Our aim was to describe the epidemiologic, clinical, and laboratory characteristics of acute parvovirus B19 infection in adults.
Material and methodsThis study describes all cases of acute parvovirus B19 infection in patients older than 18 years of age who were treated at Hospital Universitario La Paz in Madrid, Spain, in 2012.
ResultsForty-nine adults were treated for acute parvovirus B19 infection. Most were young women who were infected in the spring or early summer. In over half the cases skin lesions were key diagnostic signs. We saw the full range of types of rash of purplish exanthems that were fairly generalized; vasculitis was relatively common (in >18%). Mild or moderate abnormalities in blood counts and indicators of liver dysfunction resolved spontaneously in all but 2 immunocompromised patients, who developed chronic anemia.
ConclusionsThis is the largest case series of acute parvovirus B19 infection published to date. This infection should be suspected on observing signs of purplish skin rashes, no matter the location or pattern of distribution, or vasculitis, especially if accompanied by fever and joint pain in young women in the spring. Measures to avoid infection should be recommended to individuals at risk.
El objeto de nuestro trabajo es describir las características epidemiológicas, clínicas y analíticas de la infección aguda por parvovirus B19 en adultos.
Material y métodosPresentamos un estudio descriptivo retrospectivo de todos los casos de infección aguda por parvovirus B19, en mayores de 18 años, durante el año 2012, en el Hospital Universitario La Paz, Madrid.
ResultadosCuarenta y nueve pacientes adultos con infección aguda por parvovirus B19. La mayoría ocurrieron en mujeres jóvenes en primavera y principios de verano. La lesión cutánea fue el signo fundamental para el diagnóstico en más del 50% de los casos. Se encontraron todo tipo de exantemas purpúricos más o menos generalizados, siendo relativamente frecuente la forma de vasculitis (>18%). Las alteraciones en el hemograma y perfil hepático, leves o moderadas, se resolvieron espontáneamente, salvo en 2 pacientes inmunodeprimidos en quienes persistió una anemia crónica.
ConclusionesEs la serie más amplia de infección aguda por parvovirus B19 descrita, hasta la fecha, en la literatura. Ante exantemas purpúricos de cualquier distribución o lesiones de vasculitis, sobre todo si se acompañan de fiebre y artralgias y se presentan en mujeres jóvenes en primavera, debemos sospechar una infección aguda por parvovirus B19 y recomendar medidas para evitar el contagio a personas de riesgo.
Parvovirus B19 infection is uncommon in adults, and few cases have been described in the literature. A greater understanding of the epidemiologic, clinical, and laboratory characteristics of this infection in adults is important, as early recognition of signs and symptoms can help to prevent the spread of infection among at-risk individuals.
Parvovirus B19 is a small, nonenveloped, single-stranded DNA virus discovered by chance by Yvonne Cossart and her team in 1975 during blood screening for hepatitis B.1 It is the only member of the Parvoviridae family that can cause disease in humans. It is an airborne virus with an incubation period of 13 to 17 days.2 It has a particular tropism for red blood cell progenitors and endothelial cells, as it binds to the P antigen in the cell membranes.3
Parvovirus B19 generally infects children of school-going age at the end of the winter and is more common in regions with a temperate climate.4 In a third of cases, the infection is asymptomatic. In the remaining cases, it causes erythema infectiosum or the fifth disease, which typically presents with a malar rash that spares the nose and eye regions, giving what is known as the slapped cheek appearance. This is followed by a lacy rash on the trunk and limbs.1,5 Not all patients develop systemic manifestations, such as fever and joint pain, and when they do, they are usually mild.
Between 60% and 80% of adults have parvovirus B19 immunoglobulin (Ig) G antibodies,6,7 explaining why symptoms are much less common in adults than in children. However, in contrast to children, adults in the symptomatic stage can spread the infection,2 hence the importance of early diagnosis.8
In otherwise healthy adults, acute parvovirus B19 infection is generally self-limiting. It primarily affects women in their fourth or fifth decades and manifests most frequently as fever, rash, and symmetric, peripheral polyarticular pain.9 Skin lesions are typically purpuric and can be generalized (with a distal, symmetric distribution)10 or localized (with an asymmetric distribution).8
In patients with risk factors, acute parvovirus B19 infection can lead to serious complications, such as aplastic crisis in patients with hemoglobinopathies,11 chronic anemia in immunodepressed individuals,12,13 and hydrops fetalis in pregnant women.14
The aim of this article is to describe the typical epidemiological, clinical, and laboratory features of acute parvovirus B19 infection in adults in our setting.
