Androgenetic alopecia can be challenging to treat due to the wide range of available treatments, most of which are not based on evidence from clinical trials. In addition many of the options do not include androgenetic alopecia among the approved indications according to their summaries of product characteristics. A panel of 34 dermatologists from the Spanish Hair Disorders Society of the Spanish Academy of Dermatology and Venereology (AEDV) used the Delphi method to develop a consensus statement on the management of androgenetic alopecia. Over a 2-round process the experts agreed on 138 (86%) of the 160 proposed items, which were structured into 4 blocks of recommendations: general considerations, pharmacologic treatment, procedures and hair transplant, and special cases. The resulting consensus statement based on expert opinion of the scientific evidence can guide professionals in the routine management of androgenetic alopecia.
El tratamiento de la alopecia androgénica (AGA) puede ser complejo para el clínico debido a la amplia gama de terapias disponibles, en muchos casos con escasos ensayos clínicos disponibles, y con muchas de las opciones de tratamiento sin aprobación de uso en la AGA según su ficha técnica. Este documento de consenso sobre el manejo de la AGA se ha elaborado siguiendo un método Delphi, en el que han participado 34 dermatólogos miembros del Grupo Español de Tricología de la Academia Española de Dermatología y Venereología. Tras 2 rondas de votaciones, se consensuaron 138 de los 160 ítems propuestos (86%), estructurados en 4 bloques de recomendaciones: generalidades, tratamiento farmacológico, procedimientos y trasplante capilar, y casos especiales. Este documento de consenso se apoya en la evidencia científica disponible y en la opinión de expertos para ayudar a los profesionales en el manejo de la AGA en la práctica clínica diaria.
Androgenetic alopecia (AGA) is the most common cause of hair loss in both men and women, affecting nearly 50% of Caucasian men > 50 years old and 50% of Caucasian women > 60 years old, with a slightly lower prevalence in individuals of Black and Asian ethnicities.1,2 Despite the high prevalence of AGA, there are only 2 treatments approved by international regulatory bodies: topical minoxidil (for male and female AGA) and finasteride, both oral and topical, for male AGA. However, multiple drugs are used off-label.3,4 Additionally, several techniques for AGA treatment have been developed, such as mesotherapy with platelet-rich plasma (PRP) or low-level laser therapy, which have shown variable efficacy in the management of AGA.5–10
Despite the coexistence of multiple therapies for AGA widely used in the routine clinical practice, most of them have not undergone clinical trials to confirm their efficacy and safety profile in AGA treatment. Although these treatments are often safe, sometimes of common use in other medical areas, their side effects can be severe,11–13 which stresses the need for research into new AGA therapies.
This consensus document aims to provide guidance to health care professionals on the currently available treatments for the management of AGA in our routine clinical practice.
MethodsThis consensus document was designed and developed by members of the Spanish Hair Disorders Group (GET) of the Spanish Academy of Dermatology and Venereology (AEDV) using a modified Delphi method following RAND/UCLA recommendations,14 and in full compliance with the principles set forth in the Declaration of Helsinki.
To create the preliminary questionnaire, a scientific committee was created, including 8 participants, all dermatologists with over 5 years of clinical experience in hair disorders, and members of the GET-AEDV. A qualitative literature review was conducted in 4 thematic blocks (generalities, medical treatment of AGA, procedures and hair transplantation, and special cases). This literature review was conducted in October 2022, was limited to articles published in English and/or Spanish since 2012 indexed in the following databases: Medline (via PubMed), Embase, The Cochrane Library, MedEs, Epistemonikos, Joanna Briggs Institute Evidence Based Practice Database, and Tripdatabase.
After the literature review, a questionnaire of 160 statements was created and sent to the panelists to be answered online. A group of 34 Spanish dermatologists, all members of the GET-AEDV, participated as panelists and voted in both rounds.
A total of 2 rounds of voting were conducted. Panelists scored each statement using a Likert-type ordinal scale of 9 points (1, strongly disagree; 9, strongly agree). Responses were categorized into 3 groups: 1-3 (disagree), 4-6 (neutral), and 7-9 (agree). For an item to be considered consensually agreed or disagreed upon, it should meet the following criteria: 1) a median of responses within the range of 1-3 or 7-9; 2) less than one-third of the votes outside the range of 1-3, or 7-9, and 3) an interquartile range (IQR) ≤ 3.
