Dermatofibrosarcoma protuberans (DFSP) is a low-to-intermediate grade dermal sarcoma with the potential for deep invasion and local recurrence, but a low capacity for metastasis. DFSP is a rare entity of unknown etiology to this date.1 Its clinical presentation is heterogeneous. Initially, it is nonspecific, and then it takes on its characteristic appearance as a nodular or indurated, firm, variably colored plaque. DFSP is mainly found on the trunk, followed by the limbs.2,3 However, in some cases, it can clinically mimic other lesions.4
Histopathologically, it is a poorly circumscribed tumor of spindle cells arranged in a storiform pattern with dermal involvement. When DFSP infiltrates the subcutaneous tissue, it typically does so in a “honeycomb” pattern. Immunohistochemistry is positive for CD34 and negative for factor XIIIa, which confirms the diagnosis.3–5
Various imaging modalities can be used to study soft tissue tumors. Ultrasound is the best one to study superficial masses and their vascularity. Also, the ultrasound is a non-invasive, fast, and cost-effective tool with real-time imaging capabilities. Since DFSP is initially small and superficial, high-resolution ultrasound is useful for its diagnosis and evaluation.6 Additionally, it allows for surgical planning by assessing depth and extent.7 The treatment of choice is surgery with margin control, such as delayed Mohs micrographic surgery.3
This study aims to describe the ultrasound findings of a series of DFSP cases in which the ultrasound diagnosis led to the ultimate diagnosis.
This was an observational study that retrospectively selected cases diagnosed with DFSP via high-resolution ultrasound and confirmed through histopathological examination from 2016 through 2022 (6 years) at a reference radiology center for ultrasound study from Bogotá, Colombia by an expert dermatologist in ultrasound images from Guayaquil, Ecuador. All studies were performed with 18MHz high-resolution linear transducers: the GE Logiq P9 golf club transducer, and the Toshiba Xario linear transducer.
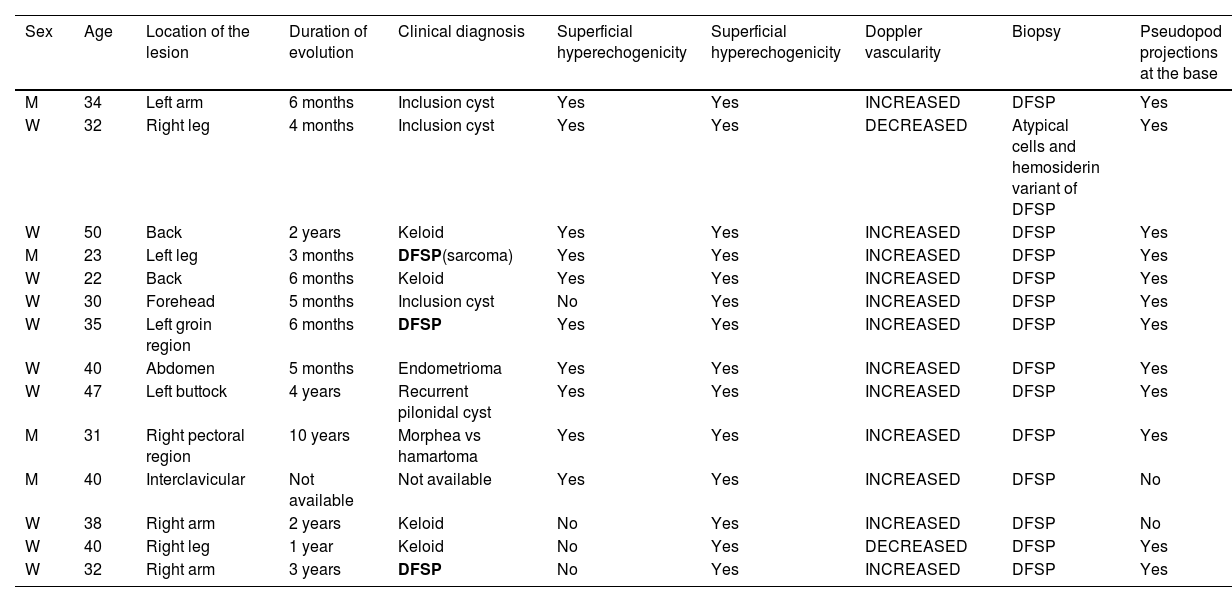
A total of 14 cases were included (table 1), with a median age of 35 years (range, 22-50 years), 10 of which (71%) involved women. The most common location was the extremities (57%). Disease progression varied from 3 months to 10 years.
