Tildrakizumab is an IL-23-inhibitor that has been approved to treat plaque psoriasis. However, few reports have become available on its efficacy profile in the real-world. Our objective was to study the mid-term efficacy of tildrakizumab in patients with moderate-to-severe psoriasis in the Spanish routine clinical practice setting. This was a retrospective multicenter study that included a total of 91 psoriatic patients on tildrakizumab. The mean Psoriasis Area and Severity Index (PASI) was 9.09 (SD, 5.30). The overall tildrakizumab survival rate was 93.47% for a mean treatment exposure of 30.18 weeks (SD, 16.57). No drug discontinuation was associated with drug tolerability, or adverse reactions. Absolute PASI ≤3 was reached by 91.3% and 96.5% of the patients on weeks 28 and 52, respectively. Response was not impacted by weight, age (>65), metabolic syndrome, presence of arthritis, or previous number of biological therapies used. Based on our own experience tildrakizumab is an effective strategy to treat plaque psoriasis and difficult-to-treat-areas.
El tildrakizumab es un inhibidor de la interleucina 23 (IL-23) aprobado para el tratamiento de la psoriasis en placas. Sin embargo, existen pocos estudios acerca de su eficacia en práctica clínica. Nuestro objetivo fue analizar la eficacia a medio plazo del tildrakizumab en los pacientes con una psoriasis moderada-grave en la práctica diaria en España. Se realizó un estudio multicéntrico incluyendo a 91 pacientes con psoriasis en tratamiento con tildrakizumab. La media del Índice de Área y Gravedad de la Psoriasis (PASI) fue de 9,09 (desviación estándar [DE] 5,30). La supervivencia global al tildrakizumab fue del 93,5% para una exposición media al tratamiento de 30,2 semanas (DE 16,6). No hubo interrupciones relacionadas con la tolerabilidad o efectos. El 91,3% y el 96,5% alcanzaron un PASI ≤ 3 en las semanas 28 y 52, respectivamente. La respuesta no se vio influida por el peso, la edad (>65), el síndrome metabólico, la presencia de artritis o el número previo de terapias biológicas. En nuestra experiencia, el tildrakizumab es un fármaco eficaz para el tratamiento de la psoriasis en placas y las áreas difíciles de tratar.
Psoriasis is a chronic inflammatory immune-mediated systemic disease manifesting in the skin, joints, or both, which is commonly associated with several important medical conditions including mood disorders, chronic kidney disease and cardiometabolic syndrome.1 Interleukin (IL)23/IL-17 axis, and IL-17 producing cells play a major role in the development of psoriasis. Immune checkpoint blockade of these cytokines and tumour necrosis factor alpha (TNFα) provided by biological therapies has revolutionized the management of severe chronic plaque psoriasis.1
Tildrakizumab is a humanized IgG1/κ type monoclonal antibody that selectively targets the p19 subunit of the cytokine interleukin 23 (IL-23). It was approved in 2018 to treat patients with moderate-to-severe cutaneous psoriasis by the main regulatory agencies (both the U.S. Food and Drug Administration [FDA] and the European Medicines Agency [EMA]). However, only the latter approved its use at a dose of 200mg in patients >90kg.2 The short- and long-term safety efficacy of tildrakizumab have been evaluated in two randomized phase III, multicenter, double blinded, placebo-controlled landmark trials (reSURFACE 1 and 2), while etanercept (reSURFACE 2) has been studied in patients with moderate-to-severe plaque psoriasis eligible for systemic or advanced therapies.3–5 The regulatory approval of medical drugs is based on strict randomized clinical trials (RCT). Still, long-term non-interventional studies are needed to determine the safety and efficacy profile in the real-world practice to bridge the gap between RCT and the routine clinical practice.6 Although, in the past few years, some authors2,5–10 have demonstrated the short- and mid-term real-world safety and efficacy profile of tildrakizumab in the management of plaque psoriasis, including difficult-to-treat-areas11 more studies are still needed. The aim of this study is to evaluate the short- and mid-term safety, efficacy, tolerability, and survival of tildrakizumab in our cohort and compare the results obtained with those previously reported in other real-world series and RCT.
