“The pharmacies are experiencing shortages of over 30 medicines” reported the Heraldo de Aragon on May 15 2014. “Warning of shortages for 170 unprofitable drugs by pharmacicsts” ran the headline in JANO on December 22 2014. “Andalusian Pharmacists denounce shortages of drugs due to drug auction” said the Andalusian edition of the ABC on April 9 2015. “Price war encourages drug shortages” claimed the Voz de Galicia on July 19 2015. And so on.
For some time now, a range of media outlets have been reporting stock-outs of some drugs. Perhaps you have been one of the physicians affected and this situation has puzzled you, alarmed you, or even caused a major setback in your daily practice. Or perhaps, without having been directly affected, these headlines have caused you a mild, though unpleasant sense of uncertainty.
One of the problems that general and hospital pharmacies face increasingly frequently is stock-outs of pharmaceutical products, including those with dermatological indications. The problem not only affects pharmacists, who cannot dispense the prescribed medication (unless it can be replaced by another product), but also impacts the work of physicians who prescribed these medications. The main losers are, however, the patients who take them.
Unfortunately, as affirmed by Sánchez Brunete, “the word patient is being used increasingly as an adjective rather than a noun.”1 In the present article, we propose a reflection on the causes and consequences of problems with drug supply. Also, with an eye on the future, we will analyze whether the suggested solutions are appropriate and then make our own specific proposal.
As the reader knows well, the Agencia Española de Medicamentos y Productos Sanitarios (AEMPS, the Spanish drug approval body), a state organization that operates under the auspices of the Spanish Ministry of Health, Social Policy, and Equality, is responsible for guaranteeing that the drugs marketed in Spain meet the criteria for quality, safety, effectiveness, and continuity of supply. Their website informs the public of drugs with supply problems. Currently, according to the AEMPS, 176 drugs approved in Spain have problems with supply.2 In 59 of these cases (33.5%), there are no other approved drugs with the same active ingredient and the same route of administration, and of these a total of 26 drugs (15%) cannot even be acquired as foreign drugs.
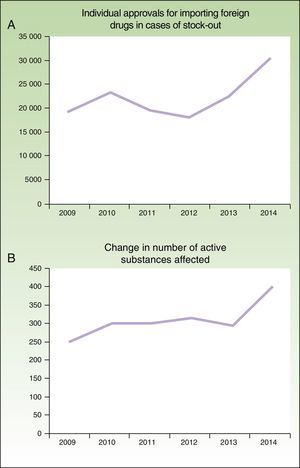
In addition, the problem has worsened in the last 6 years (Fig. 1), as noted by the AEMPS in their 2014 annual report3: “The figure is considerably higher than the previous year, in both the number of active drug substances that are imported as a foreign drug and the number of individual requests, with this increase attributable to a greater number of stock-outs.”
Number of individual approvals for importing foreign medicines in case of stock-outs (A) and number of active ingredients involved (B) between 2009 and 2014, according to the annual reports of the AEMPS for 2012, 2013, and 2014.3
In most cases, the problem can be attributed to 1 of 4 major causes:
- a)
Problems with suppliers: surprisingly drug manufacturers increasingly depend on a single supplier of starting materials (active ingredients, excipients, packaging material) in a limited number of countries such as India, China, or Brazil.
- b)
Outsourcing of production: several nations use a small percentage of world drug production, in an increasingly demanding regulatory framework, and more stringent quality metrics, and so greater investment is required. From the profitability point of view, it is therefore not viable for drug companies to manufacture their own drugs.
- c)
Growing demand for some drugs: this increase in drug demand is due, above all, to the progressive aging of the population and also to the appearance of increasingly effective treatments.
- d)
Stockpiling policies of some buyers.
One of the most recent examples on a long list of stock-outs was that of Fortecortin (Merck) in April 2014, which caused a lot of comment although the stock-out itself was not entirely novel. In fact, many of the situations described as stock-out, which is understood to mean “a problem with the supply of a drug that requires a change that has an impact on patient care and requires the use of an alternative therapeutic agent” and that therefore can lead to certain alarm within society should not actually be considered as such.
Spanish pharmacists have a very efficient tool for resolving specific problems in supply of an irreplaceable drug: pharmaceutical compounding of the drug under medical prescription (traditionally known as magistral formula). The elaboration is fully guaranteed, the product fully complies with the regulations, and the active ingredients and excipients are obtained from a supply industry bound by tight international legislation that fully guarantees the quality of the production process.
Such compounding is in fact the origin of the pharmacy profession, and one of the tasks of highest prestige associated with the job. Currently, these drugs are of unquestionable clinical importance and help provide a health service of the highest technological and professional degree.
