Specialist approaches to the diagnosis and treatment of melanoma have undergone many changes. This guideline aims to provide Spanish dermatologists with evidence-based information for resolving the most common doubts that arise in clinical practice. Members of the Spanish Oncologic Dermatology and Surgery Group (GEDOC) with experience treating melanoma were invited to participate in drafting the guideline. The group developed a new guideline on the basis of existing ones, using the ADAPTE collaboration process, first summarizing the care process and posing relevant clinical questions, then selecting guidelines with the best scores according to the AGREE II (Appraisal of Guidelines for Research and Evaluation) tool. Finally, the group searched the selected guidelines for answers to the clinical questions, drafted recommendations, and sent them for external review. The guideline is structured around 21 clinical questions chosen for their relevance to issues that make clinical decisions about the management of melanoma difficult. Evidence from existing guidelines was used to answer the questions. A limitation of this guide derives from the scarce evidence available for answering some questions. Moreover, some areas are changing rapidly, so recommendations must be updated often. The present guideline offers answers to clinical questions about the routine management of melanoma in clinical practice and provides dermatologists with a reference to guide decisions, taking into consideration the resources available and patient preferences.
El diagnóstico y tratamiento del melanoma en atención especializada es un campo en el que se han producido numerosos cambios. El objetivo de esta guía es ofrecer a los dermatólogos españoles una referencia para resolver las dudas clínicas más frecuentes basándose en la evidencia actual. Para la realización de esta Guía se escogió a miembros del Grupo Español de Dermato-Oncología y Cirugía (GEDOC) con experiencia en el tratamiento de estos tumores y con interés en participar en la elaboración de la guía. Se hizo una adaptación de las guías de práctica clínica existentes mediante el método ADAPTE: inicialmente se resumió el proceso de atención y se elaboraron las preguntas clínicas relevantes. Se seleccionaron las guías mejor puntuadas mediante el instrumento AGREE II, realizando la búsqueda de las respuestas en dichas guías y elaborando las recomendaciones. Finalmente se sometió la guía a revisión externa. La Guía se estructuró a partir de 21 preguntas clínicas que fueron seleccionadas por su relevancia, dado que se centran en aspectos que pueden plantear decisiones difíciles en el manejo del melanoma; y se han respondido empleando la evidencia obtenida de las mejores guías existentes. Entre las limitaciones de esta Guía, merece reseñarse que la evidencia es escasa para responder a algunas preguntas. En algunos aspectos el cambio es rápido y exige una actualización frecuente de la guía. Esta Guía responde a preguntas habituales sobre el manejo del melanoma en la práctica clínica diaria, sirviendo a los dermatólogos como referencia en la toma de decisiones, siempre teniendo presente los recursos y preferencias del paciente.
Skin cancer is very common in our setting and constitutes a major public health problem. The incidence of melanoma in Spain is 8.82 cases per 100 000 people-years (95% CI, 7.59-10.04 cases) and is probably rising.1 Because of its high incidence and poor prognosis, melanoma is the leading cause of skin cancer deaths in Spain, with 2.17 deaths per 100 000 person-years.1 The diagnosis and treatment of melanoma places a considerable burden on dermatologists and the health care system as a whole.2
Numerous treatments, some of them new, exist for melanoma. Their use in clinical practice varies, as does their effectiveness and associated risk of adverse events. Costs may also differ substantially. These different factors add to the complexity of clinical decision-making.
Numerous clinical practice guidelines (CPGs) exist to guide the treatment and management of melanoma, but they were designed for different settings and only partly address issues identified as important by dermatologists.
As part of the White Paper on Skin Cancer project, the Healthy Skin Foundation of the Spanish Academy of Dermatology and Venereology (AEDV) launched an initiative to create a series of CPGs on the main skin cancers in Spain by adapting existing guidelines.
In this article, we describe the development of the CPG designed to guide decision-making in the management of melanoma based on recommendations adapted to our context and on the best possible evidence.
Material and MethodsThe present CPG was developed using the ADAPTE collaboration process to adapt existing CPGs on melanoma.3 A summary of the steps involved is provided as supplementary material (Supplementary Material-Section 1).
In brief, members of the AEDV’s Spanish Oncologic Dermatology and Surgery Group (GEDOC) with experience in treating melanoma and interest in participating in the project were selected to form the panel responsible for drafting the new CPG. All the panelists declared potential conflicts of interest before the project started and their statements are included in the Conflicts of Interest section.
In defining the scope and purpose of the project, it was decided that the aim of the CPG would be to provide recommendations on controversial issues relating to the diagnosis, medical and surgical treatment, and follow-up of melanoma (Supplementary Material-Section 2). The health care setting chosen was dermatology practice in Spain and the target users, dermatologists. Prevention of melanoma was excluded from the scope of the CPG.