Sociodemographic characteristics, clinical diagnosis, and ultrasound findings.
| Sex | Age | Location of the lesion | Duration of evolution | Clinical diagnosis | Superficial hyperechogenicity | Superficial hyperechogenicity | Doppler vascularity | Biopsy | Pseudopod projections at the base |
|---|---|---|---|---|---|---|---|---|---|
| M | 34 | Left arm | 6 months | Inclusion cyst | Yes | Yes | INCREASED | DFSP | Yes |
| W | 32 | Right leg | 4 months | Inclusion cyst | Yes | Yes | DECREASED | Atypical cells and hemosiderin variant of DFSP | Yes |
| W | 50 | Back | 2 years | Keloid | Yes | Yes | INCREASED | DFSP | Yes |
| M | 23 | Left leg | 3 months | DFSP(sarcoma) | Yes | Yes | INCREASED | DFSP | Yes |
| W | 22 | Back | 6 months | Keloid | Yes | Yes | INCREASED | DFSP | Yes |
| W | 30 | Forehead | 5 months | Inclusion cyst | No | Yes | INCREASED | DFSP | Yes |
| W | 35 | Left groin region | 6 months | DFSP | Yes | Yes | INCREASED | DFSP | Yes |
| W | 40 | Abdomen | 5 months | Endometrioma | Yes | Yes | INCREASED | DFSP | Yes |
| W | 47 | Left buttock | 4 years | Recurrent pilonidal cyst | Yes | Yes | INCREASED | DFSP | Yes |
| M | 31 | Right pectoral region | 10 years | Morphea vs hamartoma | Yes | Yes | INCREASED | DFSP | Yes |
| M | 40 | Interclavicular | Not available | Not available | Yes | Yes | INCREASED | DFSP | No |
| W | 38 | Right arm | 2 years | Keloid | No | Yes | INCREASED | DFSP | No |
| W | 40 | Right leg | 1 year | Keloid | No | Yes | DECREASED | DFSP | Yes |
| W | 32 | Right arm | 3 years | DFSP | No | Yes | INCREASED | DFSP | Yes |
In bold, the cases where the clinical diagnosis was DFSP.
DFSP was only suspected in 3 cases (21%), while in the remaining 10 cases (71%), other diagnoses were considered: 4 keloids, 3 epidermal inclusion cysts, 1 pilonidal cyst, 1 morphea, and 1 endometrioma. One case had no clinical impression.
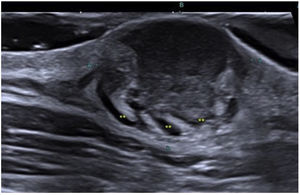
In all the cases, the ultrasound findings showed superficial hypoechogenicity, while 10 of these cases showed hyperechoic regions at the base. Pseudopod projections were a common finding (12/14; 85.7%) (Fig. 1). The color Doppler ultrasound confirmed the presence of hypervascularity in 12 cases (85.7%). In these cases, high-resolution ultrasound suggested the diagnosis, which was ultimately confirmed through histopathological study.
