Anifrolumab is an inhibitor of the type I interferon receptor subunit 1 (IFNAR1) recently approved for the management of moderate-to-severe systemic lupus erythematosus (SLE). In 2 clinical trials, it has proven effective to treat cutaneous signs. Although anifrolumab has not been indicated for cutaneous lupus erythematosus (CLE), multiple cases and case series (20 publications with a total of 78 patients) have shown good and rapid responses with this drug, both in subacute CLE and discoid lupus erythematosus, as well as in lupus panniculitis and perniosis. Two case reports of dermatomyositis have also experienced clinical improvement with anifrolumab. Clinical trials of this drug are ongoing for subacute CLE and discoid lupus erythematosus, systemic sclerosis, and progressive vitiligo. Its most common adverse effects are respiratory infections and herpes zoster. Anifrolumab may be a well-tolerated alternative in the management of CLE.
El anifrolumab es un inhibidor de la subunidad 1 del receptor del interferón tipo I (IFNAR1), recientemente aprobado para el tratamiento del lupus eritematoso sistémico (LES) moderado a grave. En dos ensayos clínicos demostró ser eficaz en el tratamiento de las manifestaciones cutáneas. Aunque el anifrolumab no ha recibido la indicación en lupus eritematoso cutáneo (LEC), múltiples casos y series de casos (20 publicaciones con 78 pacientes en total) han mostrado buenas y rápidas respuestas con este fármaco, tanto en el LEC subagudo y el lupus eritematoso discoide, como en la paniculitis y la perniosis lúpica. Dos casos clínicos con dermatomiositis (DM) también han experimentado una mejoría clínica con el anifrolumab. Se encuentran en desarrollo ensayos clínicos de este fármaco en el LEC subagudo y el lupus eritematoso discoide, la esclerosis sistémica (ES) y el vitíligo progresivo. Sus efectos adversos (EA) más frecuentes son las infecciones respiratorias y el herpes zóster. El anifrolumab puede ser una alternativa bien tolerada en el tratamiento del LEC.
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by inflammation and the production of autoantibodies, which can affect a wide variety of organs, with the skin being one of the most widely involved. Cutaneous lupus erythematosus (CLE) includes a broad spectrum of clinical forms, including acute CLE (ACLE), subacute CLE (SCLE), intermittent CLE (ICLE) or lupus tumidus, and chronic CLE (CCLE), which in turn, includes discoid lupus erythematosus (DLE), lupus pernio, and lupus panniculitis, among others.1 CLE predominantly affects young women and can have a considerable impact on quality of life.2 The treatment of lupus erythematosus is complex and often requires the use of multiple drugs, sometimes without achieving adequate disease control. Moreover, a large part of the available therapeutic arsenal consists of classic immunosuppressants and immunomodulators used off-label, with notable potential adverse effects (AE).3 In recent years, targeted therapies such as anifrolumab have been developed, emerging as promising therapeutic options due to their efficacy and safety profile.4
Type I interferon (IFN-I) plays a role in the antiviral response and acts as a bridge theraphy between innate and adaptive immunity.5–7 It has been implicated in the pathogenesis of autoimmune diseases such as SLE and CLE.8–10 The IFN-I family includes several members (13 subtypes of IFN-α, IFN-β, IFN-ɛ, IFN-κ, and IFN-ω) that act through a common receptor (IFNAR), leading to the expression of specific genes, known as the IFN-I signature (IGS).11
Anifrolumab—a human monoclonal antibody of immunoglobulin (Ig) G1 kappa targeting the IFNAR1 subunit—was approved by the Food and Drug Administration (FDA) in July 2021 and by the European Medicines Agency (EMA) in February 2022 for the treatment of moderate to severe SLE. Anifrolumab has proven effective in treating CLE in SLE patients in 2 randomized clinical trials.12,13 Additionally, multiple cases of refractory CLE have been published, showing significant improvement with anifrolumab. In this article, we review the safety and efficacy profile of this drug in the treatment of SLE and CLE and discuss its potential use in other conditions.
Materials and methodsWe conducted narrative literature review. In February 2024, searches were performed in both Spanish and English in PubMed and Google Scholar using terms such as “anifrolumab,” “skin,” “cutaneous lupus,” “discoid,” “pernio,” “panniculitis,” “tumidus,” “blistering,” “Rowell,” “ulcers,” “dermatomyositis,” “systemic sclerosis,” “scleroderma,” “morphea,” “interferonopathies,” “psoriasis,” “neutrophilic dermatosis,” “atopic dermatitis,” “lichen planus,” “pemphigus,” “pemphigoid,” “vitiligo,” “alopecia areata,” and “vasculitis.” Articles were screened based on their abstracts and selected according to relevance. A search using the term “anifrolumab” was also performed on clinicaltrials.gov. Two authors (DMT and MMP) conducted the article search and selection.