Following the ADAPTE process, we first summarized the care pathway and then formulated relevant health questions for each step of the pathway (Supplementary Material-Section 3). The most relevant questions were chosen using the ADAPTE consensus process. To retrieve existing CPGs, we performed an online search of sources and organizations that produce, collect, or disseminate guidelines, including leading dermatology and cancer academies, such as the National Guidelines Clearinghouse, the Guidelines International Network, GuíaSalud, the Institute for Clinical Systems Improvement, the UK National Institute for Health and Care Excellence (NICE), the New Zealand Guidelines Group, the Scottish Guidelines Network, the Cochrane Library, the British Academy of Dermatology, the American Academy of Dermatology (AAD), the European Academy of Dermatology, and the National Comprehensive Cancer Network (NCCN). The search strategy and dates (last revision, September 2019) are specified in the supplementary material (Supplementary Material-Section 4). The CPGs retrieved were reviewed and their methodological quality assessed using the AGREE II (Appraisal of Guidelines for Research and Evaluation) tool.4 The highest-rated CPGs were selected for further consultation.
Questions relating to melanoma stage were based on the criteria of the seventh edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, as this was the version used in the source documents.
The content of the selected CPGs was examined to prepare the recommendation matrices. References to the original sources were maintained in all cases. The panelists worked in pairs to extract data and assign levels of evidence and grades of recommendations as per the Oxford Centre for Evidence-Based Medicine system.5
Once the draft recommendations were ready, they were published on the AEDV’s website for external review by AEDV and GEDOC members, oncologists, and other potentially interested parties, such as patient associations and pharmaceutical companies. Any objections or questions raised were evaluated by the panel and, where appropriate, applied to the new CPG.
ResultsThe CPGs selected for consultation—those with the highest AGREE II quality scores and whose objectives were aligned with the scope and purpose of the new CPG—are listed in the supplementary material (Supplementary Material-Section 3).
The health questions and corresponding recommendations are described below.
Block 1: Molecular DiagnosisQuestion 1. Do new molecular biology techniques such as fluorescence in situ hybridization and comparative genomic hybridization improve diagnostic accuracy for atypical, spitzoid, or borderline melanocytic proliferations or melanocytic proliferations of uncertain significance?
Summary of EvidenceIt is difficult, and sometimes even impossible, to establish a definitive diagnosis for certain pigmented tumors. The NCCN6 and AAD7 guidelines consider that molecular techniques should be viewed as complementary investigative tools and never used in the routine diagnosis of melanoma. Their results can aid prognosis but their diagnostic value has not been adequately demonstrated.
Level of evidence: 4.
RecommendationsMolecular biology techniques are recommended as potentially useful tools in situations of high uncertainty (borderline tumors or tumors of uncertain significance). Their diagnostic value, however, is unclear. We suggest these techniques be used on a case-by-case basis and integrated with clinical and histologic findings. Their results should preferably be jointly interpreted by a group of experts.
Grade of recommendation: C
Question 2. Can gene expression profiling improve the stratification of patients with AJCC 2017 stage I-II melanoma according to risk of disease progression? Can profiling results alter treatment decisions?
Summary of EvidenceThe melanoma CPGs reviewed6–9 do not currently recommend the routine use of gene expression profiling to stratify risk of disease progression. Nor do they establish when, in whom, or for which type of genetic profile these tests are applicable.6,7 There is insufficient evidence on the validity and diagnostic value of gene expression profiling to recommend its use in clinical practice. The main use of these tests is in the context of research studies or clinical trials. The negative psychological impact of being stratified into a poor prognosis group has not been evaluated.
Level of evidence: 2b.
RecommendationsWe do not recommend gene expression profiling as a routine staging procedure in melanoma, as there is insufficient evidence of its value.
Grade of recommendation: B.
Block 2. Biopsy and Surgical Treatment of Primary MelanomaQuestion 3. Does excisional biopsy offer a correct diagnosis in a higher proportion of cases than incisional biopsy (elliptical or punch) or shave biopsy in patients with pigmented facial or acral lesions that cause cosmetic or functional impairment if completely excised?
Summary of EvidenceIncisional and shave biopsy are less reliable than excisional biopsy for the diagnosis of melanoma.10
Level of evidence: 2b.
RecommendationsWe suggest excisional biopsy for the histologic confirmation of melanoma. Nonetheless, and bearing in mind its limitations for the correct diagnosis of melanoma, partial biopsy may be used for large acral or facial lesions when there is a risk of excisional biopsy resulting in considerable aesthetic or functional impairment.6,7,9
Grade of recommendation: B.