Materials and methodsWe conducted a multicentric, non-interventional, observational, and retrospective study among 13 hospitals from the Valencian community, Spain and followed patients of our routine clinical practice from January 2020 through January 2022. Patients included were adults with moderate-to-severe cutaneous psoriasis eligible for systemic treatment, including special areas. Participants were followed for up to 52 weeks. At baseline SC tildrakizumab 100mg was administered on weeks 0 and 4, followed by a maintenance dose every 12 weeks. Cohort monitoring was performed at baseline and on weeks 4, 12, 28, 36 and 52, when available. Demographic data, previous systemic therapies and the study variables measured are shown in Table 1. Disease severity and tildrakizumab response were assessed using the Psoriasis Area and Severity Index (PASI), and the Dermatology Life Quality Index (DLQI). Disease control12 was confirmed after reaching an absolute PASI ≤3 in plaque psoriasis, or PGA ≤1 in difficult-to-treat sites on weeks 4, 12, 24, 36 and 52, when available. The safety and tolerability of tildrakizumab were evaluated at the follow-up. No substitution tools were used for missing data. Quantitative variables were expressed as mean and standard deviation (SD), and the qualitative ones as frequencies. Log-rank test was used to compare tildrakizumab efficacy and survival time in non-obese [body mass index (BMI) <30kg/m2] vs obese patients (BMI >30kg/m2), presence vs absence of psoriatic arthritis, age older or younger than 65 years, presence or absence of metabolic syndrome, biological therapy-naïve vs experienced subjects, and the number of previous courses of biological therapies received. Cox regression models were used to study the potential relationship between drug survival and patient's age, weight, presence of psoriatic arthritis, presence of metabolic syndrome and previous biological therapies used. Overall survival was estimated using the Kaplan–Meier curves. Missing data was managed as observed. Odds ratios (OR) were reported, and P values <.05 were considered statistically significant. Any reason associated with drug discontinuation was reported and considered in the analysis. Analyses were performed using IBM SPSS v21.0 statistical software package (Armonk, NY, United States).
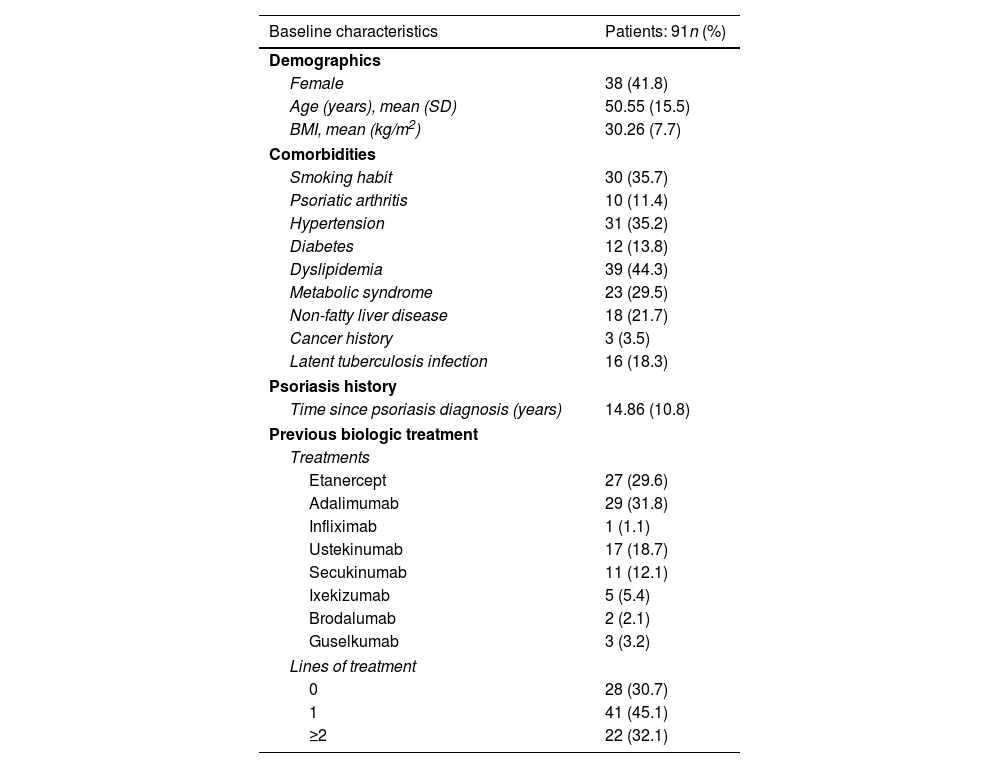
Baseline characteristics, comorbidities, and previous biological therapies used.
