Patients with melanoma appear to take extreme sun-protection measures, which could influence 25-hydroxyvitamin D [25(OH)D] levels. The aim of this study was to measure 25(OH)D levels in patients with cutaneous melanoma and identify factors associated with inadequate levels.
Material and methodsOver a period of 1 year, we prospectively measured serum 25(OH)D in patients with cutaneous melanoma and used logistic regression analysis to identify environmental, phenotypic, and genotypic factors that were associated with insufficient and deficient levels.
ResultsOf 215 patients analyzed, 8.8% had deficient 25(OH)D levels (<10ng/mL) and just 24.7% had normal levels. Insufficient levels (<30ng/mL) were associated with obesity (odds ratio [OR], 4.2; 95% confidence interval [CI], 1.3-13.3) and blood sampling in autumn/winter (OR, 2.1; 95% CI, 1.1-4). Deficient levels (<10ng/mL) were associated with obesity (OR, 7.1; 95% CI, 1.1-46.9), blood sampling in autumn/winter (OR, 9.0; 95% CI, 1.7-47.0), absence of freckles (OR, 5.4; 95% CI, 1.2-23.4), and, with marginal significance, the presence of fewer than 2 nonsynonymous melanocortin-1 receptor (MC1R) polymorphisms (OR, 5.0; 95% CI, 0.9-28.9).
LimitationsSome factors related to 25(OH)D levels, such as food, were not included in the analyses.
Conclusions25(OH)D levels should be monitored in patients with melanoma and the need for oral supplements should be contemplated where appropriate.
Los pacientes con melanoma parecen llevar al extremo las medidas de protección, lo que puede influir en los niveles de 25-hidroxivitamina D —25(OH)D—El objetivo del estudio fue evaluar los niveles de 25(OH)D en pacientes con melanoma cutáneo e identificar factores relacionados con niveles inadecuados.
Material y métodosSe midieron prospectivamente los niveles séricos de 25(OH)D en pacientes diagnosticados de melanoma cutáneo durante un periodo de seguimiento de un año. Se evaluaron qué factores ambientales, fenotípicos y genotípicos se relacionaban con niveles insuficientes y deficientes mediante regresión logística.
ResultadosDe un total de 215 pacientes solo un 24,7% tenían valores normales de 25(OH)D y un 8,8% tenían valores deficientes (<10ng/ml). La obesidad (OR: 4,2; IC 95% OR: 1,3-13,3) y la extracción de sangre realizada en otoño/invierno (OR: 2,1; IC 95% OR: 1,1-4) se asociaron a niveles insuficientes (<30ng/ml). Los niveles deficitarios (<10ng/m) se asociaron a la obesidad (OR: 7,1; IC 95% OR: 1,1-46,9), la extracción de sangre realizada en otoño/invierno (OR: 9,0; IC 95% OR: 1,7-47,0), la ausencia de efélides (OR: 5,4; IC 95% OR: 1,2-23,4) y, marginalmente, a la presencia de tener <2 polimorfismos no sinónimos en el receptor 1 de la melanocortina (MC1R) (OR: 5,0; IC 95% OR: 0,9-28,9).
LimitacionesNo se han incluido en el análisis algunos factores, como la alimentación, relacionados con los niveles de 25(OH)D.
ConclusionesSe deberían monitorizar los niveles de 25(OH)D en los pacientes con melanoma y valorar dar suplementos orales en los casos que lo precisen.
Vitamin D is considered a hormone that is essential for human homeostasis, above all for bone metabolism.1 Its relevance to other functions has been suggested by epidemiologic studies showing an association between varying degrees of diminished levels of circulating 25-hydroxyvitamin D—25(OH)D—and autoimmune processes,2–5 cardiovascular diseases,6–8 infections,9 and the development of cancer.10–13
Vitamin D insufficiency is a fairly common finding in populations the world over.14 Ninety percent of the required amount is synthesized in skin exposed to UV-B light (290–320nm).1 Dietary sources of vitamin D are mainly blue fish and eggs, plus milk products, juices, and cereals that have been supplemented with the vitamin.