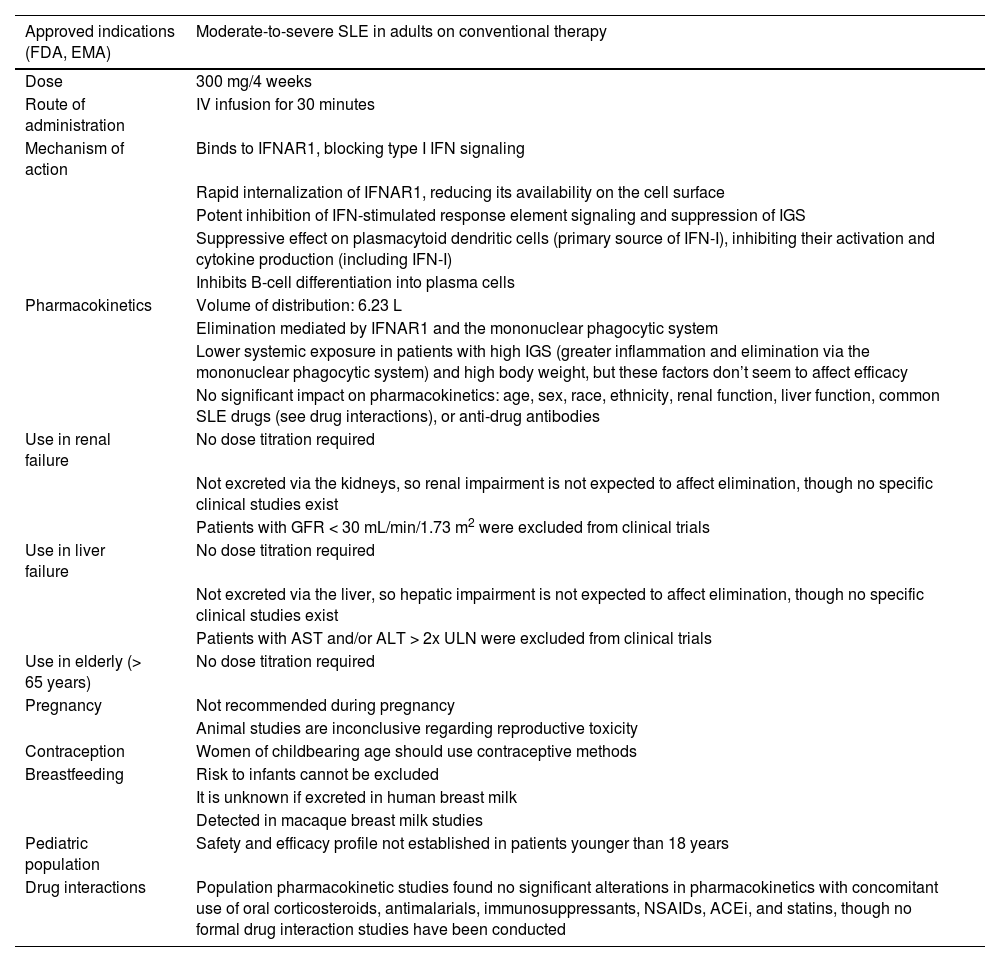
Mechanism of action and pharmacokinetics of anifrolumabMechanism of action of anifrolumabAnifrolumab is a fully human monoclonal antibody of the IgG1 kappa type that binds to IFNAR1 with high specificity and affinity, blocking IFN-I signaling. Anifrolumab induces rapid internalization of IFNAR1, reducing its availability on the cell surface.14,15 This antibody features a triple mutation (L234F/L235E/P331S) in the constant fragment crystallizable (Fc) region of the heavy chain, which reduces binding to Fcγ receptors on the cell surface and minimizes antibody-mediated effector functions—effector-null—such as antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity 14–16 (Table 1).
Characteristics of anifrolumab 12,59,72,74-77
| Approved indications (FDA, EMA) | Moderate-to-severe SLE in adults on conventional therapy |
|---|---|
| Dose | 300 mg/4 weeks |
| Route of administration | IV infusion for 30 minutes |
| Mechanism of action | Binds to IFNAR1, blocking type I IFN signaling |
| Rapid internalization of IFNAR1, reducing its availability on the cell surface | |
| Potent inhibition of IFN-stimulated response element signaling and suppression of IGS | |
| Suppressive effect on plasmacytoid dendritic cells (primary source of IFN-I), inhibiting their activation and cytokine production (including IFN-I) | |
| Inhibits B-cell differentiation into plasma cells | |
| Pharmacokinetics | Volume of distribution: 6.23 L |
| Elimination mediated by IFNAR1 and the mononuclear phagocytic system | |
| Lower systemic exposure in patients with high IGS (greater inflammation and elimination via the mononuclear phagocytic system) and high body weight, but these factors don’t seem to affect efficacy | |
| No significant impact on pharmacokinetics: age, sex, race, ethnicity, renal function, liver function, common SLE drugs (see drug interactions), or anti-drug antibodies | |
| Use in renal failure | No dose titration required |
| Not excreted via the kidneys, so renal impairment is not expected to affect elimination, though no specific clinical studies exist | |
| Patients with GFR < 30 mL/min/1.73 m2 were excluded from clinical trials | |
| Use in liver failure | No dose titration required |
| Not excreted via the liver, so hepatic impairment is not expected to affect elimination, though no specific clinical studies exist | |
| Patients with AST and/or ALT > 2x ULN were excluded from clinical trials | |
| Use in elderly (> 65 years) | No dose titration required |
| Pregnancy | Not recommended during pregnancy |
| Animal studies are inconclusive regarding reproductive toxicity | |
| Contraception | Women of childbearing age should use contraceptive methods |
| Breastfeeding | Risk to infants cannot be excluded |
| It is unknown if excreted in human breast milk | |
| Detected in macaque breast milk studies | |
| Pediatric population | Safety and efficacy profile not established in patients younger than 18 years |
| Drug interactions | Population pharmacokinetic studies found no significant alterations in pharmacokinetics with concomitant use of oral corticosteroids, antimalarials, immunosuppressants, NSAIDs, ACEi, and statins, though no formal drug interaction studies have been conducted |
NSAIDs: nonsteroidal anti-inflammatory drugs; ALT: alanine aminotransferase; AST: aspartate aminotransferase; EMA: European Medicines Agency; FDA: Food and Drug Administration; GFR: glomerular filtration rate; ACEi: angiotensin-converting enzyme inhibitors; IFN: interferon; IFN-I: type i interferon; IFNAR1: type i interferon receptor subunit 1; IGS: interferon gene signature; SLE: systemic lupus erythematosus; ULN: upper limit of normal.