Material and MethodsWe performed a retrospective, descriptive study of adult patients diagnosed with acute parvovirus B19 infection at Hospital General Universitario La Paz, in Madrid, Spain in 2012.
We included men and women aged over 18 years who tested positive for IgM antibodies to parvovirus B19 during the study period. The antibodies were measured by enzyme-linked immunosorbent assay (Biotrin).
Two patients with positive parvovirus B19 IgM antibodies were classified as false positives and excluded from the study. They both had high levels of Epstein-Barr virus (EBV) IgM, negative EBV nuclear antigen IgG, and low parvovirus B19 IgM levels. The results were therefore interpreted as a cross-reaction in patients with infectious mononucleosis.
The following variables were recorded for all patients studied: date of diagnosis; department in which the diagnosis was made (dermatology, rheumatology, internal medicine, or other), patient age and sex, personal history of interest, cutaneous and noncutaneous manifestations, blood alterations, liver profile, kidney profile, C-reactive protein (CRP) levels, antinuclear antibodies (ANAs), histologic findings in skin biopsies, and clinical outcome. The concomitant detection of IgM antibodies specific to other viruses was also recorded to investigate the possibility of cross-reactions.
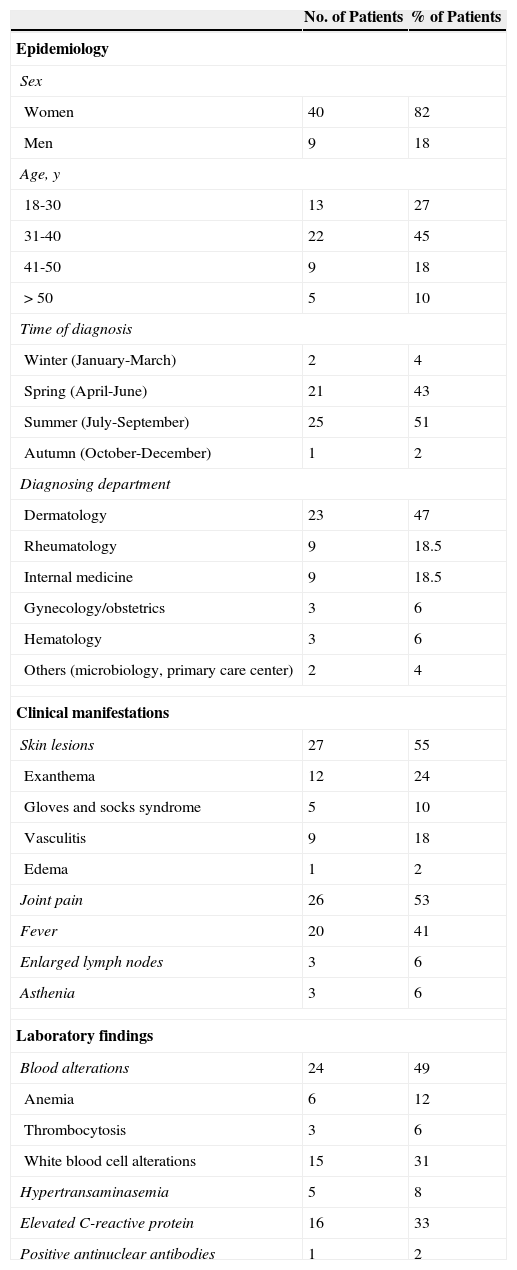
ResultsForty-nine adults (40 women and 9 men) had a positive parvovirus B19 IgM result during the study period (Table 1). Their ages ranged from 20 to 82 years and 90% were aged between 20 and 46. The mean age was 37.5 years and the mean and mode were both 36. There was a peak in incidence in June (12 cases) and July (16 cases). Only 3 cases were diagnosed in autumn and winter.