Vitamin D synthesis, and the consequent levels of circulating 25(OH)D, can be influenced by many factors, such as latitude, daily sun exposure, and seasonal changes in solar radiation among other environmental variables; in addition, skin color, the amount of skin exposed to the sun, and the use of sunscreens also play roles.15
Sun protection is recommended for preventing skin cancer in the general population, but avoidance of sun exposure is particularly important for patients who have been diagnosed with melanoma. The great majority of these patients become wary of sunlight to varying degrees, some of them adopting extreme measures of avoidance that eventually have a negative influence on circulating 25(OH)D levels.
This study aimed to assess 25(OH)D blood levels during routine follow-up in a series of patients with cutaneous melanoma in order to determine the prevalence of insufficiency or deficiency and identify possible associated factors.
Material and MethodsWe designed a retrospective observational case-control study of records for 215 patients with cutaneous melanoma evaluated in the dermatology department of the Instituto Valenciano de Oncología between March 1, 2011, and May 31, 2012. The patients resided in the autonomous community of Valencia (latitude 39°), where the UV index ranges from an average of 1 in December and January to 8 in June and July.16
Included were all patients who were seen for follow-up for whom routine blood tests were ordered.
The main variable of interest was the level of circulating 25(OH)D determined by chemiluminescence immunoassay. We used the Liaison automated analyzer (functional sensitivity under 4ng/mL; normal cutoff, 30ng/mL). The quantitative results were then categorized for analysis to reflect 3 levels, consistent with the literature: deficiency, <10ng/mL; insufficiency, 10–30ng/mL; and normal, >30–88ng/mL.17,18
Two comparisons were made in this study. In the first, suboptimal 25(OH)D levels (deficient plus insufficient) were compared to normal ones. In the second, deficient levels were compared to normal ones.
Sun exposure habits were recorded for all patients based on their answers to a previously validated structured questionnaire19 filled out during a telephone interview in which the interviewer was blinded to 25(OH)D level. The interviews took place within a month of blood sampling to minimize recall bias. The following information about the patient's melanoma phenotype and genotype was taken from the melanoma database: age, (<70 years vs ≥70 years); sex; body mass index (BMI) in the categories of low (<18.5kg/m2), normal (18.5–24.9kg/m2, overweight (25–29.9kg/m2), and obese (≥30kg/m220), hair color (red, light brown/blonde, or dark brown/black); eye color (dark or light); phototype (I–II vs III–V); and the presence or absence of freckles. We also recorded nonsynonymous polymorphisms in the melanocortin receptor 1 (MC1R) gene using the Sanger sequencing method. The gene and polymorphism distributions in our population have been described.21 For this study patients were classified as having fewer than 2 polymorphisms or 2 or more. The relative amount of weekly sun exposure during the 4 months prior to blood sampling was recorded in terms of season and hours of exposure of at least 25% of the body surface calculated with the Wallace rule of nines. The results were then distributed categorically in 3 levels: less than 6hours, 6 to 14hours, or more than 14hours. Season was recorded as autumn/winter or spring/summer.
We used contingency tables to compare the distributions of independent variables in relation to circulating 25(OH)D level. Significance was tested with Pearson's χ2 test. The degree of association between each independent variable and vitamin insufficiency and deficiency was assessed by univariate and multivariate logistic regression analysis to obtain odds ratios (ORs) and 95% CIs. All variables with a level of P<.1 entered the multivariate analysis; statistical significance was established at P<.05.
Statistical analyses were performed with SPSS, version 15.0 (Statistical Package SPSS Inc, Chicago, IL, USA).