The IV administration of anifrolumab ensures complete drug absorption. Its elimination occurs via an IFNAR1-mediated pathway—internalization and degradation of the antibody-receptor complex—as well as the mononuclear phagocytic system17–19 (Table 1). A subcutaneous formulation has been developed. Exposure after subcutaneous administration of 300mg anifrolumab is approximately 87% of that achieved with IV administration.20,21
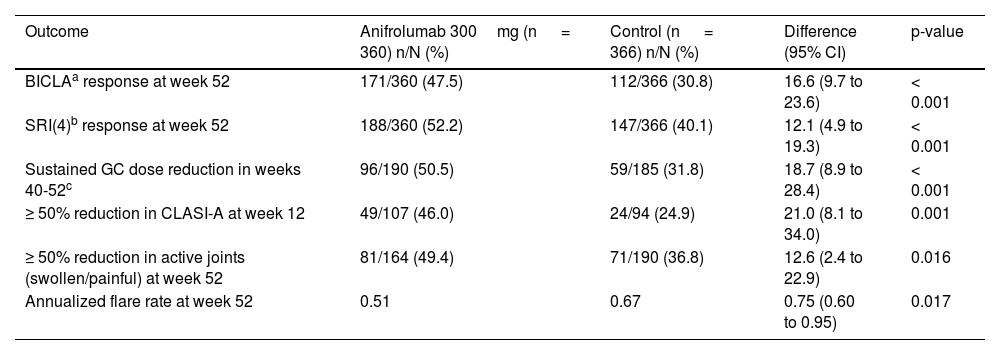
Anifrolumab in lupus erythematosusAnifrolumab in systemic lupus erythematosusResults from 2 phase III clinical trials (TULIP-1 and TULIP-2) involving 360 patients with active SLE—randomized to receive 300mg of IV anifrolumab every 4 weeks or placebo—plus conventional therapy (prednisone or equivalent, antimalarials, methotrexate, azathioprine, mycophenolate mofetil or mycophenolic acid, and/or mizoribine) have been made available. The combined data analysis at 52 weeks (Table 2) showed that anifrolumab significantly outperformed placebo in global activity markers evaluated (British Isles Lupus Assessment Group-based Composite Lupus Assessment [BICLA], Systemic Lupus Erythematosus Responder Index [SRI]). Additionally, anifrolumab was significantly superior in sustained glucocorticoid dose reduction, skin response measured by the Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI-A), joint response, and reduction in annual flare frequency.12,13,22 A phase II clinical trial (TULIP-LN) was also conducted to evaluate the efficacy profile of anifrolumab in active proliferative lupus nephritis (class III/IV).23 Although the primary endpoint was not met, this study suggested the need for an intensified regimen to achieve an adequate response. The safety and efficacy profile of such a regimen will be evaluated in a larger number of patients in a phase III clinical trial.24
Efficacy of anifrolumab in systemic lupus erythematosus: combined data from phase III clinical trials (TULIP-1 and TULIP-2) 22.
| Outcome | Anifrolumab 300mg (n = 360) n/N (%) | Control (n = 366) n/N (%) | Difference (95% CI) | p-value |
|---|---|---|---|---|
| BICLAa response at week 52 | 171/360 (47.5) | 112/366 (30.8) | 16.6 (9.7 to 23.6) | < 0.001 |
| SRI(4)b response at week 52 | 188/360 (52.2) | 147/366 (40.1) | 12.1 (4.9 to 19.3) | < 0.001 |
| Sustained GC dose reduction in weeks 40-52c | 96/190 (50.5) | 59/185 (31.8) | 18.7 (8.9 to 28.4) | < 0.001 |
| ≥ 50% reduction in CLASI-A at week 12 | 49/107 (46.0) | 24/94 (24.9) | 21.0 (8.1 to 34.0) | 0.001 |
| ≥ 50% reduction in active joints (swollen/painful) at week 52 | 81/164 (49.4) | 71/190 (36.8) | 12.6 (2.4 to 22.9) | 0.016 |
| Annualized flare rate at week 52 | 0.51 | 0.67 | 0.75 (0.60 to 0.95) | 0.017 |