Compounding pharmacists have been mitigating the effects of stock-outs for a long time, adapting the drugs to the specific characteristics of patients with special needs. In short, such professionals have been providing a service that, as far as possible, guarantees that each patient receives at a given time, and accurately, the treatment that he or she needs. Paradoxically, when the AEMPS reports an episode of stock-out, it almost never suggests customized drug formulation as a means to solve the problem. The AEMPS statements suggest resorting to foreign medicine services, changing treatment, restricting the indications for use, and forbidding exportations, but they rarely propose pharmaceutical compounding.2
However, it would be of great benefit for everybody to know the causes and, from the standpoint of our profession, we will attempt to clarify them. Such knowledge would be of widespread benefit: first, our patients would have an easier solution, the physicians would see their therapeutic arsenal extended, the compounding pharmacists would see an increased value placed on the tasks for which they have been trained and for which there is an established legal framework, and finally the Spanish authorities would be able to offer quality alternatives to their citizens while saving on costs.
Possible Causes and ImpactWhat options are available at present for patients who need one of these drugs? The information on the AEMPS website offers only one solution: obtain the drugs from outside Spain, that is, request the drug on a case-by-case basis though the Foreign Medicine Service.
Before assessing whether importing a drug from abroad is the most appropriate way of overcoming a stock-out, we should first take a look at the causes for the shortages in the first place.
How is it possible that a modern health system like ours, supplied by leading companies in the pharmaceutical industry, can experience prolonged stock-outs of certain drugs. What lies behind these interruptions in supply? And while we are asking: can anything be done to avoid these problems in the future?
Willem of Occam would have proposed using his famous razor, that is applying simplest hypothesis to explain the most complex situations. In this case, the simplest hypothesis would be that the stock-outs are the result of a worrying fragility in the production system for drugs. There are many reasons why drug production may stop in a given plant for weeks or perhaps months, for example, the lack of raw materials, down time to modernize facilities or adapt them to new quality requirements, or a change in strategy. And today, in our globalized world, a stoppage in a certain plant may lead to stock-out on a global scale. At present, a single production center (in a small settlement in Europe, the United States, or Asia) may produce a given drug for half the world, or maybe even the entire world.
In view of the growing interest in stock-outs among health professionals, in June 2013 the International Summit on Drug Shortages, organized by the International Pharmaceutical Federation (FIP), with the Canadian Pharmacists Association as co-organizers, was held with the aim of “providing a forum to discuss the causes, impacts, and solutions to the global issue of medicine shortages through a multi-stakeholder approach involving representatives from governments, healthcare practitioners and professional bodies, industry, and patients.”4
In that summit, after considering the contributions from different experts, it was concluded that the source of drug stock-outs could be classified according to factors that affect demand and those that affect distribution. The main reasons are now summarized (Table 1).
Reasons for Drug Stock-outs.
| Factors That Affect Demand | |
|---|---|
| Changes | Occasional increase in demand for an active substance for the treatment of a disease Approval of a new indication Patent expiry Political situations: wars Internal demands of emerging countries who produce the raw materials (China, India, South Africa, Brazil) |
| Purchasing capability | Prefunding of drug purchases Supplier payment Limited access to foreign currencies Delays in government payments |
| Structure | Licensing policies/changes in regulations Exclusivity rights for a producer Corruption Exportation/parallel sales |
| Factors That Affect Distribution | |
|---|---|
| Raw materials | A single source of raw materials for several drug manufacturers Deficient quality control Seasonal availability (natural products) |
| Manufacture of finished products | Deficiencies detected in quality Suspension of a manufacturer license Deficiencies/problems in the facilities of a production plant Special requirements: staff knowledge, facilities Very complex production processes Very low prices |
| Stock inventory/management | Lack of a national support inventory Elimination of minimum stock conditions |
Source: Report of the International Pharmaceutical Federation (FIP) International Summit on Drug Shortages.4
The reader will have observed that an alignment of circumstances has resulted in a system that is obliged to guarantee access to drugs when and where they are needed but that is really a house of cards. The consequences of this are lack of availability of drugs, delayed treatments, and even switches to alternative drugs that are often less effective.
The FIP summit highlighted some really interesting findings that provide evidence of the scope of the problem. In the United States alone, “data presented at the conference from the University of Utah Drug Information Center, showed an increase in the number of new shortages from 58-88 in 2002-2007 to 267 in 2011, and 204 in 2012. These new shortages are additional to unresolved shortages from previous years and therefore lead to an increase in the quarterly reports of active medicine shortages from 152 in early 2010 to 300 on early 2013. This clearly demonstrates that, despite the remedial measures taken, the issue is far from declining in the USA.”4
The results from a 2011 survey of 820 critical access hospitals were also presented.5 These showed that the physician was required to take difficult decisions, and that the patient may receive poorer care, require hospital admission, or even die (Table 2).
American Hospital Association Analysis of the Data From a Survey of 820 Critical Access Hospitals.
| Factor | Consequence |
|---|---|
| The physician must make difficult decisions | The physician must decide which patients to treat or in which patients treatment must be suspended |
| Worsening in the state of health of the patient | Admission to hospital, death |
| Generation of additional expenses | More expensive alternative drugs Additional staff expenditure |
| Negative impact on resource allocation | Staff need extra time to find solutions Decrease in direct health care activities for the patients |
Source: Report from the International Pharmaceutical Federation (FIP) International Summit on Drug Shortages.4
At this point, we would like to ponder whether acquiring a drug from neighboring countries from time to time is the best solution in the face of an increasingly global and simultaneous problem of stock-outs, or whether, in contrast, it would be worthwhile anticipating these problems and exploring other complementary options, analyzing in depth their potential for avoiding future emergencies.