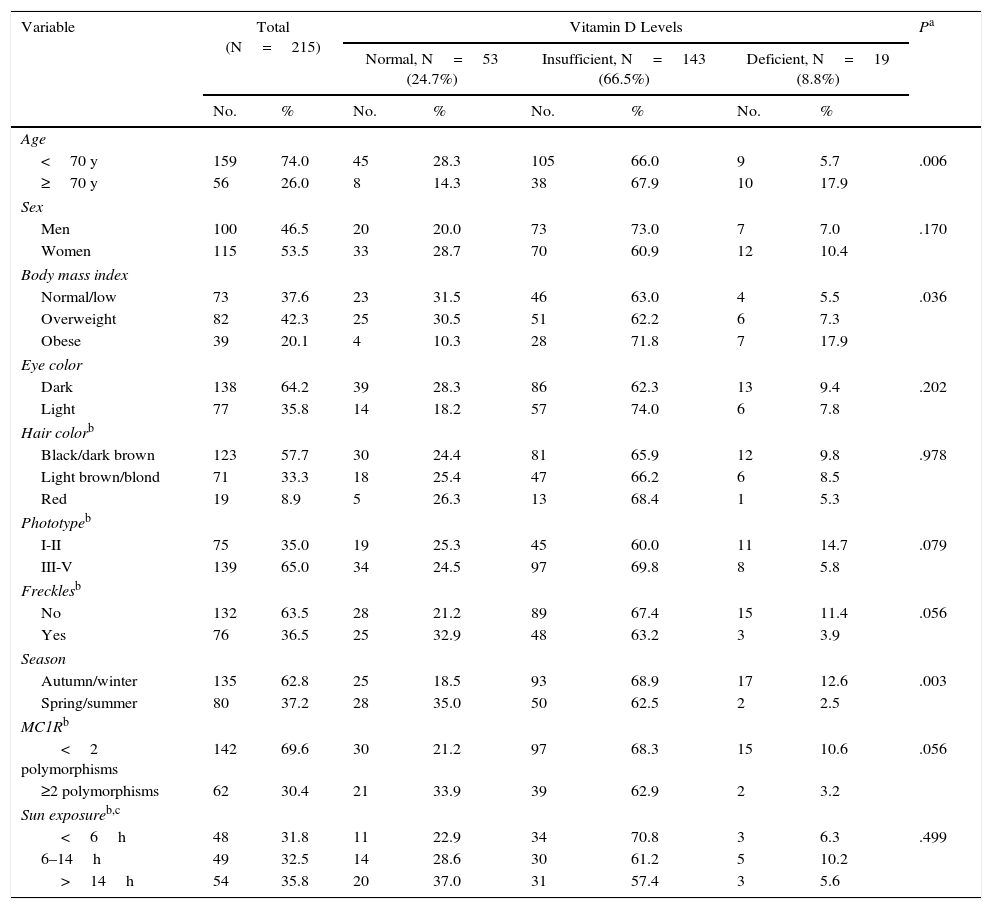
ResultsA total of 250 patients diagnosed with melanoma were included. Five declined to participate and 30 could not be contacted to answer the questionnaire. The final patient series therefore included data for 215. Patient characteristics and all study variables including 25(OH)D levels are shown in Table 1. The median age at the time samples were extracted was 56 years (range, 19–93 years). The proportion of overweight patients was 42.3%. No patients were in the low BMI category, dark eyes and black or dark brown hair predominated. A majority had no freckles.
Characteristics of the Study Population According to Circulating Vitamin D Levels.