BICLA: British Isles Lupus Assessment Group-based Composite Lupus Assessment; BILAG: British Isles Lupus Assessment Group; CLASI-A: Cutaneous Lupus Erythematosus Disease Area and Severity Index (activity scale); GC: glucocorticoids; CI: confidence interval; PGA: Physician Global Assessment; SLEDAI-2K: Systemic Lupus Erythematosus Disease Activity Index 2000; SRI: Systemic Lupus Erythematosus Responder Index.
Defined by reduction in all severe disease activity (BILAG-2004 A) or moderately severe disease activity (BILAG-2004 B) at baseline to lower levels (BILAG-2004 B, C, or D and C or D, respectively) without worsening in other organ systems; no worsening of disease activity based on SLEDAI-2K and PGA scores; no use of restricted drugs, and no discontinuation of the intervention. The BILAG-2004 index includes a total of 9 systems (constitutional, mucocutaneous, cardiorespiratory, GI, ophthalmic, renal, and hematologic). The SLEDAI-2K includes 24 items: 16 clinical and 8 laboratory (urinary casts, hematuria, proteinuria, pyuria, hypocomplementemia, thrombocytopenia, leukopenia, and elevated DNA binding).
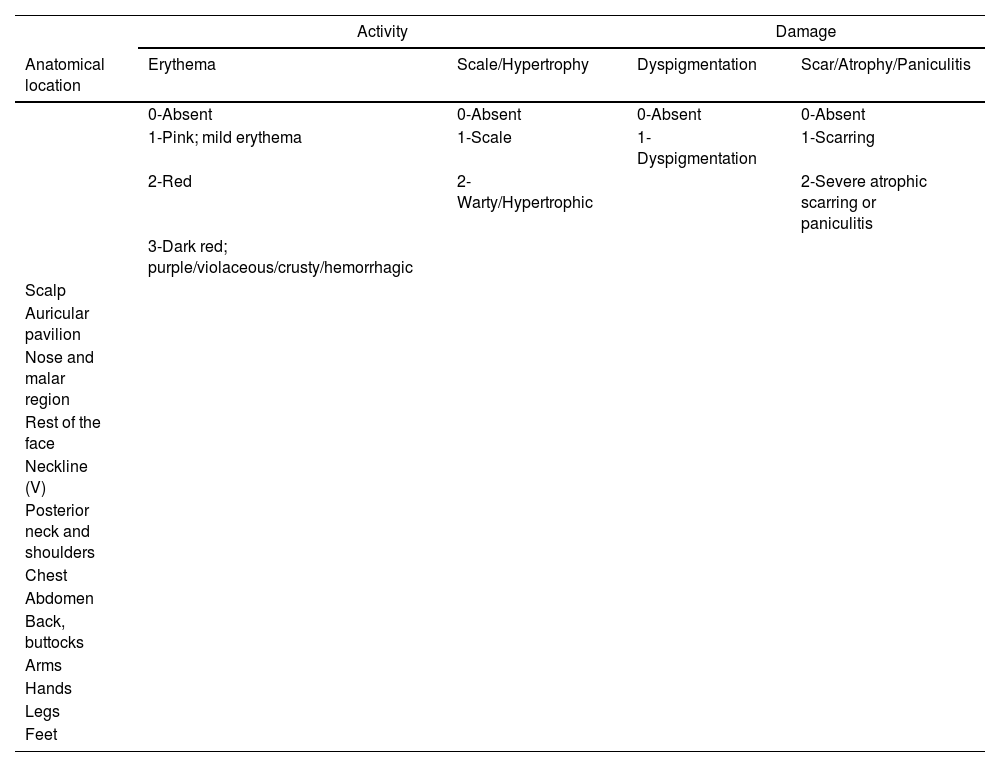
As previously mentioned, IV anifrolumab demonstrated efficacy in treating CLE in patients with SLE in the phase III clinical trials TULIP-1 and TULIP-2. At 12 weeks, a ≥ 50% reduction in the CLASI-A scale (Table 3) was observed in 46% of patients on anifrolumab vs 24.9% on placebo (p <0.001). The skin response was a secondary endpoint in both clinical trials, and the characteristics of mucocutaneous involvement were not detailed, preventing inference about which mucocutaneous phenotypes respond best to anifrolumab.12,13,22,25
Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI)78
| Activity | Damage | |||
|---|---|---|---|---|
| Anatomical location | Erythema | Scale/Hypertrophy | Dyspigmentation | Scar/Atrophy/Paniculitis |
| 0-Absent | 0-Absent | 0-Absent | 0-Absent | |
| 1-Pink; mild erythema | 1-Scale | 1-Dyspigmentation | 1-Scarring | |
| 2-Red | 2-Warty/Hypertrophic | 2-Severe atrophic scarring or paniculitis | ||
| 3-Dark red; purple/violaceous/crusty/hemorrhagic | ||||
| Scalp | ||||
| Auricular pavilion | ||||
| Nose and malar region | ||||
| Rest of the face | ||||
| Neckline (V) | ||||
| Posterior neck and shoulders | ||||
| Chest | ||||
| Abdomen | ||||
| Back, buttocks | ||||
| Arms | ||||
| Hands | ||||
| Legs | ||||
| Feet | ||||
| Mucous membranes | Dyspigmentation |
|---|---|
| Damage to mucous membranes | Document duration of dyspigmentation after resolution of active lesions |
| 0-Absent | • Dyspigmentation usually persists for < 12 months (the previous score is maintained) |