Characteristics of Acute Parvovirus B19 Infection in Adults.
| No. of Patients | % of Patients | |
|---|---|---|
| Epidemiology | ||
| Sex | ||
| Women | 40 | 82 |
| Men | 9 | 18 |
| Age, y | ||
| 18-30 | 13 | 27 |
| 31-40 | 22 | 45 |
| 41-50 | 9 | 18 |
| >50 | 5 | 10 |
| Time of diagnosis | ||
| Winter (January-March) | 2 | 4 |
| Spring (April-June) | 21 | 43 |
| Summer (July-September) | 25 | 51 |
| Autumn (October-December) | 1 | 2 |
| Diagnosing department | ||
| Dermatology | 23 | 47 |
| Rheumatology | 9 | 18.5 |
| Internal medicine | 9 | 18.5 |
| Gynecology/obstetrics | 3 | 6 |
| Hematology | 3 | 6 |
| Others (microbiology, primary care center) | 2 | 4 |
| Clinical manifestations | ||
| Skin lesions | 27 | 55 |
| Exanthema | 12 | 24 |
| Gloves and socks syndrome | 5 | 10 |
| Vasculitis | 9 | 18 |
| Edema | 1 | 2 |
| Joint pain | 26 | 53 |
| Fever | 20 | 41 |
| Enlarged lymph nodes | 3 | 6 |
| Asthenia | 3 | 6 |
| Laboratory findings | ||
| Blood alterations | 24 | 49 |
| Anemia | 6 | 12 |
| Thrombocytosis | 3 | 6 |
| White blood cell alterations | 15 | 31 |
| Hypertransaminasemia | 5 | 8 |
| Elevated C-reactive protein | 16 | 33 |
| Positive antinuclear antibodies | 1 | 2 |
Three patients were receiving immunosuppressants to treat an underlying disease and classified as immunosuppressed. One was receiving azathioprine and hydroxychloroquine to treat systemic lupus erythematosus, while the other 2 were receiving periodic infusions of infliximab to treat rheumatoid arthritis in one case and hidradenitis suppurativa in the other. A fourth patient was considered to be immunocompromised due to advanced age and multiple comorbidities. Five patients were identified as at risk because of pregnancy.
