaarticular new bone formation (1 point), rheumatoid factor negativity (1 point), and nail dystrophy (1 point).
The introduction of biologic drugs has brought an important change in the management of RA. Anti-TNF-α agents (etanercept, infliximab and adalimumab) are now widely prescribed for these patients and the same drugs are also used to treat patients with severe psoriasis or psoriatic arthritis. However, paradoxically, these agents have been known to trigger an outbreak of psoriasis or psoriasiform dermatitis.109 The incidence of psoriasis induced by TNF-α-blocking therapy in patients with RA is estimated to be between 2.3% and 5%.110
In short, the diagnosis of coexistent psoriasis and RA represents a real challenge for both the dermatologist and the rheumatologist, and the association has both therapeutic and prognostic implications.
ConclusionsIn conclusion we note that, despite the fact that psoriasis is a relatively common disease in the general population, its simultaneous occurrence with connective tissue or rheumatic diseases is quite rare. However, the implications of such associations are important. In recent years, we have gained a better understanding of the etiology and pathogenesis of both psoriasis and the connective tissue diseases, an advance that has facilitated the detection of the common pathogenic pathways that shape the clinical characteristics of these associations and inform the appropriate therapeutic approach for each case. Because of the particular clinical characteristics of these associations and, more especially, the therapeutic approaches required in this setting, individual assessment of each patient is essential and this will often require collaboration between the rheumatologist and the dermatologist.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Cuesta-Montero L, Belinchón I. Conectivopatías y psoriasis. Actas Dermosifiliogr. 2011;102:487-97.