| 1-Lesion or ulceration | • Dyspigmentation usually persists for > 12 (the previous score is doubled) |
| Alopecia | |
| Recent hair loss (last 30 days) | |
| 0-No | |
| 1-Yes | |
| Non-scarring alopecia | Scarring alopecia |
| 0-Absent | 0-Absent |
| 1-Diffuse; non-inflammatory | 3-In 1 quadrant |
| 2-Focal or patchy in 1 quadrant | 4-In 2 quadrants |
| 3-Focal or patchy in > 1 quadrant | 5-In 3 quadrants |
| 6-Involves the entire scalp | |
| Total activity score (CLASI-A) | Total damage score (CLASI-D) |
The CLASI consists of 2 independent scales: one for activity (CLASI-A) and one for damage (CLASI-D). CLASI-A considers skin involvement (erythema, scaling/hypertrophy), mucosal involvement, and non-scarring alopecia. CLASI-D considers dyspigmentation, scarring/atrophy/paniculitis, and scarring alopecia. Revised CLASI (RCLASI) increases the accuracy of existing parameters and adds new indices (such as edema/infiltration or subcutaneous nodule/plaque) to include the full spectrum of cutaneous lupus erythematosus (CLE)79.
Table modified from Bonilla-Martinez et al.78
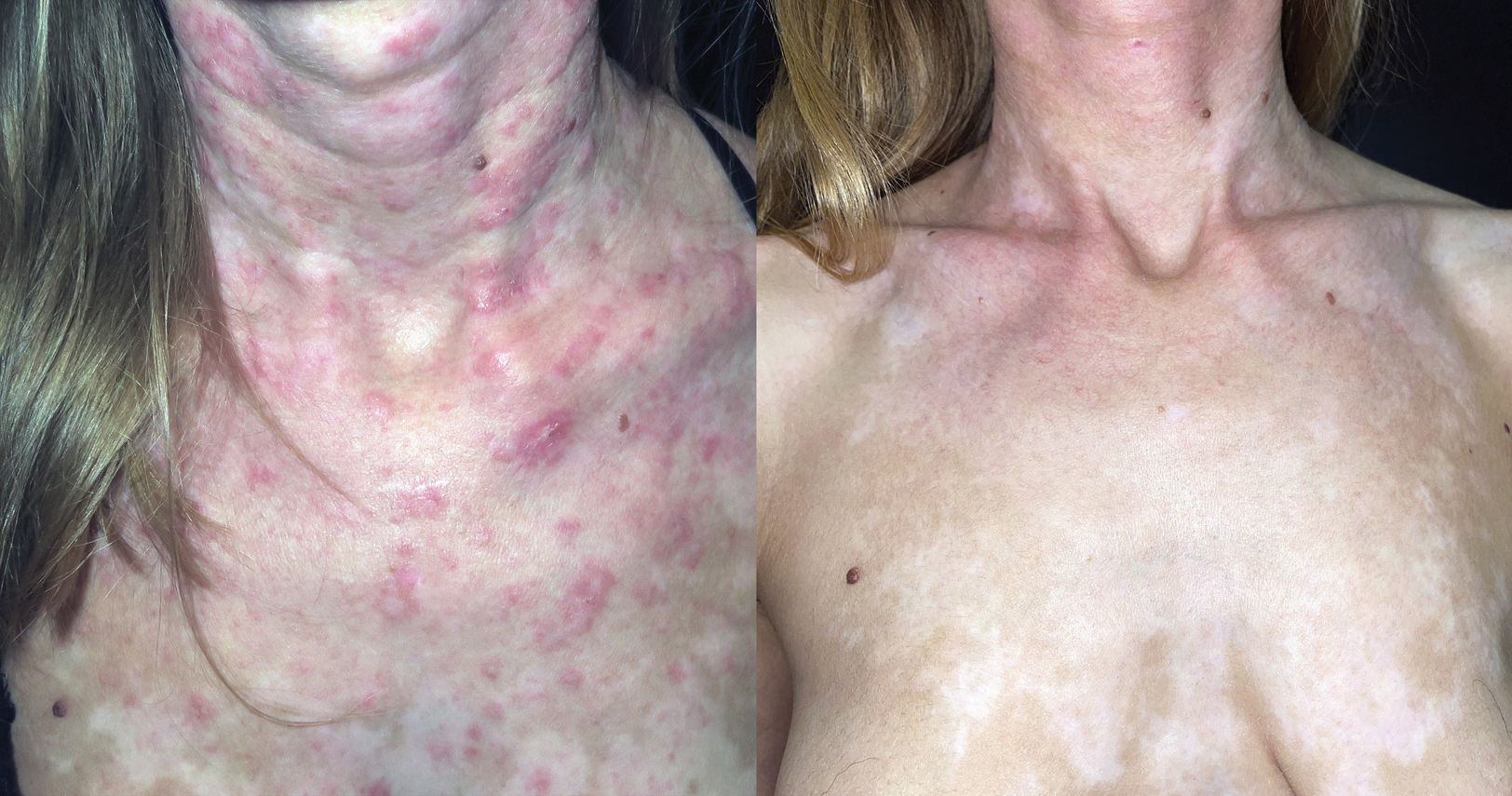
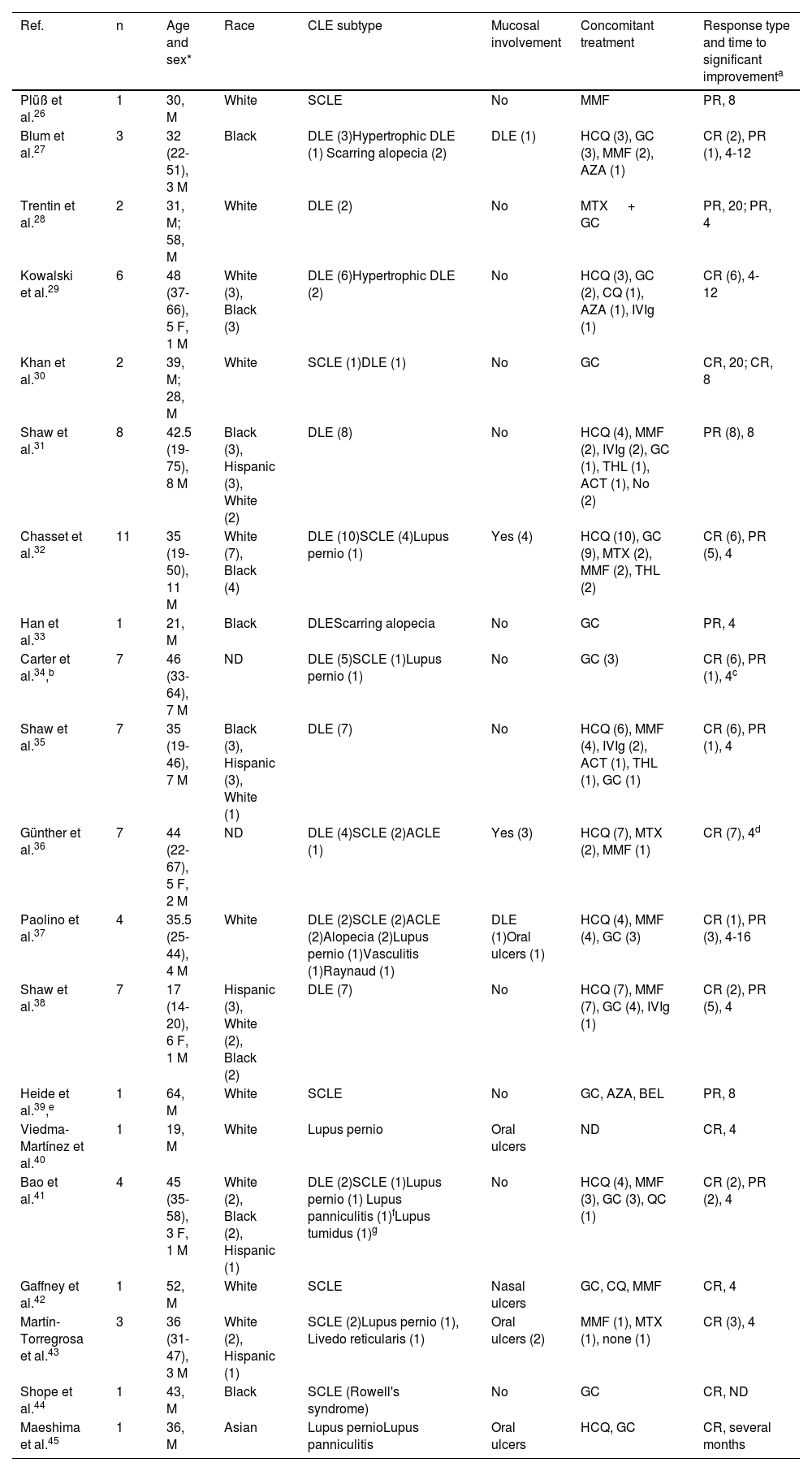
Multiple case reports and small case series of patients with CLE on anifrolumab have been published (20 reports with a total of 78 individuals) (Table 4).26–45 We found 13 articles on its use in DLE, including a total of 58 subjects. Of the patients for whom response data were available, 30 (62.5%) achieved a complete response after initiating the drug, while 18 (37.5%) had a partial response. In SCLE (Figure 1), 11 articles were published with a total of 17 patients, with a complete response in 10 (76.9%) and a partial response in 3 (23.1%). Anifrolumab produced rapid responses in both DLE and SCLE, generally observable within the first 2 treatment cycles (4 to 8 weeks). For lupus pernio, we found 7 reports involving 7 patients, with 4 complete responses and 2 partial responses.32,34,37,40,41,43,45 In all cases, significant clinical improvement was achieved within the first 4 to 12 weeks of treatment. For lupus panniculitis, we found 2 reports involving two patients, with 1 complete and 1 partial response.41,45 Regarding mucosal lesions, 9 reports disclosed a total of 22 individuals (9 with DLE, 5 with oral ulcers, and 1 with a nasal ulcer) who used anifrolumab for mucosal lesions, though in many cases, neither the characteristics of the mucosal involvement nor the response were specified.27,32,35–37,40,42,43,45 In articles focused on mucosal lesion treatment, rapid responses were obtained after a single infusion.31,36 To date, only 1 case of anifrolumab use in lupus tumidus41 and in Rowell's syndrome44 has been reported, both with complete responses.