Skin lesions, present in 27 patients (55.1%), were the most common clinical finding. Joint pain was the most common systemic manifestation of infection and was reported by 26 patients (53.1%). Most of these patients described symmetric, polyarticular pain in the phalanges, wrists, ankles, elbows, and knees. Several patients, however, had monoarthritis affecting the central axis of the body, and reported cervical pain, lower back pain, and hip pain. Twenty patients (40.8%) had fever, but their temperature generally remained under 38.5°C. Important diagnostic clues in our series included enlarged lymph nodes in the lateral cervical chains (3 cases), weakness or asthenia (3 cases), and swelling of the feet and hands (1 case).
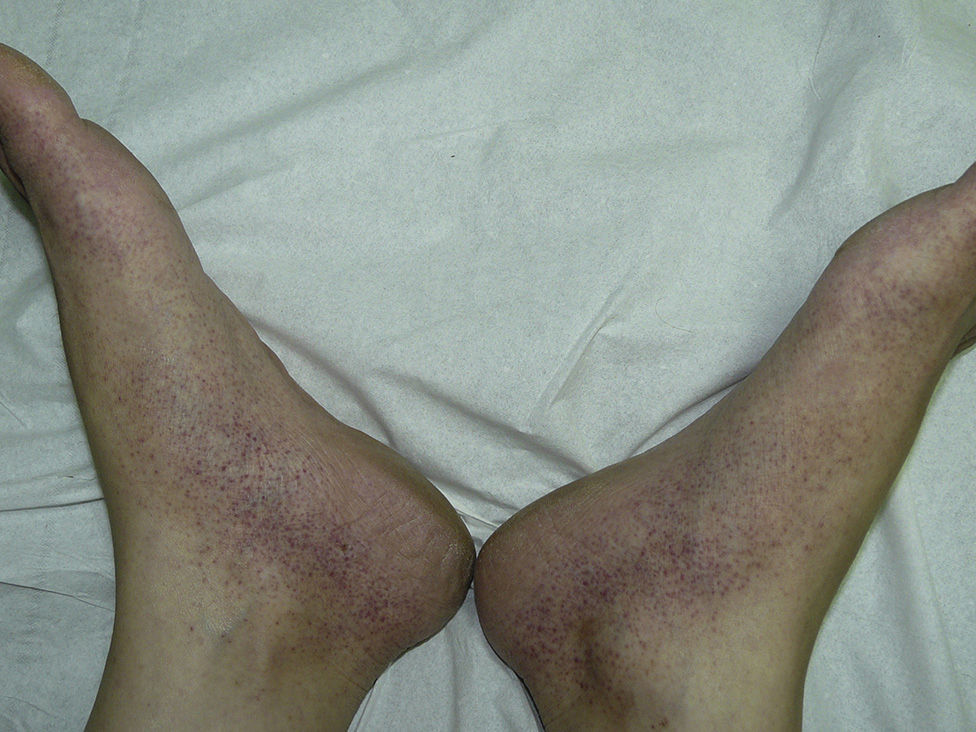
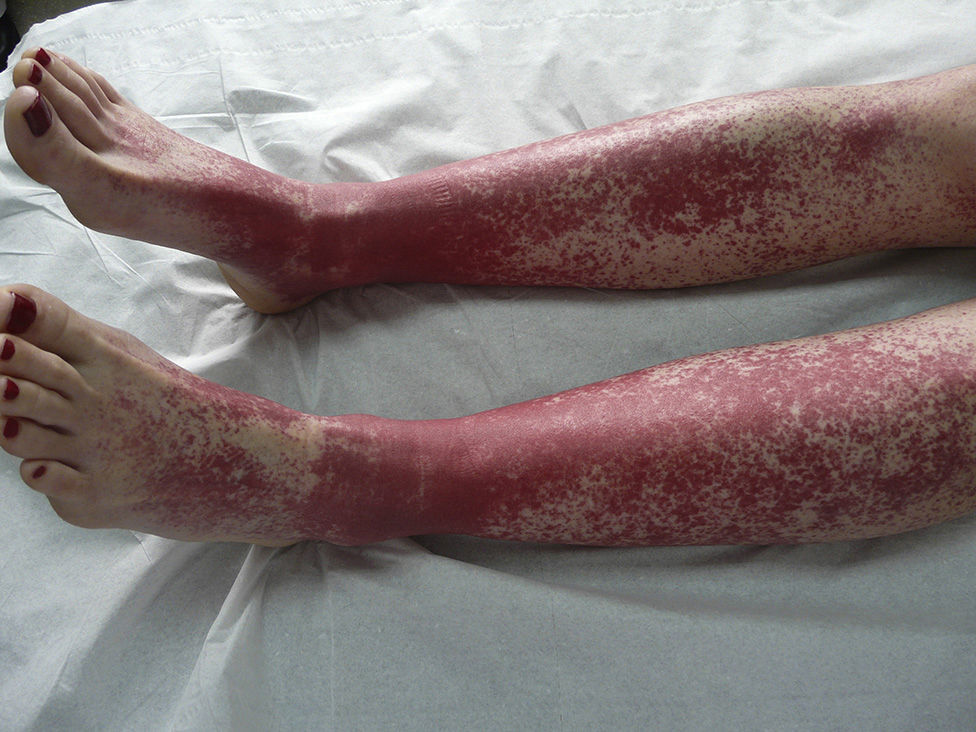
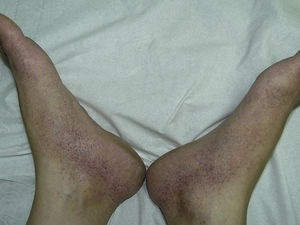
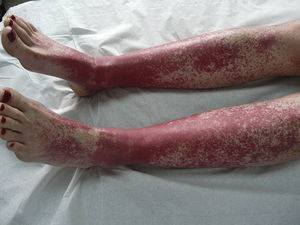
The most common skin lesion was purpuric exanthema, which was largely symmetric and present on the trunk and limbs (12 cases). Six patients had purpuric lesions consistent with the papular-purpuric gloves and socks syndrome (Fig. 1), and 9 patients had palpable purpura clinically consistent with vasculitis on the anterior surface of the lower limbs (Fig. 2). One patient had intense swelling of the legs, feet, forearms, and hands, with epidermal detachment, erosions, and purpura.