| Variable | Total (N=215) | Vitamin D Levels | Pa | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal, N=53 (24.7%) | Insufficient, N=143 (66.5%) | Deficient, N=19 (8.8%) | |||||||
| No. | % | No. | % | No. | % | No. | % | ||
| Age | |||||||||
| <70 y | 159 | 74.0 | 45 | 28.3 | 105 | 66.0 | 9 | 5.7 | .006 |
| ≥70 y | 56 | 26.0 | 8 | 14.3 | 38 | 67.9 | 10 | 17.9 | |
| Sex | |||||||||
| Men | 100 | 46.5 | 20 | 20.0 | 73 | 73.0 | 7 | 7.0 | .170 |
| Women | 115 | 53.5 | 33 | 28.7 | 70 | 60.9 | 12 | 10.4 | |
| Body mass index | |||||||||
| Normal/low | 73 | 37.6 | 23 | 31.5 | 46 | 63.0 | 4 | 5.5 | .036 |
| Overweight | 82 | 42.3 | 25 | 30.5 | 51 | 62.2 | 6 | 7.3 | |
| Obese | 39 | 20.1 | 4 | 10.3 | 28 | 71.8 | 7 | 17.9 | |
| Eye color | |||||||||
| Dark | 138 | 64.2 | 39 | 28.3 | 86 | 62.3 | 13 | 9.4 | .202 |
| Light | 77 | 35.8 | 14 | 18.2 | 57 | 74.0 | 6 | 7.8 | |
| Hair colorb | |||||||||
| Black/dark brown | 123 | 57.7 | 30 | 24.4 | 81 | 65.9 | 12 | 9.8 | .978 |
| Light brown/blond | 71 | 33.3 | 18 | 25.4 | 47 | 66.2 | 6 | 8.5 | |
| Red | 19 | 8.9 | 5 | 26.3 | 13 | 68.4 | 1 | 5.3 | |
| Phototypeb | |||||||||
| I-II | 75 | 35.0 | 19 | 25.3 | 45 | 60.0 | 11 | 14.7 | .079 |
| III-V | 139 | 65.0 | 34 | 24.5 | 97 | 69.8 | 8 | 5.8 | |
| Frecklesb | |||||||||
| No | 132 | 63.5 | 28 | 21.2 | 89 | 67.4 | 15 | 11.4 | .056 |
| Yes | 76 | 36.5 | 25 | 32.9 | 48 | 63.2 | 3 | 3.9 | |
| Season | |||||||||
| Autumn/winter | 135 | 62.8 | 25 | 18.5 | 93 | 68.9 | 17 | 12.6 | .003 |
| Spring/summer | 80 | 37.2 | 28 | 35.0 | 50 | 62.5 | 2 | 2.5 | |
| MC1Rb | |||||||||
| <2 polymorphisms | 142 | 69.6 | 30 | 21.2 | 97 | 68.3 | 15 | 10.6 | .056 |
| ≥2 polymorphisms | 62 | 30.4 | 21 | 33.9 | 39 | 62.9 | 2 | 3.2 | |
| Sun exposureb,c | |||||||||
| <6h | 48 | 31.8 | 11 | 22.9 | 34 | 70.8 | 3 | 6.3 | .499 |
| 6–14h | 49 | 32.5 | 14 | 28.6 | 30 | 61.2 | 5 | 10.2 | |
| >14h | 54 | 35.8 | 20 | 37.0 | 31 | 57.4 | 3 | 5.6 | |
Abbreviation: MC1R, melanocortin-1 receptor gene.
There was a statistically significant association between 25(OH)D level in blood and age, BMI, and season of sampling. The presence or not of freckles and MC1R polymorphisms were also marginally related (Table 1).