Real-life experience with anifrolumab in the treatment of CLE26–45.
| Ref. | n | Age and sex* | Race | CLE subtype | Mucosal involvement | Concomitant treatment | Response type and time to significant improvementa |
|---|---|---|---|---|---|---|---|
| Plüß et al.26 | 1 | 30, M | White | SCLE | No | MMF | PR, 8 |
| Blum et al.27 | 3 | 32 (22-51), 3 M | Black | DLE (3)Hypertrophic DLE (1) Scarring alopecia (2) | DLE (1) | HCQ (3), GC (3), MMF (2), AZA (1) | CR (2), PR (1), 4-12 |
| Trentin et al.28 | 2 | 31, M; 58, M | White | DLE (2) | No | MTX + GC | PR, 20; PR, 4 |
| Kowalski et al.29 | 6 | 48 (37-66), 5 F, 1 M | White (3), Black (3) | DLE (6)Hypertrophic DLE (2) | No | HCQ (3), GC (2), CQ (1), AZA (1), IVIg (1) | CR (6), 4-12 |
| Khan et al.30 | 2 | 39, M; 28, M | White | SCLE (1)DLE (1) | No | GC | CR, 20; CR, 8 |
| Shaw et al.31 | 8 | 42.5 (19-75), 8 M | Black (3), Hispanic (3), White (2) | DLE (8) | No | HCQ (4), MMF (2), IVIg (2), GC (1), THL (1), ACT (1), No (2) | PR (8), 8 |
| Chasset et al.32 | 11 | 35 (19-50), 11 M | White (7), Black (4) | DLE (10)SCLE (4)Lupus pernio (1) | Yes (4) | HCQ (10), GC (9), MTX (2), MMF (2), THL (2) | CR (6), PR (5), 4 |
| Han et al.33 | 1 | 21, M | Black | DLEScarring alopecia | No | GC | PR, 4 |
| Carter et al.34,b | 7 | 46 (33-64), 7 M | ND | DLE (5)SCLE (1)Lupus pernio (1) | No | GC (3) | CR (6), PR (1), 4c |
| Shaw et al.35 | 7 | 35 (19-46), 7 M | Black (3), Hispanic (3), White (1) | DLE (7) | No | HCQ (6), MMF (4), IVIg (2), ACT (1), THL (1), GC (1) | CR (6), PR (1), 4 |
| Günther et al.36 | 7 | 44 (22-67), 5 F, 2 M | ND | DLE (4)SCLE (2)ACLE (1) | Yes (3) | HCQ (7), MTX (2), MMF (1) | CR (7), 4d |
| Paolino et al.37 | 4 | 35.5 (25-44), 4 M | White | DLE (2)SCLE (2)ACLE (2)Alopecia (2)Lupus pernio (1)Vasculitis (1)Raynaud (1) | DLE (1)Oral ulcers (1) | HCQ (4), MMF (4), GC (3) | CR (1), PR (3), 4-16 |
| Shaw et al.38 | 7 | 17 (14-20), 6 F, 1 M | Hispanic (3), White (2), Black (2) | DLE (7) | No | HCQ (7), MMF (7), GC (4), IVIg (1) | CR (2), PR (5), 4 |
| Heide et al.39,e | 1 | 64, M | White | SCLE | No | GC, AZA, BEL | PR, 8 |
| Viedma-Martínez et al.40 | 1 | 19, M | White | Lupus pernio | Oral ulcers | ND | CR, 4 |
| Bao et al.41 | 4 | 45 (35-58), 3 F, 1 M | White (2), Black (2), Hispanic (1) | DLE (2)SCLE (1)Lupus pernio (1) Lupus panniculitis (1)fLupus tumidus (1)g | No | HCQ (4), MMF (3), GC (3), QC (1) | CR (2), PR (2), 4 |
| Gaffney et al.42 | 1 | 52, M | White | SCLE | Nasal ulcers | GC, CQ, MMF | CR, 4 |
| Martín-Torregrosa et al.43 | 3 | 36 (31-47), 3 M | White (2), Hispanic (1) | SCLE (2)Lupus pernio (1), Livedo reticularis (1) | Oral ulcers (2) | MMF (1), MTX (1), none (1) | CR (3), 4 |
| Shope et al.44 | 1 | 43, M | Black | SCLE (Rowell's syndrome) | No | GC | CR, ND |
| Maeshima et al.45 | 1 | 36, M | Asian | Lupus pernioLupus panniculitis | Oral ulcers | HCQ, GC | CR, several months |
ACT: acitretin; AZA: azathioprine; BEL: belimumab; CLASI-A: Cutaneous Lupus Erythematosus Disease Area and Severity Index; CQ: chloroquine; GC: glucocorticoids; M: male; HCQ: hydroxychloroquine; IVIg: IV immunoglobulins; ACLE: acute cutaneous lupus erythematosus; SCLE: subacute cutaneous lupus erythematosus; DLE: discoid lupus erythematosus; F: female; MMF: mycophenolate mofetil or mycophenolic acid; MTX: methotrexate; ND: not documented; QC: quinacrine/mepacrine; CR: complete response; Ref.: reference; PR: partial response; THL: thalidomide.
Partial response is defined as ≥ 50% reduction in CLASI-A. Complete response is defined as CLASI-A of 0. Time to significant improvement is expressed in weeks.
One treatment discontinuation due to herpes zoster complicated by otic zoster with sensorineural hearing loss.
Significant CLASI-A reduction after 4 weeks of treatment with anifrolumab. Rapid resolution of erythema and scaling in DLE at 4 weeks. Clinically evident improvement in lupus pernio lesions at 12 weeks.
Mucosal lesions responded as quickly as skin lesions and did not recur during the observation period.
Refractory subacute cutaneous lupus erythematosus. A) Woman with multiple erythematous plaques on the neck and chest, refractory to topical corticosteroids, prednisone, hydroxychloroquine, mepacrine, methotrexate, belimumab, and rituximab. B) Complete response after starting anifrolumab 300mg every 4 weeks.