Question 4: Is incisional/partial biopsy versus excisional biopsy associated with worse survival outcomes in patients with suspected melanoma?
Summary of EvidenceMultiple studies have demonstrated that partial biopsy is not associated with worse survival outcomes in melanoma.7,9,10
Level of evidence: 2b.
RecommendationsWe suggest excisional biopsy for confirming a diagnosis of melanoma and as a first-line treatment for primary tumors. Nonetheless, there are cases, such as large tumors on the face or at an acral location, where excisional biopsy would produce a large surgical defect requiring reconstruction with a skin flap or graft. An incisional biopsy may be used for diagnostic confirmation in such cases. Incisional biopsy has not been demonstrated to be associated with worse survival outcomes or an increased risk of metastasis.7,9
Grade of recommendation: B.
Question 5. Does surgical excision with 10-mm versus 5-mm margins reduce local recurrence rates in patients with histologically confirmed melanoma in situ (stage 0)?
Summary of EvidenceNo randomized controlled trials have compared surgical excision with 5-mm and 10-mm margins in melanoma in situ. The recommendation for 5-mm margins in this setting was based on expert consensus, but appears to have been validated by the results of several later studies. Excision margins of 5 mm, however, may not be adequate in some clinical situations.7,9,10
Level of evidence: 5.
RecommendationsWe recommend excision with 5-mm margins for most melanomas in situ, as this can be considered adequate for achieving proper local control. Nevertheless, we suggest using wider margins or, where possible, surgery with margin control in certain clinical situations, especially lentigo maligna on the head.
Grade of recommendation: D.
Block 3. Treatment of Lentigo MalignaQuestion 6. Does excision with 10-mm margins versus excision with 5-mm margins or surgery with microscopic margin control result in lower local recurrence rates in patients with histologically confirmed lentigo maligna?
Summary of EvidenceWhile surgical procedures for the treatment of lentigo maligna have not been compared in randomized controlled trials, numerous studies in the CPGs revised6,7,10 suggest that:
Margins larger than 5 mm may be necessary to achieve histologically clear margins in large lentigo malignas due to subclinical extension.
Surgery with microscopic margin control is associated with higher cure rates and lower recurrence rates than conventional surgery.
Recommended excision margins are based on clinical margins measured by the surgeon, not on histologic margins measured by the pathologist.
Level of evidence: 4.
RecommendationsFor conventional excision we suggest using margins wider than 5 mm (and up to 10 mm) whenever possible.
Also, when possible, we suggest using techniques that allow exhaustive evaluation of histologic margins: serial excision with permanent cut paraffin-embedded sections or Mohs micrographic surgery.
We recommend evaluating the center of the permanent tumor specimen to correctly identify and stage any invasive component that might be present.
Grade of recommendation: C.
Question 7. How do radiotherapy, imiquimod therapy, cryotherapy, and observation compare in terms of their effect on survival in patients with histologically confirmed lentigo maligna not amenable to surgery?
Summary of EvidenceJust 1 randomized clinical trial comparing imiquimod monotherapy and imiquimod plus tazarotene 0.1% gel in the treatment of lentigo maligna has been performed. As the tumors were excised after the trial, there are no data on clinical cure rates. With the same limitations, 37% of patients in a phase II study achieved histologic cure with imiquimod.
The systematic review by Read et al.11 reported recurrence rates of around 13% for radiotherapy based on data from retrospective observational studies. The respective rates for imiquimod and laser therapy were 25% and 35%, although 100% recurrence has been reported for laser therapy in prospective studies. Recurrences rates with cryotherapy were not described. In general, the validity of the evidence is poor.
Level of evidence: 4.
RecommendationsWe suggest using surgical treatment whenever possible. When not possible, radiotherapy and imiquimod can be considered, although they appear to be associated with higher recurrence rates requiring closer follow-up. Laser therapy appears to be associated with worse outcomes.
Very little data exist on cryotherapy and laser therapy, rendering them largely unadvisable.
Clinical observation of large lentigo malignas may be adequate in very old patients with comorbidities not amenable to intensive therapy. If significant clinical or dermoscopic changes are detected during follow-up, suspicious areas should be biopsied to check for invasive disease.
Grade of recommendation: C.
Block 4. Staging and Follow-upQuestion 8. Do imaging and/or laboratory tests versus clinical examination and history taking improve overall survival in patients diagnosed with melanoma?
Summary of EvidenceImaging and/or laboratory tests are useful for the early detection of clinically occult metastasis, particularly in patients with stage III or higher melanoma. These tests are particularly important for correct staging and ensuring early initiation of appropriate systemic therapy. Additional tests have not been demonstrated to directly improve survival, and false positive rates must be taken into account.