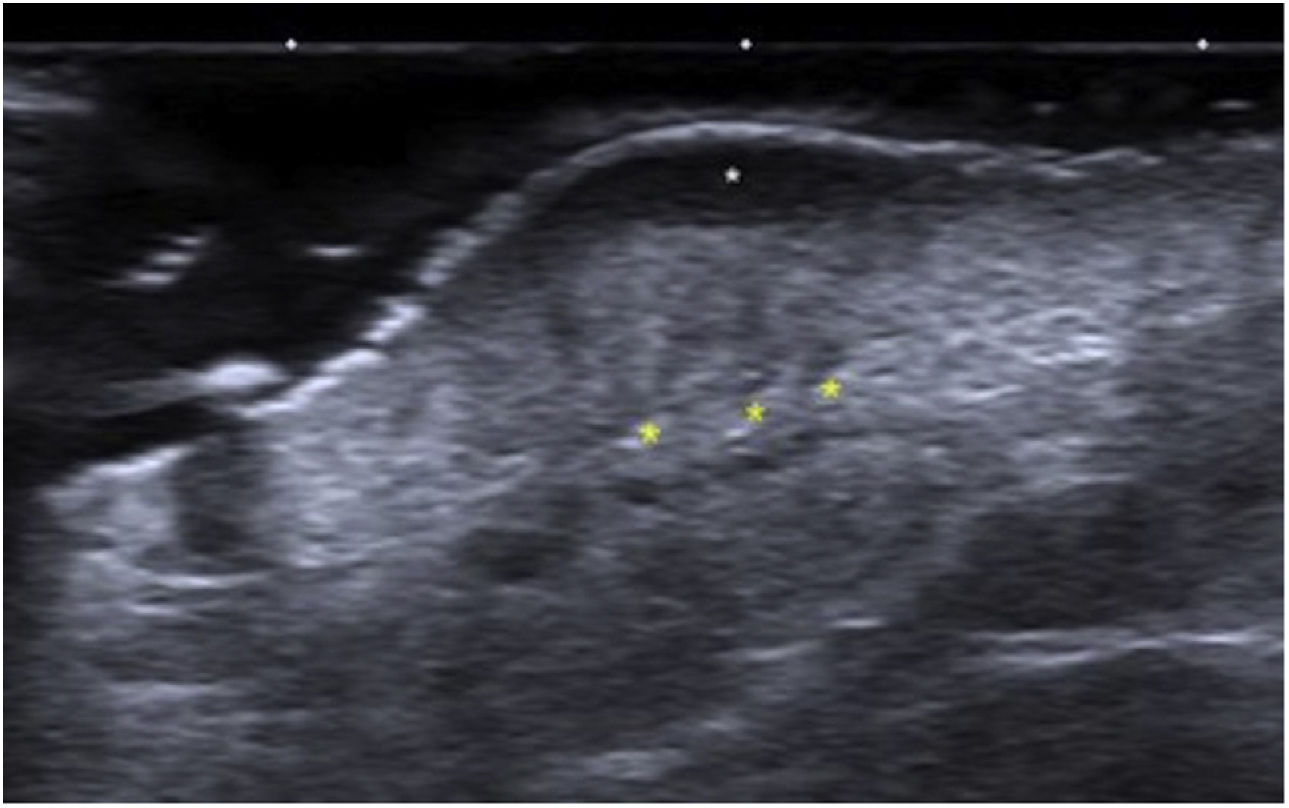
Ultrasound findings of DFSP: Grayscale ultrasound, longitudinal view showing the classic image of a DFSP with an oval mass, poorly defined borders, and a hypoechoic band in the dermis and hypodermis (white arrow). At the base, the lesion is hyperechoic with adjacent borders and pseudopod projections (yellow markers).
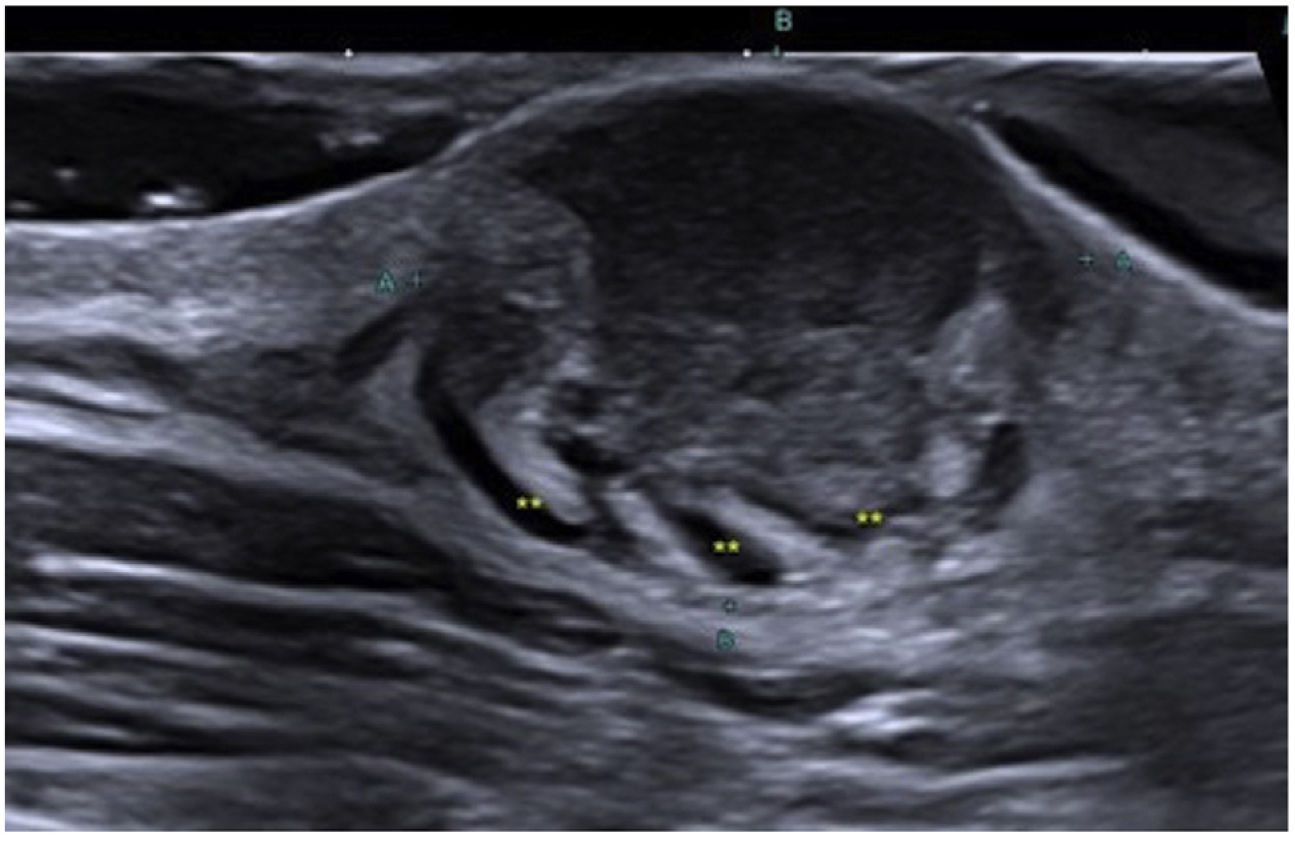
Ultrasound allows for the characterization and differentiation of superficial soft tissue masses.3 Diago et al. found 4 patterns of ultrasound invasion of DFSP, with good histopathological correlation. As it happened with our cases, the most common pattern (53.3%) was the presence of an oval hypoechoic mass with pseudopod or digitiform projections and posterior hyperechoic regions (Fig. 1) that correlated with the spread of tumor cells and fibroblasts scattered across the subcutaneous tissue forming the characteristic honeycomb pattern. We should mention that digitiform projections have an asymmetric and unpredictable growth, which makes them highly indicative or pathognomonic. Such structures were found in most of our cases (Fig. 2). The second most common pattern was the oval hypoechoic pattern with pseudopod projections but without posterior hyperechoic regions (20%), which correlated with spread along the subcutaneous septum. Finally, they revealed the presence of a mixed invasive pattern without deep projections (16.7%), or dermal/subcutaneous