Type I interferon (IFN-I) signaling pathway appears to play a significant role in the pathogenesis of dermatomyositis (DM). Several studies have corroborated the presence of an elevated IFN-I gene signature (IGS) in the blood, muscle, and skin of these patients, with a correlation between disease activity and IGS in blood.46–48 These findings have made the IFN-I signaling pathway a therapeutic target to consider. To date, 2 cases of DM with cutaneous involvement refractory to multiple therapies and responsive to anifrolumab have been documented.49,50 The first case involved a patient with juvenile DM with extensive photosensitive skin rash and myositis, refractory to multiple drugs, including tofacitinib and IV immunoglobulins. The addition of anifrolumab led to a notable improvement 72hours after the first infusion. At the 56-day follow-up, the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI-A) score decreased from 38 to 9. The second case was an individual with paraneoplastic DM. Due to the refractoriness of skin signs, anifrolumab was initiated, resulting in considerable improvement of skin involvement after the 1st dose. We have not found any clinical trials underway to assess the efficacy of anifrolumab in the treatment of DM. Although a phase III clinical trial is recruiting participants to investigate the subcutaneous use anifrolumab to treat polymyositis, patients with DM have been excluded.51
Systemic sclerosisSeveral studies have shown elevated IFN-I plasma levels in patients with systemic sclerosis (SSc), as well as a prominent IGS in both blood and skin.52–55 Although the pathogenic role of IFN-I in SSc vasculopathy and fibrosis and the mechanisms involved are still to be elucidated, the IFN-I signaling pathway emerges as a potential therapeutic target. A phase I clinical trial evaluated the safety, pharmacokinetics, and pharmacodynamics of IV anifrolumab in patients with SSc, but no efficacy data were provided.17 Currently, a phase III clinical trial is underway to determine the efficacy and safety of subcutaneous anifrolumab in patients with SSc.56 We have not found any published studies either on the use of this drug in SSc.
InterferonopathiesInterferonopathies are hereditary autoinflammatory diseases characterized by increased IFN-I signaling, including Aicardi-Goutières syndrome, STING-associated vasculopathy with onset in infancy (SAVI), chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE), familial lupus pernio, monogenic forms of SLE, trichohepatoenteric syndrome, and X-linked reticulate pigmentary disorder.57 Given the mechanism of action of anifrolumab, it could be a therapeutic option in these cases. However, we have not found any articles on the use of anifrolumab to treat these conditions.
Non-lupus perniosisA systemic polarization of IFN-I is observed in the pathophysiology of perniosis, though the exact mechanisms remain unclear. This association is observed in lupus pernio, but also in seasonal perniosis and in perniosis due to SARS-CoV-2 infection (COVID-19).43,58 We have not found any studies on anifrolumab in refractory seasonal perniosis or COVID-19-associated perniosis.
SafetyIn the TULIP-1 and TULIP-2 clinical trials, a higher rate of AEs was reported in the anifrolumab group vs the control group (86.9% vs 79.4%). The most frequent AEs were nasopharyngitis (16.3%), upper respiratory tract infections (15.5%), bronchitis (9.8%), and herpes zoster (6.1%). Most AEs were mild to moderate in intensity. Serious AEs occurred in 11.8% of patients on anifrolumab, although the incidence was not higher than in the control group. Two deaths due to pneumonia were reported. The rates of malignancy were comparable to placebo (1.3% vs 0.6%).12,13,19,59,60 The safety and tolerability of subcutaneous anifrolumab were similar to those of IV anifrolumab.21
Anifrolumab infusions are generally well tolerated. Infusion-related reactions occurred in 9.4% of cases, typically mild to moderate in intensity. The most common symptoms were headache, nausea, vomiting, and asthenia. A hypersensitivity reaction occurred in 2.6% of patients, most being mild to moderate. A case of anaphylaxis has been documented.12,13,19,59
DiscussionAnifrolumab has the highest level of evidence (level of evidence 1a/A) for the treatment of SLE, according to the Oxford Centre for Evidence-Based Medicine classification. In the 2023 update of the European League Against Rheumatism (EULAR) recommendations for the treatment of SLE, anifrolumab has been added to the therapeutic arsenal as a second-line therapy (Table 5) .61 Anifrolumab could be initiated in patients who remain unresponsive to antimalarials, with or without associated glucocorticoids, or in those unable to down titrate glucocorticoid doses to acceptable levels for chronic use. Most experts agreed that it is not essential to use a conventional immunosuppressant before starting anifrolumab or belimumab. These recommendations position anifrolumab as a second-line therapy for skin signs of SLE, refractory to topical agents, antimalarials, and/or systemic glucocorticoids.
2023 Recommendations from the European Alliance of Associations for Rheumatology (EULAR) for the management of patients with systemic lupus erythematosus (SLE) 61
| General principles |
| A. SLE requires multidisciplinary and individualized management, with patient education and joint decision-making, taking into account costs for both the patient and society. |
| B. SLE activity should be assessed at each visit (the frequency of visits is left to the physician's discretion), with evaluation of organ damage (at least annually), using validated tools. |
| C. Non-pharmacological interventions, including sun protection, smoking cessation, a healthy and balanced diet, regular exercise, and measures to promote bone health, are important for improving long-term outcomes. |
| D. Pharmacological interventions are guided by patient characteristics, type and severity of organ involvement, treatment-related damage, comorbidities, risk of progressive organ damage, and patient preferences. |
| E. Early diagnosis of SLE (including serological evaluation), periodic screening for organ involvement (especially nephritis), rapid initiation of treatment aiming for remission (or low disease activity if remission is not possible), and strict adherence to treatment are essential to prevent flares and organ damage, improve prognosis and quality of life. |
| Recommendations* |
| 1. Hydroxychloroquine is recommended for all patients (1b/A), unless contraindicated, at a dose of 5mg/kg of actual body weight per day (2b/B), but also individualized based on the risk of exacerbation (2b/B) and retinal toxicity. |
| 2. Glucocorticoids, if necessary, should be dosed according to the type and severity of organ involvement (2b/C) and down titrated to a maintenance dose of ≤ 5mg/day (prednisone equivalent) (2a/B), and, if possible, withdrawn; in patients with moderate-to-severe disease, IV methylprednisolone pulses (125 up to 1000mg/day for 1 to 3 days) may be considered (3b/C). |
| 3. In patients who do not respond to hydroxychloroquine (alone or in combination with glucocorticoids) or those unable to down titrate glucocorticoids to acceptable chronic doses, the addition of immunomodulators/immunosuppressants (e.g., methotrexate [1b/B], azathioprine [2b/C], or mycophenolate [2a/B]) and/or biologics (e.g., belimumab [1a/A] or anifrolumab [1a/A]) should be considered. |