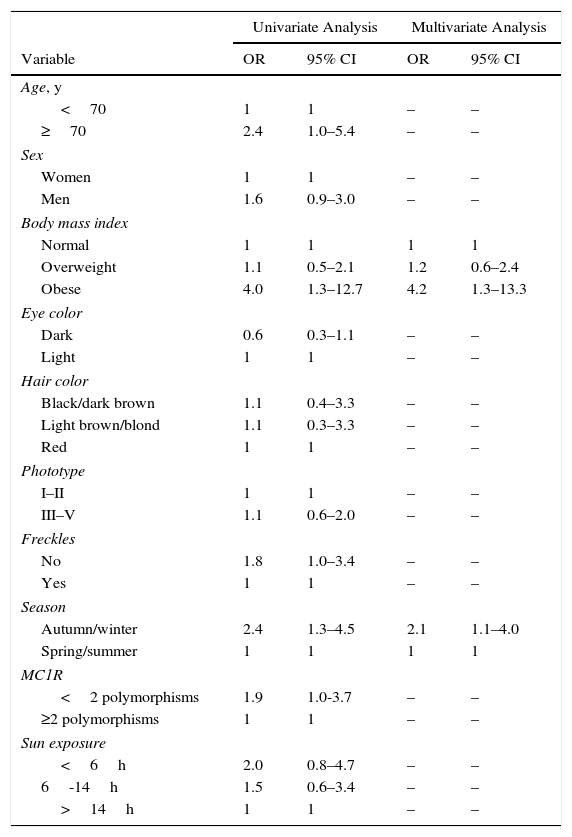
We first studied variables in relation to inadequate 25(OH)D levels (deficiency plus insufficiency). Age, obesity, and blood sampling in the autumn or winter were associated in the univariate analysis (Table 2). After multivariate analysis only obesity (OR, 4.2; 95% CI, 1.3–13.3) and autumn/winter sampling (OR, 2.1; 95% CI, 1.1–4) remained in the model (Table 2).
Results of Univariate and Multivariate Logistic Regression Analyses of Variables Related to 25-Hydroxyvitamin D Insufficiency and Deficiency (vs Normal Values).
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variable | OR | 95% CI | OR | 95% CI |
| Age, y | ||||
| <70 | 1 | 1 | – | – |
| ≥70 | 2.4 | 1.0–5.4 | – | – |
| Sex | ||||
| Women | 1 | 1 | – | – |
| Men | 1.6 | 0.9–3.0 | – | – |
| Body mass index | ||||
| Normal | 1 | 1 | 1 | 1 |
| Overweight | 1.1 | 0.5–2.1 | 1.2 | 0.6–2.4 |
| Obese | 4.0 | 1.3–12.7 | 4.2 | 1.3–13.3 |
| Eye color | ||||
| Dark | 0.6 | 0.3–1.1 | – | – |
| Light | 1 | 1 | – | – |
| Hair color | ||||
| Black/dark brown | 1.1 | 0.4–3.3 | – | – |
| Light brown/blond | 1.1 | 0.3–3.3 | – | – |
| Red | 1 | 1 | – | – |
| Phototype | ||||
| I–II | 1 | 1 | – | – |
| III–V | 1.1 | 0.6–2.0 | – | – |
| Freckles | ||||
| No | 1.8 | 1.0–3.4 | – | – |
| Yes | 1 | 1 | – | – |
| Season | ||||
| Autumn/winter | 2.4 | 1.3–4.5 | 2.1 | 1.1–4.0 |
| Spring/summer | 1 | 1 | 1 | 1 |
| MC1R | ||||
| <2 polymorphisms | 1.9 | 1.0-3.7 | – | – |
| ≥2 polymorphisms | 1 | 1 | – | – |
| Sun exposure | ||||
| <6h | 2.0 | 0.8–4.7 | – | – |
| 6-14h | 1.5 | 0.6–3.4 | – | – |
| >14h | 1 | 1 | – | – |
Abbreviation: MC1R, melanocortin-1 receptor gene.