oval hypoechoic tumors (10%). The ultrasound has sensitivity and specificity rates of 81.8% and 100%, respectively for the detection of deep invasion, with a positive predictive value of 83.3%, which stresses its importance in assessing tumor spread.8
Zou et al. described the ultrasound differences between primary and recurrent tumors. The former involve the dermis and hypodermis (86.4%) but not deeper structures; they exhibit pseudopod projections (50%), while the latter have oval (28.4%), lobulated (22.9%), and irregular (34.3%) morphology, with no difference in echogenicity. Hypervascularity can be observed in both groups, a common finding in our cases.
Additionally, ultrasound helps exclude other diagnoses.9,10 For example, epidermal cysts are often associated with internal floating echogenicity without blood flow; pilomatricomas show calcification, and lipomas and dermatofibromas low or no vascularity at all.6
In conclusion, the high-resolution ultrasound findings of DFSP included superficial hypoechogenicity, hyperechogenicity at the base, pseudopod projections, and hypervascularity. These findings are key regarding the ultrasound diagnosis of DFSP, especially when the clinical presentation mimics other lesions.
Conflicts of interestNone declared
We wish to thank Dr. Hugo Dominguez Menoscal, dermatologist experienced in the use of ultrasounds from Guayaquil, Ecuador for his collaboration and help while conducting this study.