| Baseline characteristics | Patients: 91n (%) |
|---|---|
| Demographics | |
| Female | 38 (41.8) |
| Age (years), mean (SD) | 50.55 (15.5) |
| BMI, mean (kg/m2) | 30.26 (7.7) |
| Comorbidities | |
| Smoking habit | 30 (35.7) |
| Psoriatic arthritis | 10 (11.4) |
| Hypertension | 31 (35.2) |
| Diabetes | 12 (13.8) |
| Dyslipidemia | 39 (44.3) |
| Metabolic syndrome | 23 (29.5) |
| Non-fatty liver disease | 18 (21.7) |
| Cancer history | 3 (3.5) |
| Latent tuberculosis infection | 16 (18.3) |
| Psoriasis history | |
| Time since psoriasis diagnosis (years) | 14.86 (10.8) |
| Previous biologic treatment | |
| Treatments | |
| Etanercept | 27 (29.6) |
| Adalimumab | 29 (31.8) |
| Infliximab | 1 (1.1) |
| Ustekinumab | 17 (18.7) |
| Secukinumab | 11 (12.1) |
| Ixekizumab | 5 (5.4) |
| Brodalumab | 2 (2.1) |
| Guselkumab | 3 (3.2) |
| Lines of treatment | |
| 0 | 28 (30.7) |
| 1 | 41 (45.1) |
| ≥2 | 22 (32.1) |
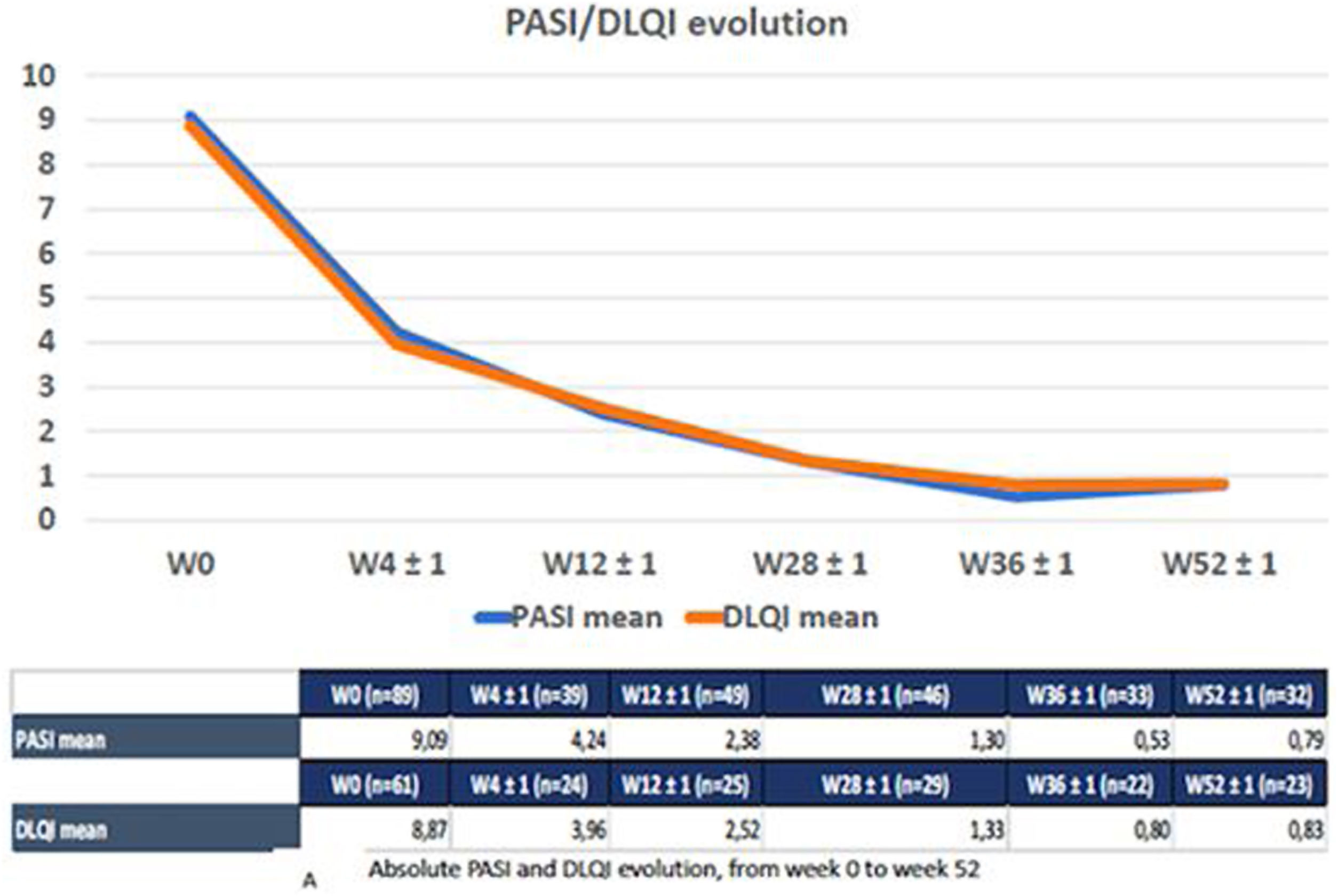
A total of 91 patients (53 men) with moderate-to-severe psoriasis treated with tildrakizumab were included. Baseline characteristics of the cohort are shown in Table 1. The participants’ mean age was 50.6 (SD, 15.5). The mean psoriasis history since diagnosis was 14.8 years (SD, 10.8), and the mean BMI, 30.26 (SD, 7.7). A total of 35 patients (45.5%) were obese and 30 subjects (35.7%) were smokers. Regarding psoriasis phenotypes, 85.7% of the patients showed classical psoriasis plaques, while considering only psoriasis in hard-to-treat-areas; scalp, nail, palmoplantar and genital involvement was present in 28.7%, 16.1%, 8% and 6.9% of the study participants, respectively. Only one patient showed erythrodermic psoriasis. Among the comorbidities evaluated, only 10 patients (11.4%) had psoriatic arthritis. Regarding cardiovascular risk factors, 35.2%, 13.8%, 44.3% and 29.5% of the patients had arterial hypertension, diabetes, dyslipidemia, and metabolic syndrome, respectively. Mood disorders (depression or anxiety) were presents in 25 patients (27.2%). Three patients had a past medical history of neoplasm, but did not relapse while on therapy. Additionally, 17 patients were diagnosed of latent tuberculosis (TB) infection (16 of them were on chemoprophylaxis), while no patients developed active infections. The prevalence of main comorbidities and other psoriasis-related diseases is shown in Table 1. Methotrexate was the most widely used (65%) classical systemic treatment. A total of 28 patients (30.7%) were biological therapy-naïve vs 41 (45.1%) and 15 (16.5%) participants