After the 1st round of voting, the scientific committee met for an interim analysis, reediting several statements that had not reached consensus. These statements underwent a 2nd round of voting. Panelists were briefed on the responses from the 1st round before casting their votes in the 2nd one.
After completing the 2nd round, the scientific committee drafted the consensus document, assigning a level of evidence (LE) to each recommendation, and a grade of recommendation (GR), following the recommendations issued by the Center for Evidence-Based Medicine at Oxford.15
ResultsIn the 1st round, a total of 130 items were agreed upon. After the 1st round, a total of 8 recommendations were re-edited, and after the 2nd round, a total of 12 additional recommendations were agreed upon. After the 2 rounds of the Delphi method, 138 out of the proposed 160 items were agreed upon (86%) (see supplementary material, tables A.1-A.4).
Generalities on the management of androgenetic alopeciaPanelists considered the treatment of AGA to be complex due to several factors, including the limited number of approved treatments available, and the lack of clinical trials demonstrating the safety and efficacy profile of many therapies used in the routine clinical practice. (table A.1 of the supplementary data). It is essential to establish a good doctor-patient relationship to outline realistic expectations regarding treatment outcomes, discuss different therapeutic alternatives and potential side effects with the patient. Treatment should be individualized, because one of the most important factors for therapeutic success in the management of AGA is to improve compliance by individualizing the therapeutic strategy used. In the case of using an off-label drug, it was agreed that the health care professional has a duty to inform the patient so he/she can give his/her consent (verbal or written). In fact, this should be documented in the patient's health record. It was agreed that to assess the efficacy of a treatment, it should be maintained for, at least, 6 to 12 months. Finally, it was decided that the physician should rule out the association of AGA with other types of alopecia and scalp diseases that may require specific treatment, as well as underlying causes that may exacerbate alopecia in the case of female AGA.
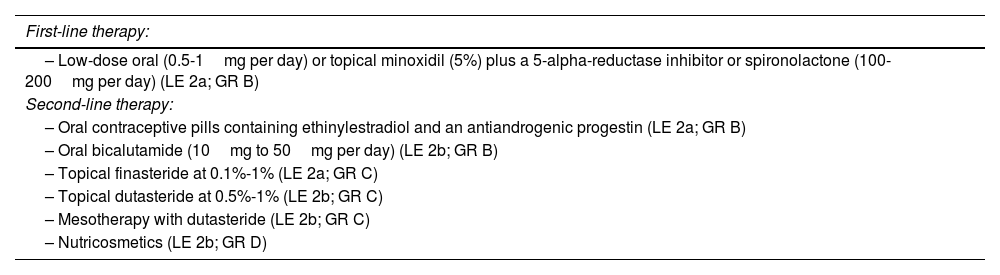
Medical treatment of AGAIn this set of proposals, consensus recommendations were reached on the management of both male AGA (MAGA) (Table 1) and female AGA (FAGA) (Table 2); 5-alpha-reductase inhibitors were recommended as the first-line therapy for MAGA, with a preference for oral dutasteride 0.5mg over oral finasteride 1mg due to its greater efficacy and similar safety profile, despite being an off-label indication. It was agreed that low-dose oral minoxidil is more effective than the topical formulation and is the most effective treatment for FAGA. Additionally, it was agreed that oral minoxidil can be considered as a first-line therapy in patients with MAGA and FAGA. On the other hand, the combination of topical or oral minoxidil plus a 5-alpha-reductase inhibitor, or spironolactone was considered the most effective option to treat FAGA in premenopausal women. In postmenopausal women, the combination of topical or oral minoxidil plus 5-alpha-reductase inhibitors was considered the most effective option.
Recommendations for the pharmacological treatment of MAGA.
| First-line therapy: |
|---|
| Oral dutasteride 0.5mg/day alone or in combination with low-dose oral minoxidil (2.5-5mg/day) or topical minoxidil (5%) (LE 1a; GR A) |
| Second-line therapy: |
| Oral finasteride 1mg/day (LE 1a; GR A) |
| Topical finasteride at 0.1%-1% (LE 2a; GR C) |
| Topical dutasteride at 0.5%-1% (LE 2b; GR C) |
| Mesotherapy with dutasteride (LE 2b; GR C) |
| Nutricosmetics (LE 2b; GR D) |
GR, grade of recommendation; MAGA, male androgenetic alopecia; LE, level of evidence.