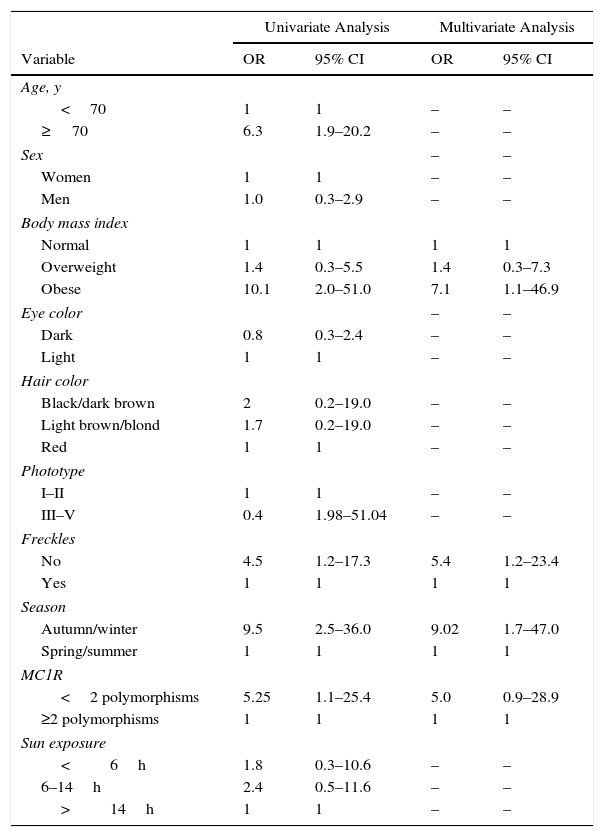
Second, we compared variables in relation to 25(OH)D deficiency versus normal values (Table 3). Age 70 years or more, obesity, phototype III–V, absence of freckles, autumn or winter sampling, and the presence of fewer than 2 MC1R polymorphisms were associated in the univariate analysis. On multivariate analysis 3 variables remained significantly associated with 25(OH)D deficiency: obesity (OR, 7.1; 95% CI, 1.1–46.9), blood extraction in the autumn or winter (OR, 9.0; 95% CI, 1.7–47.0), and absence of freckles (OR, 5.4; 95% CI, 1.2–23.4). We also included the presence of fewer than 2 MC1R polymorphisms in the model, although the level of significance was marginal (OR, 5.0; 95% CI, 0.9–28.9) (Table 3).
Results of Univariate and Multivariate Logistic Regression Analyses of Variables Related to 25-Hydroxyvitamin D Deficiency (vs Normal Values).
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variable | OR | 95% CI | OR | 95% CI |
| Age, y | ||||
| <70 | 1 | 1 | – | – |
| ≥70 | 6.3 | 1.9–20.2 | – | – |
| Sex | – | – | ||
| Women | 1 | 1 | – | – |
| Men | 1.0 | 0.3–2.9 | – | – |
| Body mass index | ||||
| Normal | 1 | 1 | 1 | 1 |
| Overweight | 1.4 | 0.3–5.5 | 1.4 | 0.3–7.3 |
| Obese | 10.1 | 2.0–51.0 | 7.1 | 1.1–46.9 |
| Eye color | – | – | ||
| Dark | 0.8 | 0.3–2.4 | – | – |
| Light | 1 | 1 | – | – |
| Hair color | ||||
| Black/dark brown | 2 | 0.2–19.0 | – | – |
| Light brown/blond | 1.7 | 0.2–19.0 | – | – |
| Red | 1 | 1 | – | – |
| Phototype | ||||
| I–II | 1 | 1 | – | – |
| III–V | 0.4 | 1.98–51.04 | – | – |
| Freckles | ||||
| No | 4.5 | 1.2–17.3 | 5.4 | 1.2–23.4 |
| Yes | 1 | 1 | 1 | 1 |
| Season | ||||
| Autumn/winter | 9.5 | 2.5–36.0 | 9.02 | 1.7–47.0 |
| Spring/summer | 1 | 1 | 1 | 1 |
| MC1R | ||||
| <2 polymorphisms | 5.25 | 1.1–25.4 | 5.0 | 0.9–28.9 |
| ≥2 polymorphisms | 1 | 1 | 1 | 1 |
| Sun exposure | ||||
| <6h | 1.8 | 0.3–10.6 | – | – |
| 6–14h | 2.4 | 0.5–11.6 | – | – |
| >14h | 1 | 1 | – | – |
Abbreviation: MC1R, melanocortin-1 receptor gene.
This study of a series of 215 patients found that only 25% of those with a diagnosis of melanoma had adequate circulating 25(OH)D levels, at least in terms of reference values established for bone metabolism homeostasis. We also found that obesity and seasonal changes were the factors that most affected the outcome. Darker skin, reflected by the absence of freckles and MC1R polymorphism pattern, were especially related to deficiency.