who had previously received, at least, 1 and 2 courses of biological therapies, respectively. Adalimumab was the most previously prescribed biological therapy (29 patients, 31.8%) in the biological therapy-experienced group, followed by etanercept (29.6%) and ustekinumab (18.7%). Guselkumab therapy had failed in three patients (Table 1). The mean baseline PASI was 9.09 (SD, 5.30), body surface area was 11.5 (SD, 11.3), and the Dermatology Life Quality Index (DLQi) was 8.87 (SD, 5.42). The overall survival rate of tildrakizumab was 93.5% for a mean treatment exposure of 30.2 weeks (SD, 16.6), with only six drug withdrawals (three secondary failures and three primary failures). No drug discontinuation was associated with drug tolerability or adverse events. No association was found either between the different variables collected and these withdrawals, such as age, BMI, metabolic syndrome, presence of psoriatic arthritis, or previous experience with biological therapies. On week 28, 91.3% and 71.7% of the 46 patients who came to our office for evaluation, reached absolute PASIs ≤3 and ≤1, respectively, with a mean DLQI of 1.3 (SD, 2.42). On treatment week 52, patients who completed that treatment period were assessed (n=32), 96.5% and 65.2% of whom reached absolute PASIs ≤3 and 0, respectively, with a mean DLQI of 0.83 (SD, 1.47). The mean PASI and DLQi responses on weeks 4, 12 and 36 are shown in Fig. 1. The overall effectiveness and survival rates associated with age, BMI, metabolic syndrome, presence or absence of psoriatic arthritis, being biological therapy-naïve or not, and the number of previous biological therapies received were analyzed. Nonsignificant differences were seen in the log-rank-tests (Supplementary data. Figs. 2A/2B, 3A/3B and 4). No severe adverse events associated with the administration or exposure to tildrakizumab were reported either.
Absolut PASI and DLQI evolution from week 0 to week 52. The reduced number of patients seen between weeks 0 and 52 is mainly associated with patients not having reached that phase of the follow-up period yet. Only six patients experienced drug discontinuations, three due to primary failure, and three due to secondary failure.