Recommendations for the pharmacological treatment of FAGA.
| First-line therapy: |
|---|
| – Low-dose oral (0.5-1mg per day) or topical minoxidil (5%) plus a 5-alpha-reductase inhibitor or spironolactone (100-200mg per day) (LE 2a; GR B) |
| Second-line therapy: |
| – Oral contraceptive pills containing ethinylestradiol and an antiandrogenic progestin (LE 2a; GR B) |
| – Oral bicalutamide (10mg to 50mg per day) (LE 2b; GR B) |
| – Topical finasteride at 0.1%-1% (LE 2a; GR C) |
| – Topical dutasteride at 0.5%-1% (LE 2b; GR C) |
| – Mesotherapy with dutasteride (LE 2b; GR C) |
| – Nutricosmetics (LE 2b; GR D) |
FAGA, female androgenetic alopecia; GR, grade of recommendation; LE, level of evidence.
It was agreed that second-line pharmacological therapies for MAGA should include topical finasteride/dutasteride and dutasteride in mesotherapy, while second-line therapies for FAGA should include topical formulations of 5-alpha-reductase inhibitors, oral contraceptives, oral bicalutamide, dutasteride in mesotherapy, and PRP. Oral cyproterone acetate should only be considered as an alternative treatment for FAGA after exhausting other therapeutic options, as its use has been shown to increase the risk of meningioma. Regarding nutricosmetic products, it was recommended to individualize their use depending on each case due to their variable efficacy.
Regarding the side effects of medical therapy for AGA (Table 3), it was agreed that these are often mild, rare, and some highly controversial. It was decided that periodic blood tests are not necessary except for some of cases illustrated in Table 4. It was agreed that pregnancy should be avoided during treatment with 5-alpha-reductase inhibitors, bicalutamide, and other anti-androgens, due to their potential teratogenicity, and for a variable time after discontinuation (Table 5).
Side effects of pharmacological treatment for AGA.
| 5-alpha-reductase inhibitors: |
| – Reduced libido |
| – Erectile dysfunction |
| – Gynecomastia |
| – Decreased ejaculate volume |
| – Feminization of male fetus |
| – GI discomfort |
| – Migraine |
| Oral minoxidil: |
| – Hypertrichosis |
| – Dizziness |
| – Hypotension |
| – Tachycardia (especially within the first 24hours after starting treatment) |
| – Edema |
| Topical minoxidil: |
| – Local side effects (often due to its formulation with propylene glycol) |
| Spironolactone: |
| – Menstrual irregularities |
| – Edema |
| – Electrolyte disturbances |
| Bicalutamide: |
| – Menstrual irregularities |
| – Elevated liver enzymes |
| – GI discomfort |
| – Edema |
AGA, androgenetic alopecia.
Recommendations for periodic lab testing in the management of AGA.
| 5-alpha-reductase inhibitors: |
| – Periodic lab testing is not recommended for patients without significant comorbidities (LE 5, GR D) |
| – PSA level monitoring is advised for men > 50 years (> 40 years for black male patients) prior to and after initiating treatment with 5-alpha-reductase inhibitors (LE 5, GR D) |
| Minoxidil: |
| – Periodic lab monitoring is not necessary for patients without significant comorbidities (LE 5, GR D) |
| Spironolactone: |
| – In women with kidney diseases > 45 years who are on spironolactone therapy, lab testing to monitor renal function and blood ion levels is advised (LE 5, GR D) Bicalutamide: |
| – Patients on bicalutamide require lab testing every 3 to 6 months to monitor liver enzyme and plasma lipid levels throughout the entire treatment (LE 5, GR D) |
AGA, androgenetic alopecia; GR, grade of recommendation; LE, level of evidence.
Recommendations for contraceptive treatment in premenopausal women on pharmacological treatment for FAGA.
| – In premenopausal women on dutasteride therapy, it is mandatory to use oral contraceptives (preferably with antiandrogenic activity) for up to 6 months after discontinuing treatment (LE 5, GR D) |
| – In premenopausal women on finasteride therapy, it is mandatory to use oral contraceptives (preferably with antiandrogenic activity) for up to 1 month after discontinuing treatment (LE 5, GR D) |
| – In premenopausal women on spironolactone therapy, it is mandatory to use oral contraceptives (preferably with antiandrogenic activity) for up to 1 month after discontinuing treatment (LE 5, GR D) |
| – Premenopausal women on bicalutamide therapy should avoid pregnancy during treatment and up to 2 months after its discontinuation (LE 5, GR D) |
FAGA, female androgenetic alopecia; GR, grade of recommendation; LE, level of evidence.