The finding that melanoma patients have high rates of circulating 25(OH)D insufficiency has been previously described in the literature. A Barcelona study of 81 patients recently diagnosed with melanoma found that 68% had insufficient levels; the only associated factor was season in which blood had been sampled.22 Our larger case series evaluating patients during follow-up found a slightly higher rate of insufficiency, suggesting that the use of sun protection measures may increase after diagnosis and sometimes become extreme, potentially affecting 25(OH)D levels. In any case, the percentage of patients with insufficiency exceeds the 33% reported for the general Spanish population.23 It is also useful to remember that 8.8% of patients in our series had levels below 10ng/mL (deficiency), slightly more than has been reported in other studies done inside or outside Spain.14,23,24 There has been universal consensus for some years now that circulating vitamin D levels should be measured using the metabolite 25(OH)D. Epidemiologic studies or randomized controlled trials should be interpreted and compared to ours very cautiously, however, given that in addition to variations in findings across studies there will also be variation in the precision and accuracy of measurements.22 Furthermore, most studies enroll subjects over 65 years of age given that they are seeking information about bone metabolism.
Our finding of an inverse relationship between vitamin D levels and BMI is consistent with previous reports25–27 and supports the assertion that this vitamin is sequestered in adipose tissue. Suboptimal levels have been implicated in the development of melanoma, a higher Breslow index, higher recurrence rates, and even shorter survival, given that vitamin D seems to inhibit tissue invasion and local micrometastases during the early stages of tumor development28–30; later stages seem to remain unaffected, however. More recently, low levels of vitamin D during melanoma follow-up have seemed not to worsen prognosis.31 However, vitamin D supplementation did seem to confer an adjuvant benefit in the treatment of high-risk melanoma in one clinical trial, supporting the possibility that this treatment could delay recurrence and improve the prognosis overall.32
Certain limitations apply to the interpretation of our findings. First, we did not record some variables that might have influenced vitamin D levels. Of particular interest would have been information about diet and the use of sunscreens as well as the UV index during periods of exposure. Information on smoking and physical exercise would also have been of interest. Second, we did not study genetic polymorphisms in genes involved in the metabolism of vitamin D: the vitamin D receptor gene, for example, not only influences levels of the vitamin but has also been directly linked to the development and progression of melanoma.33,34 Third, the size of our sample may have been too small to detect a statistically significant influence of some variables on 25(OH)D level.
Finally, we did not analyze the time elapsed since diagnosis, although this factor could have had an impact on modifying patients’ habits in the direction of relaxing their practice of preventive measures over the years. However, the authors’ own clinical experience suggests that patients do not substantially drift in their habits over the course of long term follow-up.
In conclusion, although there is ongoing debate on what 25(OH)D blood levels are actually necessary and what dose is required for reaching those levels, our findings suggest that this variable should be evaluated in obese persons and patients with melanoma, especially during the autumn and winter. Supplementation should be considered for patients with insufficiency, at least in the interest of supporting adequate bone metabolism. The possibility that supplementation confers an additional benefit by improving prognosis in melanoma and decreasing the risk of other diseases further argues in favor of this measure.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that the procedures followed adhered to the ethical guidelines of the responsible committee on human experimentation and comply with the Declaration of Helsinki of the World Medical Association.
Data confidentialityThe authors declare that they followed their hospitals’ regulations regarding the publication of patient information and that written informed consent for voluntary participation was obtained for all patients.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects referred to in this article. The signed forms are in the possession of the corresponding author.
AuthorshipS. Hernández Ostiz and M.D. Pérez Ramada have contributed equally to this work.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Hernández-Ostiz S, Pérez-Ramada MD, Ortiz B, Requena C, Ribas G, Aznar E, et al. Niveles de 25-hidroxivitamina D en pacientes con melanoma y factores asociados con su insuficiencia. Actas Dermosifiliogr. 2016;107:758–764.