Patients with moderate-to-severe cutaneous psoriasis treated with tildrakizumab in the routine clinical practice differed significantly from those included in the reSURFACE clinical trials 1–2,3,4 where inclusion criteria were strict, both highly pretreated patients, and commonly excluded fragile participants. Currently, different real-world series assessing the safe and efficacy of tildrakizumab have been published.2,5–10 Our results are similar to those obtained from previous real-world series, and slightly higher than those obtained from the reSURFACE clinical trials 1–2 (72% and 43% of the patients included maintained PASI 90 and PASI 100 responses, respectively, on week 52)3–5 and from the Tilot trial.6 The mean PASI in our cohort was lower 9.09 (SD, 5.30) than that reported in the reSURFACE trials (20.5 [SD 7.6]). However, our number of participants who used biological therapies was higher (69.3% vs 17.9%) and no-washout period was required.3,4 Regarding biological therapy-naïve patients, in our cohort, the rate (30.7%) was higher than that reported by Ruiz-Villaverde et al. (5%),5 and lower than that reported by Caldarola et al. (60%),10 and as occurred in these series, in our study, all special differences regarding effectiveness were reported. An interesting piece of information from our series is that three patients who had developed a previous solid malignancy did not experience cancer reactivation at the 52-week follow-up. This patient profile was also reported by Ruiz-Villaverde et al.5 and Wei et al.,2 but was not included in the RCT, which is why this finding could contribute to reinforce the safe use of this drug in this fragile population. In our series, the overall efficacy and survival were not impacted by age, BMI, metabolic syndrome, presence of psoriatic arthritis, being biological therapy-naïve or not, or by the number of previous biologicals therapies received. On the other hand, some authors5 reported that a shorter duration of the disease, scalp involvement and a low number of previous biological therapies could be associated with a great drug survival and better patient outcomes. This high degree of effectiveness was also confirmed in difficult-to-treat-areas, specially scalp and nails.6,11,13 The mean limitation of our study was its retrospective design, lack of control group, and the sample size, although we believe that it is suitable for comparison purposes with other reported real-world series.
ConclusionsBased on our own results, tildrakizumab may represent an effective and safe strategy for the management of patients with moderate-to-severe cutaneous psoriasis, including patients with several comorbidities. Future studies should be conducted to assess the effectiveness of tildrakizumab vs other IL-23 inhibitors.14–17
Conflict of interestThe authors declare that they have no conflict of interest.