Level of evidence: 2a.
RecommendationsWe recommend performing additional tests in patients with early-stage melanoma (stages I-II) if signs or symptoms suspicious for metastasis are detected. In patients with advanced disease, additional tests should be carried out to ensure correct staging and appropriate treatment.
Grade of recommendation: B
Question 9: Do imaging and/or laboratory tests versus self-examination and clinical examination at follow-up visits improve overall survival in patients diagnosed with and under treatment for melanoma?
Summary of EvidenceRoutine performance of additional tests may not be cost-effective in patients with early-stage melanoma. Nevertheless, some studies have demonstrated better survival in patients following the detection of occult metastasis amenable to early treatment with curative intent (lower tumor burden or surgically resectable tumor). Studies analyzing this question are from before the adjuvant therapy era.
Level of evidence: 2a.
RecommendationsPeriodic follow-up with proper physical examination and history taking aimed at detecting signs of metastasis is recommended in early-stage melanoma. Imaging and laboratory tests may be more useful from stage IIB on. Grade of recommendation: B.
We recommend periodic lymph node ultrasound evaluation in patients with sentinel lymph node (SLN) micrometastases who are not treated with complete lymph node dissection (CLND). Grade of recommendation: A.
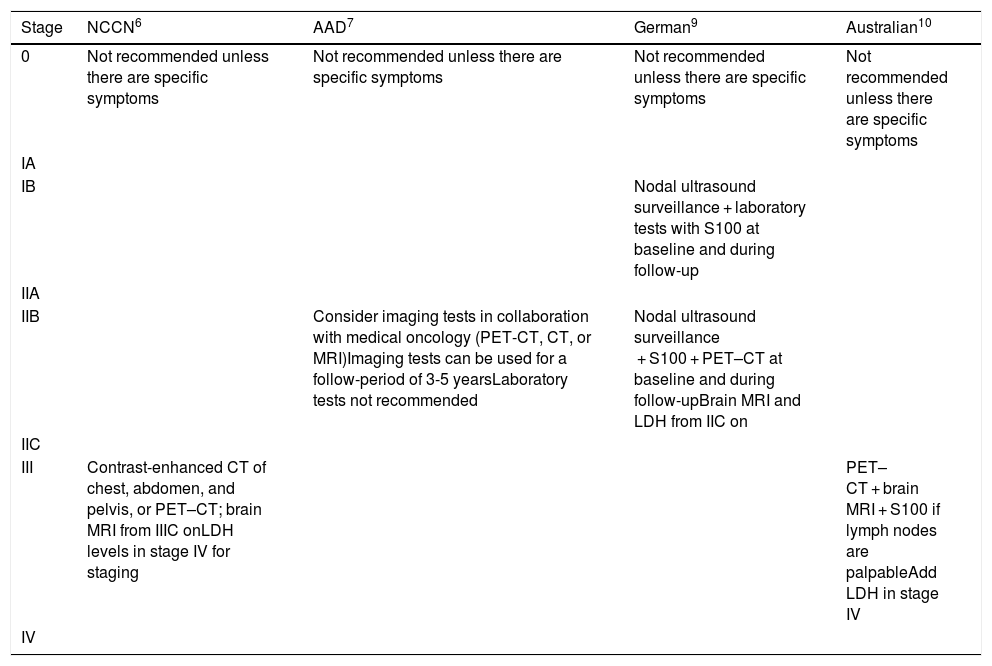
The follow-up approaches recommended by the different guidelines are described in Table 1.
Additional Tests at Baseline and During Follow-up: Guideline Recommendations.
| Stage | NCCN6 | AAD7 | German9 | Australian10 |
|---|---|---|---|---|
| 0 | Not recommended unless there are specific symptoms | Not recommended unless there are specific symptoms | Not recommended unless there are specific symptoms | Not recommended unless there are specific symptoms |
| IA | ||||
| IB | Nodal ultrasound surveillance + laboratory tests with S100 at baseline and during follow-up | |||
| IIA | ||||
| IIB | Consider imaging tests in collaboration with medical oncology (PET-CT, CT, or MRI)Imaging tests can be used for a follow-period of 3-5 yearsLaboratory tests not recommended | Nodal ultrasound surveillance + S100 + PET–CT at baseline and during follow-upBrain MRI and LDH from IIC on | ||
| IIC | ||||
| III | Contrast-enhanced CT of chest, abdomen, and pelvis, or PET–CT; brain MRI from IIIC onLDH levels in stage IV for staging | PET–CT + brain MRI + S100 if lymph nodes are palpableAdd LDH in stage IV | ||
| IV |
Abbreviations: AAD, American Academy of Dermatology; CT, computed tomography;, LDH, lactate dehydrogenase;, MRI, magnetic resonance imaging; NCCN, National Comprehensive Cancer Network; PET, positron emission tomography.