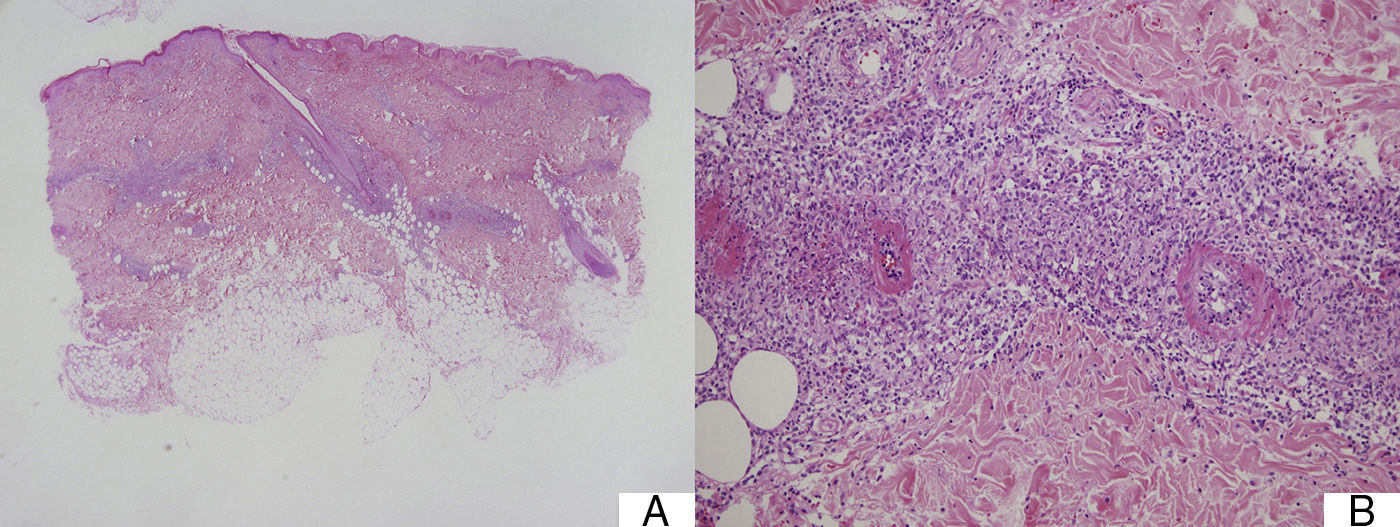
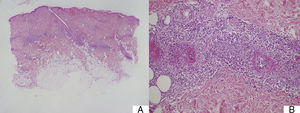
Suspected cases of vasculitis were confirmed by histologic examination of skin biopsy samples. The examination revealed superficial perivascular inflammation that also affected the deep dermis in 1 case, dilated vessels with endothelial swelling, fibrinoid necrosis of vessels, and nuclear dust (leukocytoclasis) (Fig. 3).
A, Mixed superficial and deep perivascular inflammation (panoramic view, hematoxylin-eosin, original magnification ×2). B, Higher magnification view of fibrinoid necrosis of vessel, perivascular inflammatory infiltrate, and nuclear dust or leukocytoclastia (hematoxylin-eosin, original magnification ×10).
Serology tests were ordered by the following departments: dermatology (23 cases), internal medicine (9 cases), rheumatology (9 cases), hematology (3 cases), gynecology (3 cases), and microbiology (1 cases); 1 test was also ordered by a primary care center.
Blood tests revealed 6 cases of mild anemia (12.2%), 4 of which resolved spontaneously. Two immunocompromised patients—the elderly patient with multiple comorbidities and a 75-year-old patient with rheumatoid arthritis under treatment with infliximab—were referred to the hematology department with persistent anemia. White blood cell alterations—mainly lymphopenia, monocytosis, and neutrophilia—were detected in 15 patients (30.6%). Three patients (6.1%) had thrombocytosis. CRP levels were mildly to moderately elevated in 15 patients (30.6%). There were 5 cases (10.2%) of mild to moderate elevations of transaminase levels, which normalized spontaneously. One patient tested positive for ANAs, despite previous negative tests. It should be noted, however, that an ANA test was ordered only for 21 patients (Table 1).