In this section, consensus recommendations were reached regarding procedures currently available for the management of AGA. Mesotherapy with PRP (Table 6) and low-level laser therapy were considered to have proven but variable efficacy as adjunctive therapies, and their use should be individualized depending on each case. Among the treatments used in mesotherapy, dutasteride at concentrations of 0.01% or 0.05% has shown the highest efficacy in studies, while there is limited evidence regarding mesotherapy with finasteride and minoxidil. Mesotherapy with vitamins like biotin has not demonstrated efficacy. Microneedling can be used along with topical therapies such as minoxidil to enhance percutaneous penetration and, therefore, efficacy. The administration of the botulinum toxin has been poorly studied regarding it effectiveness, and its use should be individualized depending on each case.
Recommendations for PRP therapy.
| – A protocol of 3 monthly sessions is advised (LE 5, GR D) |
| – Subsequently, it is recommended to have a session every 3 to 6 months as maintenance treatment (LE 5, GR D) |
| – PRP sessions can vary from 2mL to 4mL of volume per session (LE 5, GR D) |
| – Activating PRP prior to its administration is not recommended (LE 5, GR D) |
GR, grade of recommendation; LE, level of evidence; PRP, platelet-rich plasma.
Hair transplantation was agreed upon as an option for patients with FAGA and MAGA, provided that the alopecia is stable, and patients have enough hair follicles in the donor area. It can be considered as the sole treatment in elderly patients with established alopecia. However, it is not recommended in young patients, or in those who have not received treatment for their alopecia with clinical progression. Before recommending hair transplantation, the clinician should assess the patient's expectations, as well as his/her medical history, ruling out the presence of body dysmorphic disorder, or unrealistic expectations.
Special casesRecommendations for the management of AGA in pediatric and adolescent patients, elderly patients with comorbidities, and pregnant patients, or those planning to concieve are illustrated in Table 7.
Recommendations for the management of AGA in special cases.
| Pediatric/Adolescent patients |
| General recommendations: |
| – They should be evaluated by a pediatric endocrinologist even if they do not present signs of early puberty (LE 5, GR D) |
| – Adjuvant treatments such as platelet-rich plasma therapy or low-level laser therapy can be considered in adolescent patients with MAGA/FAGA, although there is not enough evidence on their efficacy or safety profile in this population (LE 5, GR D) |
| Management of MAGA in pediatric/adolescent patients: |
| – Topical minoxidil at 2% to 5% plus topical finasteride (0.25% to 1%) is the treatment of choice for adolescent patients with MAGA (LE 4, GR D) |
| – In case of an unsatisfactory response to topical minoxidil, the use of a 5-alpha-reductase inhibitor in adolescent patients with MAGA can be considered (LE 4, GR D) |
| – Before starting treatment with a 5-alpha-reductase inhibitor in adolescent males, we should make sure that all secondary sexual characteristics have developed (in the case of finasteride), or that they have reached 18 years of age (in the case of dutasteride) (LE 5, GR D) |
| Management of FAGA in pediatric/adolescent patients: |
| – Topical minoxidil is the treatment of choice in adolescent women with FAGA (LE 4, GR D) |
| – In case of an unsatisfactory response to topical minoxidil in adolescent women with FAGA, the use of low-dose oral minoxidil, or oral anti-androgens may be considered (LE 5, GR D) |
| – The use of oral contraceptives for FAGA treatment in adolescents before the onset of pubarche is not recommended (LE 5, GR D) |
| – In adolescent females with FAGA showing a poor response to topical minoxidil, oral treatment with spironolactone is considered the first choice, especially in cases associated with seborrhea, acne, or hirsutism (LE 5, GR D) |