Question 10. Does follow-up by a dermatologist versus follow-up by a primary care physician or self-examination improve the early detection of a second melanoma or recurrence in patients who underwent surgical excision 5 or more years ago and have not developed metastasis?
Summary of EvidenceThe likelihood of melanoma recurrence generally decreases with time, but never disappears. Tumors recur sooner in patients with more advanced disease. The likelihood of recurrence is highest within 3 years of diagnosis of the initial tumor and decreases considerably after 5 years. It becomes much lower after 10 years.
The risk of developing a second melanoma ranges from 2% to 10%. Second melanomas tend to occur within a few years of the initial diagnosis, although they have been known to appear after more than 30 years. Patients with dysplastic nevi or a family history of melanoma have a higher risk.
Level of evidence: 5.
RecommendationsWe recommend close follow-up for 5 years after diagnosis to check for recurrences and for 10 years to check for second melanomas. Follow-up intervals will vary according to stage at diagnosis and individual risk. After 10 years, we suggest annual follow-up by a dermatologist, except in the case of higher-risk patients, where the timing of follow-up visits will vary according to their risk profile (fair skin, multiple melanocytic nevi, dysplastic nevus syndrome, family history of melanoma).
Grade of recommendation: D.
Block 5. Sentinel Lymph Node and CLNDQuestion 11. Does SLN biopsy versus observation improve overall survival in melanoma patients with a Breslow thickness of less than 0.8 mm?
Summary of EvidenceNo studies have analyzed the effect of SLN biopsy on overall or disease-free survival in patients with a primary melanoma with a Breslow thickness of less than 0.8 mm.
Level of evidence: 5
RecommendationsSLN biopsy should not be recommended in patients with a primary melanoma with a Breslow thickness of less than 0.8 mm in the absence of other high-risk histologic features.6,7,10,12
We suggest SLN biopsy be performed in patients with a primary melanoma with a Breslow thickness of less than 0.8 mm if there are other high-risk histologic features in the primary tumor (ulceration, high mitotic rate [> 2 / mm2], lymphovascular invasion, Clark level IV-V).6,7,12
Grade of recommendation: D.
Question 12. Does CLND versus follow-up with periodic ultrasound surveillance improve overall survival in patients with a positive SLN biopsy?
Summary of EvidenceThe effect of immediate CLND in patients with a positive SLN biopsy has been evaluated in 2 clinical trials: the Multicenter Selective Lymphadenectomy Trial-II13 and the DeCOG-SLT trial.14 Neither of the trials demonstrated a survival benefit for CLND versus periodic ultrasound surveillance and CLND on detection of nodal metastasis. The trials also described a higher frequency of sequelae (mainly lymphedema) in patients who underwent immediate CLND compared with those assigned to ultrasound surveillance after a positive SLN biopsy.
In addition, both trials demonstrated that between 75% and 88% of immediate CLNDs do not identify additional nodal metastases.13,14
Level of evidence: 1b.
RecommendationsFor patients with low-risk metastasis (SLN tumor burden ≤ 1 mm), we recommend replacing immediate CLND with ultrasound surveillance of the nodal basin followed by CLND if metastasis is detected.13,14
Grade of recommendation: A.
Question 13. Does information on number of lymph nodes involved, size of metastatic deposit, and location of metastasis in the lymph node provide relevant prognostic information for patients with nodal metastasis and should it be included in the pathology report?
Summary of EvidenceNodal tumor burden has been identified as a predictor of recurrence-free and melanoma-specific survival. The latest edition of the AJCC melanoma staging (TNM) system has maintained nodal involvement (N) as a key staging and prognostic factor.15
Other pathologic factors, such as extracapsular extension, are still useful for guiding decisions regarding the need for adjuvant radiotherapy.6
Level of evidence: 2a.
RecommendationsWe recommend recording the following information in pathology reports for patients who undergo SLN biopsy: lymph node region involved, number of metastatic lymph nodes, number of nonmetastatic lymph nodes, size of largest metastatic deposit (mm), location of metastasis within the lymph node, and presence of extracapsular extension. The pathology report should clearly differentiate SLNs from secondary or non-SLNs if removed.
In the case of patients who undergo CLND, we recommend including the following information in the pathology report: lymph node basin involved, number of metastatic lymph nodes, number of nonmetastatic lymph nodes, presence of matted nodes, and presence of extracapsular extension.
Grade of recommendation: B.