Parvovirus B19 infection was self-limiting in 75.5% of patients. In 20.4% of cases, systemic corticosteroids were used to provide symptomatic relief. This treatment was particularly effective in patients with clinical features of vasculitis and in the patient with intense swelling. Nine patients improved following the administration of 12mg of intramuscular betamethasone. The patient with vasculitis of the superficial and deep vessels on histology required tapering doses of prednisone, starting at 0.5mg/kg/d, with reductions of 10mg every 10 days. The 2 older patients are still being monitored for chronic anemia. The 5 pregnant women underwent weekly ultrasound examinations, and no complications were observed.
Positive IgM antibodies against EBV were observed in 18.4% of cases; 6.1% of patients had a positive borderline IgM result, 8.1% had a negative borderline IgM result, and 6.1% had an equivocal IgM result. In all cases, however, IgM titers were much higher for parvovirus B19 and practically negligible for EBV, indicating that EBV IgM positivity was the result of a cross-reaction. None of the patients tested positive for cytomegalovirus IgM antibodies. Serology tests for measles and rubella were ordered in very few cases.
DiscussionWe have described the epidemiologic, clinical, and laboratory characteristics of acute parvovirus B19 infection in 49 adults. Based on our review of the literature, this is the largest such series published to date. The high incidence of cases in such a short space of time lends weight to the theory that parvovirus B19 infection occurs in epidemic cycles.15–17
Coinciding with previous reports, we observed that acute parvovirus B19 infection is more common in young women6 and occurs more frequently in late spring and in summertime.18
The literature suggests that systemic manifestations such as joint pain and fever are more common than skin lesions in adults,19–21 but in our series, skin lesions were the most common clinical finding.
The heterogeneous distribution of purpuric exanthemas observed in our patients is also consistent with previous reports of acute parvovirus B19 infection in adults.2,8 This infection is considered to be one of the main causes of papular-purpuric gloves and socks syndrome, first described in 1990,22 and is in fact believed to be responsible for 80% of all cases. Associations with other viruses, namely, EBV, cytomegalovirus, hepatitis virus B, varicella-zoster virus, human herpes viruses 6 and 7, measles virus, rubella virus, and coxsackievirus, have also been described.10 Papular-purpuric gloves and socks syndrome consists of erythema and swelling of the feet and hands that can cause pruritus, pain, and a burning sensation. The erythema is sharply demarcated at the wrists and ankles, and progresses to purpuric lesions that spread centripetally and may be accompanied by mucosal lesions and systemic involvement.23–25 The histologic features of papular-purpuric gloves and socks syndrome are nonspecific and include varying degrees of epidermal spongiosis, foci of parakeratosis, a mild perivascular infiltrate around the superficial dermal vascular plexus, and extravasated red blood cells around the superficial capillaries of the dermis.26 Over 70 cases of papular-purpuric gloves and socks syndrome associated with parvovirus B19 have been reported to date,10 and our series adds a further 6 cases.
Parvovirus B19 has been implicated in the etiology of vasculitis,25 but few cases have been described. Clearly, the most remarkable finding in our series is the high frequency of skin lesions clinically consistent with vasculitis in patients with no other evident cause of this inflammation. Over 18% of patients had palpable purpura on the lower limbs, and one had necrotic lesions, indicating the severity of the condition.
Based on existing reports, joint pain and fever are the most common manifestations of acute parvovirus B19 infection in adults.18–20 While these conditions were common in our series, they were outnumbered by skin lesions. Other signs and symptoms described in the literature, such as enlarged lymph nodes, weakness or asthenia, and swelling,19 should raise suspicion of acute parvovirus B19 infection in patients with a compatible clinical presentation.
Anemia was detected in 12.2% of our patients; this rate is much lower the rate of over 80% reported elsewhere.20 The condition, however, was, as is commonly described, self-limiting in immunocompetent patients. The fact that anemia persisted in 2 immunocompromised patients in our series may be related to the chronification of infection (which has been previously described in immunodepressed patients), as the onset of anemia coincides with the detection of parvovirus IgM antibodies in blood samples. White blood cell alterations—particularly lymphopenia, monocytosis, and neutrophilia—were the most common blood alterations detected in our series, and contrasting with previous reports, we observed thrombocytosis (3 cases) rather than thrombocytopenia.19 Also, although transient elevation of transaminases is very common, affecting almost 90% of patients in other series,19 only 10.2% of the patients in our series had raised transaminase levels.