| 4. In patients with life- or organ-threatening disease, IV cyclophosphamide should be considered (2b/C); in refractory cases, rituximab should be considered (2b/C). |
| 5. Active cutaneous disease treatment should include topical agents (glucocorticoids, calcineurin inhibitors) (2b/B), antimalarials (hydroxychloroquine, chloroquine) (1a/A), and/or systemic glucocorticoids (4/C) as needed, with methotrexate (1b/B), mycophenolate (4/C), anifrolumab (1a/A), or belimumab (1a/B) considered as second-line therapies. |
| 6. In active neuropsychiatric SLE, glucocorticoids and immunosuppressants should be considered for inflammatory signs (1b/A) and antiplatelets/anticoagulants for atherothrombotic/antiphospholipid antibody-related signs (2b/C). |
| 7. For acute treatment of severe autoimmune thrombocytopenia, high-dose glucocorticoids (including IV methylprednisolone pulses) should be considered (4/C), with or without IV immunoglobulin (4/C) and/or rituximab (2b/B) and/or high-dose IV cyclophosphamide (4/C), followed by maintenance therapy with rituximab (2b/B), azathioprine (2b/C), mycophenolate (2b/C), or cyclosporine (4/C). |
| 8. Patients with active proliferative lupus nephritis should receive low-dose IV cyclophosphamide (EuroLupus regimen) (1a/A) or mycophenolate (1a/A) and glucocorticoids (IV methylprednisolone pulses followed by lower oral doses); combination therapy with belimumab (either with cyclophosphamide or mycophenolate [1b/A]) or calcineurin inhibitors (especially voclosporin or tacrolimus, along with mycophenolate [1b/A]) should be considered. |
| 9. After renal response, lupus nephritis treatment should continue for, at least, 3 years (2b/B); patients initially on mycophenolate alone or along with belimumab or a calcineurin inhibitor should continue on these drugs (1a/A), while azathioprine or mycophenolate should replace cyclophosphamide in those initially treated with cyclophosphamide alone (1a/A) or in combination with belimumab (1a/A). |
| 10. In patients at high risk of renal failure (defined as reduced GFR, histological presence of crescents or fibrinoid necrosis, or severe interstitial inflammation), high-dose IV cyclophosphamide (NIH regimen) (1a/A) plus IV methylprednisolone pulses should be considered. |
| 11. In SLE patients who achieve sustained remission, a gradual reduction in treatment should be considered, with glucocorticoids being withdrawn first (2a/B). |
| 12. Thrombotic antiphospholipid syndrome-related SLE should be treated with long-term vitamin K antagonists after the first unprovoked arterial or venous thrombotic episode (1b/B); low-dose aspirin (75 to 100mg/day) should be considered in SLE patients without antiphospholipid syndrome but with a high-risk antiphospholipid antibody profile (2a/B). |
| 13. Vaccination for infection prevention (herpes zoster, human papillomavirus, influenza, COVID-19, and pneumococcus), bone health management, nephroprotection, cardiovascular risk reduction, and cancer screening should be considered (5/D). |
EULAR: European Alliance of Associations for Rheumatology; GFR: glomerular filtration rate; SLE: systemic lupus erythematosus; NIH: National Institutes of Health.
Although both belimumab and anifrolumab have shown efficacy in mucocutaneous signs of SLE,25,62 only anifrolumab clinical trials evaluated the CLASI. As discussed throughout this review, anifrolumab has also been shown in multiple case series to be a good alternative for various forms of CLE, including DLE, SCLE, lupus panniculitis, and lupus pernio, among others.26–45 It is notable for its rapid action, significantly reducing CLE lesions within the first few months of treatment (Figure 1). This rapid improvement was not only seen in the skin signs of SCLE and DLE but also in mucosal lesions, both ulcers and DLE of mucosa.27,32,35–37,40,42,43,45 This contrasts with belimumab, which takes approximately 20 weeks to achieve a clinical response.62
Due to its inhibition of type I Interferon (IFN-I) action, anifrolumab could be a therapeutic alternative in other connective tissue diseases, such as DM and SSc, and in interferonopathies. However, current evidence is too limited to make recommendations in this regard. IFN-I is also involved in other dermatological conditions like psoriasis,63,64 morphea,65 vitiligo,66 alopecia areata,67 and lichen planus.68 Currently, clinical trials for anifrolumab in SCLE, CCLE,69 polymyositis51, Sjögren's syndrome,70 and progressive vitiligo71 are underway.
Anifrolumab has demonstrated to be a safe and well-tolerated drug in clinical trials. Its most common AEs are respiratory infections, typically mild to moderate, and herpes varicella-zoster infection.11,12 Most cases of herpes zoster were cutaneous, mild to moderate in severity, and resolved with antiviral treatment without having to discontinue the drug. However, cases of severe herpes zoster, including 1 case of transverse myelitis, have been reported.59,72 Due to the increased risk of infection, anifrolumab should be used with caution in patients with chronic infections, a history of recurrent infections, or other infection risk factors. It should not be initiated in patients with an active, clinically significant infection. Prior to starting therapy, it is advisable to perform viral serologies (HIV, hepatitis B and C viruses), screen for latent tuberculosis, and update the vaccination schedule, including vaccination against herpes varicella-zoster.73 Concomitant use of live or attenuated vaccines should be avoided.
LimitationsThis review is narrative and not a systematic review or meta-analysis. Moreover, except for pivotal clinical trials, studies on the treatment of CLE with anifrolumab are based solely on case series, and randomized studies are needed to support them. Additionally, the potential uses of anifrolumab in conditions other than CLE are proposed based on the pathophysiology of these diseases and the mechanism of action of anifrolumab. To date, aside from some cases of DM treated with anifrolumab, there is no solid evidence supporting its use.
ConclusionsAnifrolumab is a newly approved drug for the treatment of refractory SLE, which has proven to be rapid and effective in treating CLE in patients with SLE. Additionally, although it may be considered an alternative for treating various forms of CLE, randomized clinical trials are still lacking. The mechanism of action of anifrolumab positions it as a promising therapy for other conditions in which IFN-I signaling is implicated, such as DM, SSc, or interferonopathies.
Confirmation of originalityThe authors confirm that the work presented in this manuscript is original and has not been published elsewhere, either in whole or in part. The content of this manuscript has not been submitted for publication to any other journal.
Patient consentInformed written consent was obtained from the patient included in this review for the publication of her clinical details, images, and any identifiable information. The consent forms are in possession of the corresponding author and are available for review by the editorial team.
FundingNone declared.
Conflicts of interestNone declared.