| – When starting treatment with 5-alpha-reductase inhibitors in adolescent patients with FAGA, it is essential to advise them on the importance of avoiding pregnancy, and initiating contraceptive methods if necessary (LE 5, GR D) |
| Elderly and patients and with comorbidities |
| General recommendations: |
| – In elderly patients, or those with significant comorbidities, treatment should be coordinated with the patient's primary care physician and/or specialists (LE 5, GR D) |
| – The patient's past medical history, and his/her usual treatment should be considered when prescribing systemic treatment for AGA (LE 5, GR D) |
| – 5-alpha-reductase inhibitors and topical therapies, as well as adjuvant procedures, are the safest therapeutic options for elderly patients and those with comorbidities (LE 5, GR D) |
| 5-alpha-reductase inhibitors: |
| – Overall, they are safe for patients older than 65 years (LE 4, GR D) |
| Oral minoxidil: |
| – Caution should be observed when prescribing this drug in patients older than 65 years (LE 5, GR D) |
| – It is not recommended in patients with a history of cardiac arrhythmias due to the risk of tachycardia involved (LE 4, GR C) |
| – First choice in women with FAGA and a history of breast cancer (LE 5, GR D) |
| Spironolactone: |
| – Caution is advised when prescribing this drug in patients older than 65 years, and those with a history of kidney disease, or electrolyte disorders (LE 4, GR C) |
| – First choice in women with FAGA and a history of breast cancer (LE 5, GR D) |
| Bicalutamide: |
| – Contraindicated in patients with liver failure (LE 4, GR C) |
| Pregnant patients, lactation, and patients planning to conceive |
| Pregnancy: |
| – Overall, pharmacological treatment is not recommended during pregnancy (LE 5, GR D) |
| – Topical therapy or mesotherapy with drugs is also not recommended due to potential systemic absorption (LE 5, GR D) |
| – Low-level laser therapy and PRP therapy may be considered if clinically necessary (LE 5, GR D) |
| – Specific nutricosmetics that have proven safe during pregnancy can be administered (LE 5, GR D) |
| Lactation: |
| – Topical and oral minoxidil are drugs that can be used during lactation (LE 4, GR D) |
| – Finasteride or dutasteride are ill-advised during lactation (LE 5, GR D) |
| – Specific nutricosmetics with proven safety during lactation can be administered (LE 5, GR D) |
| Fertility desire |
| – In female patients with FAGA planning to conceive, both 5-alpha-reductase inhibitors, and other hormonal therapies are contraindicated (LE 5, GR D) |
| – There is no need to discontinue topical or oral minoxidil therapy in women with FAGA months before pregnancy (LE 5, GR D) |
| – Male patients planning to conceive on 5-alpha-reductase inhibitors, and without a clinical history of infertility, or sexual side effects, can continue treatment (LE 5, GR D) |
| – As a precautionary measure, the use of condoms as a barrier method in sexual relations with a pregnant woman whose male partner is on 5-alpha-reductase inhibitor therapy is advised (LE 5, GR D) |
AGA, androgenetic alopecia; FAGA, female androgenetic alopecia; GR, grade of recommendation; MAGA, male androgenetic alopecia; LE, level of evidence; PRP, platelet-rich plasma.
This consensus document provides guidelines on the management of AGA and aims to assist in the management of this condition, which is characterized by its high prevalence in our environment.
Regarding the general aspects of AGA management, we should mention that despite the fact that there is a growing number of therapeutic tools currently available, management can be complex due to several factors: the limited number of approved treatments for AGA, the wide variety of off-label treatments available, the lack of clinical trials on different treatments, as well patient preferences and expectations.3,4,16–18 In cases where off-label therapies are necessary, and while considering ethical and legal implications, it is recommended to inform the patient and obtain his/her verbal or written consent, documenting everything in the patient's health record.