None of the patients in our series developed serious disease, although the literature contains isolated reports of acute parvovirus B19 infection associated with acute fulminant hepatitis,27 acute glomerulonephritis,28 acute encephalitis and peripheral neuropathy,29 myocarditis, and aplastic anemia in both immunocompetent and immunocompromised patients.
Parvovirus B19 infection has been reported in association with a wide variety of autoimmune disorders, including juvenile rheumatoid arthritis, reactive arthritis, systemic lupus erythematosus, dermatomyositis, polymyositis, systemic sclerosis, primary biliary cirrhosis, and autoimmune cytopenia.30–32 According to Lunardi et al., 30 parvovirus B19 might not only have a lytic effect on endothelial cells and a cytotoxic effect on platelet progenitors, but also lead to the production of antibodies that, through cross-reactivity, could attack autoantigens and possibly induce autoimmune disease. This theory would explain the high frequency of vasculitis in our series. A systematic study of autoantibodies involving more patients followed for a longer time is needed to demonstrate that parvovirus B19 infection is capable of inducing not only an autoimmune response but also an autoimmune disorder.
Disease outcome was, as expected,6 favorable in our series, with clinical manifestations resolving spontaneously in under 2 weeks in most cases. Possible signs of persistent infection were present in immunodepressed patients, coinciding with observations in the majority of reports published.13
Little has been published on the treatment of acute parvovirus B19 infection, probably because when the infection does cause symptoms, these tend to be self-limiting. A number of articles have described the successful use of immunoglobulin infusion therapy in the treatment of persistent infection.13,31 Long-term administration of systemic corticosteroids has been associated with an increased risk of infections.33 However, in a systematic review of parvovirus B19 infection associated with neurological manifestations, Barah et al.34 reported that a combined regimen of systemic corticosteroids and immunoglobulin infusion therapy was effective.34 In our series, clinical improvement occurred rapidly in patients treated with systemic corticosteroids.
Pregnant women with acute parvovirus B19 infection must undergo regular examinations to monitor for fetal distress. The examinations should be weekly up to the end of pregnancy, as the risk of fetal involvement can persist for months after the resolution of symptoms.14
We detected a high rate of cross-reactivity with EBV, with slightly positive, borderline, and equivocal IgM found in 38.7% of patients. Cross-reactions between parvovirus B19 and EBV, cytomegalovirus, rubella, and measles have been previously reported.35,36
Early detection of acute parvovirus B19 infection in adults can result in important epidemiological benefits. The infection should be suspected in any patient with a purpuric rash, regardless of its distribution, and particularly if the patient is a young woman, seen in the spring or summer months, with fever and joint pain. The presence of skin lesions, presenting as either nonpalpable purpura (papular-purpuric gloves and socks syndrome) or palpable purpura (probably vasculitis), is particularly suggestive. The differential diagnosis should include other viral infections and conditions involving purpuric lesions, such as capillaritis, which manifests as purpuric lesions on the legs and also occurs in young women in the spring and summer. Capillaritis, however, does not tend to affect the dorsal or plantar surfaces of the feet and is not accompanied by fever, joint pain, or general malaise.
Our findings are likely to be affected by some bias due to the descriptive and retrospective nature of the study. Although to our knowledge our series is the largest yet published on adult parvovirus B19 infection, even larger studies are required to be able to draw conclusions that can be more accurately extrapolated to our setting. Also needed are prospective studies that apply standardized protocols for ordering serology tests and that span several years to cover the epidemic nature of parvovirus B19 infection.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Rodríguez Bandera AI, Arenal MM, Vorlicka K, Bravo-Burguilllos ER, Vega DM, Díaz-Arcaya CV. Estudio retrospectivo de 49 casos de infección aguda por parvovirus B19 en adultos. Actas Dermosifiliogr. 2015;106:44–50.