As pharmacists, we believe that this is a golden opportunity to highlight the value of individualized medicine, a resource already available to our health system, although one that both physicians and patients have never really trusted.
Pharmacopeias such as the United States one refer to pharmaceutical compounding as “essential in pharmacy practice and essential for an appropriate health protection system.”6
Among the uses of pharmaceutical compounding recognized by the General Council of Spanish Pharmacist Associations are the following7:
- 1.
Cover therapeutic gaps: the appearance of industrial medications has simplified the catalogue of available pharmacotherapeutic options. Pharmaceutical compounding can cover therapeutic gaps. This is essential in certain patients or diseases for which industrially available drugs specific for their needs are not available.
- 2.
Solve problems of stock-outs/withdrawal of industrially produced drugs: pharmaceutical compounding guarantees continuity of treatments more quickly than other options, such as importing foreign drugs, with notable costs for the patient or Spanish National Health System.
- 3.
Facilitate administration to the patient: this is associated with an improvement in therapeutic adherence and, therefore, improved therapeutic outcomes.
- 4.
Replacement of excipients in case of allergies, poor tolerability, interactions, interference with analysis, or physical-chemical incompatibilities with other products.
- 5.
Customization of treatment to the characteristics of the patient: dose adjustment or modification of the pharmaceutical form to adapt it to the individual patient characteristics and with the most appropriate dosing regimen. The variability of response to drugs is the norm, as demonstrated by pharmacogenomics and nanopharmacy, and not the exception. Moreover, customization of treatment is important for improving the pharmaceutical care of the patient.
- 6.
Reduce the risk of possible adverse reactions, which helps reduce the risk of onset of possible adverse drug reactions.
- 7.
In the case of patients admitted to hospital, the hospital pharmacy services can attend to the therapeutic needs of a significant percentage of patients for whom commercial options are not available.
Accessibility as well as quality are thus guaranteed. Given the specific regulations, pharmacies that do not have the means for pharmaceutical compounding with guaranteed quality should subcontract the process to other specialized pharmacies. Thus, the patient always accesses the service by going directly to his or her reference pharmacy, where he or she will also receive all the information on the conditions of access to the drug and its use.
Some Examples of Replacement Pharmaceutical Compounding for Dermatological Drugs With Stock-Outs or Those That Have Been Deregistered for Commercial ReasonsTable 3 shows some of the compounded products that we are currently producing as a result of stock-outs of the commercial product.
Current Examples of Pharmaceutical Compounding in Dermatology.
| Product | Indication | Formula |
|---|---|---|
| Polaramine | Skin allergies | Chlorpheniramine maleate 4mg For 1cap. no. 30 (4mg of chlorpheniramine maleate is equivalent to 2mg of dexchlorpheniramine) |
| Wartec cream | Condyloma acuminatum | Podophyllotoxin 0.15% O/W emulsion qs 5g |
| Wartec solution | Condyloma acuminatum | Podophyllotoxin 0.15% O/W emulsion qs 5g |
| Fulcin | Dermatomycosis | Griseofulvin 125/500mg For 1cap. no. 30 |
| Sulfona® | Herpetiform dermatitis | Dapsone 100mg per 1cap. no. 30 Dapsone 50mg per 1cap. no. 30 |
| Nalcrom® | Mastocytosis | Disodium chromogicate-100 and 200mg For 1 cap. no. 30 |
| Oxoralen® | Viligo | 8-MOP (methoxsalen) 10mg For 1 cap. no. 30 |
| Celesemine® syrup | Allergic and inflammatory disorders | Betamethasone 3mg Chlorpheniramine 48mg (instead of dexchlorpheniramine maleate 24mg) Pediatric syrup, qs 60mL 1dose=5mL |
If individualized drugs are already successfully used for personalizing treatments, why shouldn’t the authorities, physicians, and pharmacists explore their potential in other situations such as stock-outs?
It is important to note that these are not second rate drugs, but are supported by the most robust evidence within the pharmaceutical industry. Production, with application of all the appropriate know-how, of a drug tailored to the patient's needs, would also demonstrate, once more, that in this globalized world with economies of scale, sometimes it is the small things that define our humanity. Thus, pharmaceutical compounding is at least necessary to provide a coherent and integral care system.
Finally, we make a call to the health authorities, as well as the physicians and patients themselves, to be aware of this instrument—pharmaceutical compounding of individualized medicines—which can contribute in its small way to the quality and overall sustainability of our beloved health system. It is essential that the system itself places trust in its own health professionals, including of course the pharmacists, because they are its most valuable resource and the only ones able to compensate and complement the gaps left by the large drug companies. Such individuals will always genuinely have the best interests of the patients at heart.
Please cite this article as: Abarca Lachén E, Marro Ramón D. El desabastecimiento de los medicamentos: ¿qué hay detrás? Causas, consecuencias y una buena alternativa. Actas Dermosifiliogr. 2016;107:178–182.