Question 14. Does SLN biopsy versus observation improve overall survival in patients with regional cutaneous disease (satellitosis, in-transit metastasis) detected at diagnosis or during follow-up?
Summary of EvidenceNo studies have demonstrated that SLN biopsy improves overall survival or regional control in melanoma patients with satellitosis or in-transit metastases.
SLN biopsy can be used for restaging purposes in such cases. Its value is thus prognostic, not therapeutic.
Level of evidence: 5.
RecommendationsWe do not recommend routine SLN biopsy in patients with resectable satellite or in-transit metastases because it does not improve survival or alter treatment decisions for the majority of patients.
Grade of recommendation: D.
Block 6: Adjuvant TherapyQuestion 15. Does adjuvant high-dose interferon therapy improve overall survival in patients with high-risk melanoma (stages IIB, IIC or III/IV after excision of metastasis and in the absence of visible disease)?
Summary of EvidenceA meta-analysis of 17 clinical trials that analyzed adjuvant interferon-alpha in patients with stage II and III melanoma showed an increase in overall survival compared with observation only (hazard ratio [HR], 0.91; 95% CI, 0.85-0.97; P = .003; number needed to treat [NNT], 35 patients [95% CI, 21-108] to prevent 1 death in 5 years).16 Not all studies, however, have demonstrated an improvement in overall survival for interferon therapy, which in addition is associated with considerable toxicity.
Level of evidence: 1b.
RecommendationsConsidering the efficacy demonstrated for immune checkpoint inhibitors and BRAF/MEK inhibitors as adjuvant therapies in clinical trials, we suggest that interferon-alpha should not be considered an alternative in this setting.
Grade of recommendation: A.
Question 16. Does adjuvant therapy with immune checkpoint inhibitors (anti-cytoxic T-lymphocyte antigen-4 [CTLA-4] and anti-programmed cell death 1 [PD-1] antibodies) improve overall survival in patients with high-risk melanoma (stages IIB, IIC or III/IV after excision of metastasis and in the absence of visible disease)?
Summary of EvidenceCompared with placebo, high-dose ipilimumab (10 mg/kg every 3 weeks, 4 doses, followed by a maintenance dose every 3 months for a maximum of 3 years) has been demonstrated to improve overall survival (5-year survival 65% vs. 54%; HR, 0.72 [0.58-0.88]; P = .001) in patients with stage IIIA > 1 mm or IIIB/C disease without in-transit metastasis (seventh edition of AJCC Cancer Staging Manual criteria).17
Studies conducted so far with the anti-PD-1 antibodies nivolumab and pembrolizumab as adjuvant therapy have demonstrated statistically significant improvements in disease-free survival compared with ipilimumab and placebo, respectively.18,19 Severe adverse effects were less common with nivolumab. No data were published for overall survival, even though this was a primary endpoint.
Level of evidence: 1b.
RecommendationsThere is no evidence showing an overall survival benefit for adjuvant therapy with anti-PD-1 antibodies, although, as recognized in the 2 pivotal trials,18,19 longer follow-up times are probably needed. Nonetheless, if it is decided to use checkpoint inhibitory immunotherapy, we recommend using anti-PD-1 antibodies as adjuvant therapy as they are associated with better disease-free survival and have a better toxicity profile than ipilimumab. Nivolumab and pembrolizumab are considered suitable options for adjuvant therapy in patients with melanoma and nodal involvement.6,10 The level of evidence and strength of recommendation for both nivolumab and pembrolizumab would be 1A for patients with IIIB or IIIC melanoma.6,10 It would also be 1A for patients with stage IIIA melanoma with lymph node metastases larger than 1 mm (pembrolizumab trial, KEYNOTE-05418) and for those with distant metastases (stage IV) after excision with clear margins (nivolumab, CheckMate 238 trial19).6,10 Other candidates for this therapy would be patients with excisable in-transit metastases, although in this case, the level of evidence and strength of recommendation would be lower.
Grade of recommendation: A.
Question 17. Does targeted adjuvant therapy with BRAF inhibitors improve overall survival in patients with high-risk melanoma (stages IIB, IIC or III/IV after excision of metastasis and in the absence of visible disease)?
Summary of EvidenceCombined treatment with the BRAF and MEK inhibitors dabrafenib and trametinib has been demonstrated to improve overall survival compared with placebo in patients with melanoma carrying the BRAF V600E or V600 K mutation with stage IIIA > 1 mm, IIIB, or IIIC disease. With a median follow-up of 2.8 years, the overall 3-year survival rate was 86% in the combined dabrafenib and trametinib group versus 77% in the placebo group (HR, 0.57; 95% CI, 0.42-0.79; P = .0006).20 The difference, however, was not significant as it did not reach the specified limit of P = .000019.