This consensus positions oral dutasteride as the first-choice drug for MAGA despite being an off-label indication (Table 1). This is due to dutasteride greater efficacy profile compared to finasteride, with a similar safety profile.19–21 Similarly, low-dose oral minoxidil is recommended as the first choice for both FAGA and MAGA due to its superior efficacy compared to topical minoxidil and few adverse events reported so far.11,22–26 The combination of topical or oral minoxidil with an antiandrogen (5-alpha-reductase inhibitor, or spironolactone) is considered the first-line therapy for FAGA (Table 2) despite finasteride and dutasteride being contraindicated in the product label. Regarding topical minoxidil, despite being one of the only 2 approved treatments for AGA, panelists suggested sparing its use for the early stages of the disease, given the drug's greater efficacy in oral formulation.27
In terms of hormonal treatment, panelists positioned spironolactone as the first-line therapy for FAGA in premenopausal patients;28 second-line options include oral contraceptives and bicalutamide.29–31 Due to the potential risk of meningioma, cyproterone acetate is not recommended in the management of FAGA, except for cases unresponsive to other drugs.32,33 Individualizing the use of nutricosmetic products, including those with natural antiandrogenic activity, is advised due to their low efficacy.34
While most AGA treatments are safe, and serious side effects are rare, there are several drugs whose prescription requires periodic monitoring through lab tests, such as bicalutamide for the potential risk of hepatotoxicity, or spironolactone in patients with a past medical history of kidney disease or electrolyte disorders (Table 3).35
Mesotherapy and other technological interventions are adyuvants to pharmacological therapy for AGA36,37, although their effectiveness has proven low/moderate in some former studies. PRP treatment,9,38–40 and low-energy laser therapy5–7,41 have proven efficacy in the management of MAGA/FAGA but with highly variable results, thus requiring individualized use in each case. An administration scheme for PRP has been agreed upon, recommending an initial regimen of 3 monthly sessions, without plasma activation prior to the administration (Table 6). No consensus was ever reached on the best low-energy laser treatment regimen, as the application protocols for different light systems are not well established, nor their combination with other mesotherapy treatments. However, they should be administered, at least, 3 times a week to assess their effectiveness. Several recommendations regarding procedures such as mesotherapy, microneedling, and treatments with botulinum toxin did not reach consensus due to the lack of scientific evidence to date, which stresses the need for further studies to validate their safety and efficacy profile.
Hair transplantation is becoming increasingly common in the routine clinical practice,42–44 and this document supports its use in selected patients. Before recommending hair transplantation, it is necessary to make sure that alopecia has stabilized, which is ill-advised in young patients. However, it can be considered even as the only treatment in elderly patients with established alopecia.
This document is a useful guide for special and challenging cases for dermatologists, such as the management of pediatric patients, older patients with comorbidities, or pregnant women (Table 7). A multidisciplinary approach, with assessments by pediatric endocrinology, is advised for pediatric/adolescent patients with AGA.45 Similarly, the importance of staying coordinated with primary care physicians and/or specialists is emphasized in the case of elderly patients, or those with significant comorbidities. During pregnancy, we should mention that most pharmacological treatments should be avoided, although specific nutricosmetics designed and approved for pregnancy, low-power laser therapy, and platelet-rich plasma treatment can be considered if clinically necessary.
ConclusionsThe management of AGA can be complex due to the wide range of therapies currently available, often with variable evidence regarding their efficacy and safety profile. This consensus document is based on the scientific evidence available to date, and expert opinions to help professionals in the management of AGA in the routine clinical practice.
FundingThis article has been funded by the Spanish Hair Disorders Group of the Spanish Academy of Dermatology and Venereology.
Conflicts of interestDr. Sergio Vañó-Galván declares being an advisor to Lilly and Pfizer. The remaining authors declared no conflicts of interest whatsoever.
The authors wish thank Dr. Bernadette Pfang, Dr. Pablo Rivas, and Content Ed Net for their help while drafting this manuscript.
Rubina Alves, Salvador Arias-Santiago, Anne Barrutia Borque, Víctor Cabezas Calderón, Francisco M. Camacho Martínez, Andrea Combalia, Francisco Javier del Boz González, María Antonia Fernández Pugnaire, Ramón Fernández-Miranda, Juan Ferrando Barberá, Blanca Ferrer Guillón, Pablo Miguel Fonda Pascual, Javier Forteza Muñoz, Azael Freites Martínez, Rocío Gil Redondo, Elena González Guerra, Ramón Grimalt Santacana, Aurora Guerra Tapia, Alejandro Lobato Berezo, Paola Maldonado Cid, Pablo Martín Carrasco, Teresa Meyer González, José María Mir Bonafé, Joan Francesc Mir-Bonafé, Jose Carlos Moreno Giménez, Ramón Pigem Gasos, Cristina Pindado Ortega, María Librada Porriño Bustamante, Luis Puig Sanz, Ana Rita Rodrigues Barata, Marta Rubio Lombraña, Daniela Subiabre Ferrer, Cristian Valenzuela Oñate, and Virginia Velasco Tamariz