Level of evidence: 1b.
RecommendationsBased on the data for the groups treated in the COMBI-AD trial, which evaluated the combined use of dabrafenib and trametinib as adjuvant therapy, the AEDV’s recommendation would be to consider this treatment for all patients carrying the BRAF V600 mutation with stage III disease at diagnosis or following recurrence.20 Although the trial only included patients with lymph node metastases larger than 1 mm, dabrafenib/trametinib combined therapy is now used in patients with nodal metastases of any size. The recommendation could also be applied to patients with completely excised in-transit metastases.
Grade of recommendation: A.
Question 18. Does adjuvant radiotherapy versus observation improve overall survival in patients who have undergone therapeutic CLND and have a high risk of local recurrence (extracapsular extension or ≥ 2-4 lymph nodes or largest maximum diameter of ≥ 3-4 cm)?
Summary of EvidenceAdjuvant radiotherapy of the lymphatic drainage basin does not improve overall survival in patients with melanoma and lymph node metastases treated with CLND but considered to have a high risk of nodal recurrence (≥ 1 parotid node, ≥ 2 cervical or axillary nodes, ≥ 3 inguinal nodes; ≥ 3 cm in cervical lymph nodes, ≥ 4 cm in axillary or inguinal lymph nodes, or evidence of extracapsular extension). It does, however, improve local control (statistically significant reduction in nodal recurrence).21 This conclusion is based on the results of a study conducted before the use of current adjuvant therapy. Current findings might be different.
Level of evidence: 1b.
RecommendationsWe suggest that adjuvant radiotherapy could be considered in patients with melanoma and lymph node metastases who have a high risk of nodal recurrence after surgery as it has been demonstrated to improve local disease control.21 The potential adverse effects associated with radiotherapy should be evaluated in all cases, particularly fibrosis and limb edema, which is more severe in the lower limbs of patients who receive radiotherapy to the groin region.6,9,10
Grade of recommendation: A.
Block 7: Treatment of Locoregional Disease and Surgical Treatment of Regional Disseminated and/or Distant diseaseQuestion 19. Compared with observation, does treatment with surgery, isolated limb perfusion, electrochemotherapy, radiotherapy, intralesional interleukin 2, imiquimod, oncolytic virus therapy, rose bengal, or systemic therapy improve overall survival? And does it improve quality of life?
Summary of EvidenceNo overall survival benefit has been demonstrated for local treatments in melanoma. There is also no evidence of their effect on patient quality of life. In general, the preference is to surgically excise satellite or in-transit metastases whenever possible. A wide range of other treatment possibilities exists.
Level of evidence: 2b.
RecommendationsWe recommend excising satellite or in-transit metastases with clear margins whenever possible. A number of alternative treatments can be contemplated when surgery with clear margins is not possible, including isolated limb perfusion, intralesional treatment (with interleukin 2, Bacillus-Calmette-Guérin, bengal rose, T-VEC), topical immunotherapy (imiquimod, diphencyprone), radiotherapy, local ablation (carbon dioxide laser therapy), electrochemotherapy, and cryotherapy. Systemic therapies can be considered in patients with multiple lesions.
Grade of recommendation: B.
Question 20. Does excision of metastasis improve overall survival in patients with stage III or IV melanoma? And does it improve patient quality of life?
Summary of EvidenceThere is general consensus that CLND with curative intent is necessary to achieve regional control in patients with nodal metastasis. Similarly, surgical excision is preferred to observation for isolated metastasis or oligometastasis.
Level of evidence: 4.
RecommendationsWe recommend considering surgical excision of solitary or multiple lymphatic or visceral metastases, including brain metastases, assuming they are surgically accessible.
Grade of recommendation: C.
Block 8: Medical Treatment of Metastatic DiseaseQuestion 21. Do immune checkpoint inhibitors (anti-CTLA-4, anti-PD-1) and targeted therapy with BRAF inhibitors improve overall survival in patients with stage III and IV melanoma not amenable to surgery?
Summary of EvidenceTreatment with immune checkpoint inhibitors, whether anti-CTLA-4 (ipilimumab) or anti-PD-1 (nivolumab, pembrolizumab) antibodies or a combination of both (nivolumab/ipilimumab), has been demonstrated to improve overall survival in patients with advanced melanoma (unresectable stage III or stage IV) regardless of whether or not they have a BRAF mutation.
- -
Ipilimumab (3 mg/kg every 3 weeks, 4 cycles) versus peptide vaccine gp100, median overall survival 10.1 vs. 6.4 months (HR, 0.66, P = .003); ipilimumab (10 mg/kg) and dacarbazine (850 mg/m2) in weeks 1, 4, 7, and 10, followed by dacarbazine only every 3 weeks up to week 22 versus dacarbazine (850 mg/m2) in the same weeks, median overall survival 11.2 vs. 9.1 months (HR, 0.72, P < .001).22–24
- -
Nivolumab (3 mg/kg every 2 weeks) versus dacarbazine (1000 mg/m2 every 3 weeks), median overall survival 37.5 vs. 11.2 months; HR, 0.46 (95% CI, 0.36-0.59; P < .001).25,26
- -
Pembrolizumab (10 mg/kg every 3 weeks) versus ipilimumab (3 mg/kg every 3 weeks, 4 cycles), median overall survival not reached vs. 16 months; HR, 0.68 (95% CI, 0.53-0.86; P = .0008).27,28
- -
Nivolumab/ipilimumab (nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks, 4 doses, followed by nivolumab 3 mg/kg every 2 weeks) versus ipilimumab (3 mg/kg every 3 weeks, 4 doses), median overall survival > 60 months and not reached vs. 19.9 months; HR, 0.54 (95% CI, 0.44-0.67; P < .001).29–32
- -
Nivolumab (nivolumab 3 mg/ kg every 2 weeks) versus ipilimumab (3 mg/kg every 3 weeks, 4 doses), median overall survival 36.9 vs. 19.9 months; HR, 0.65 (95% CI, 0.53-0.79; P < .001).29–32
Different combinations of BRAF and MEK inhibitors (dabrafenib/trametinib, vemurafenib/cobimetinib, encorafenib/binimetinib) have been demonstrated to improve overall survival in patients with advanced BRAF-mutant melanoma (unresectable stage III or stage IV):
- -
Dabrafenib/trametinib versus dabrafenib, median overall survival 25.1 vs. 18.7 months; HR, 0.71 (95% CI, 0.55-0.92; P = .0107)33; dabrafenib/trametinib versus vemurafenib, median overall survival not reached vs. 17.2 months; HR, 0.69 (95% CI, 0.53-0.89; P = .005).34
- -
Vemurafenib/cobimetinib versus vemurafenib, median overall survival 22.3 vs. 17.4 months; HR, 0.70 (95% CI, 0.55-0.90;, P = .005).35
- -
Encorafenib/binimetinib versus vemurafenib, median overall survival 33.6 vs. 16.9 months; HR, 0.61 (95% CI, 0.47-0.79; P < .0001).36,37
Level of evidence: 1b.
RecommendationsWe recommend using either nivolumab or pembrolizumab (anti-PD-1 antibodies) or nivolumab combined with ipilimumab to treat advanced non-BRAF-mutant melanoma (unresectable stage III or stage IV).6,10,38
In patients with advanced BRAF-mutant melanoma, we recommend one of the available combinations of BRAF and MEK inhibitors (dabrafenib/trametinib, vemurafenib/cobimetinib, or encorafenib/binimetinib) or nivolumab or pembrolizumab (anti-PD-1 antibodies), or the combination nivolumab/ipilimumab. The criteria for choosing one drug class over another (BRAF vs. immune checkpoint inhibitor) have not been defined, but the general preference is to begin with a BRAF inhibitor in patients with a high tumor burden who need a rapid response, as immune checkpoint inhibitors take longer to exert their effect.6,10,38
Grade of recommendation: A.
DiscussionThis CPG on the management of melanoma, adapted from a number of recently published guidelines, is designed to guide decision-making in the field of dermato-oncology in Spain.
The main strength of our study is the use of a rigorous, reproducible method for adapting existing recommendations on the management of melanoma to our setting. An additional strength is that the recommendations were reviewed by external experts from several disciplines.
One possible limitation is that all the panelists responsible for drawing up the recommendations were dermatologists, but the scope and purpose of the project was to create a CPG to be used by dermatologists practising in Spain. The guidelines consulted were multidisciplinary and the draft versions of our CPG were revised by experts from several disciplines.
To retain its validity, the content of this guideline should be revised in the next 3 years.
As with any guideline, the recommendations are not binding; they should be applied flexibly with consideration of local availability of resources, physician experience, and patient preferences.
FundingThe AEDV White Paper on Cancer was fully funded by the AEDV Health Skin Foundation. No external or pharmaceutical companies (except those invited to participate in the external revision and submit their comments like other interested parties) were involved in its production.
This guideline is part of the AEDV’s Health Skin Foundation's White Paper on Cancer.
Please cite this article as: Botella-Estrada R, Boada-García A, Carrera-Álvarez C. Guía de práctica clínica de melanoma de la Academia Española de Dermatología y Venereología. ACTAS Dermo-Sifiliográficas 2021;112:142–152.