Primary cutaneous lymphomas (PCL) are uncommon. Observations based on the first year of data from the Spanish Registry of Primary Cutaneous Lymphomas (RELCP, in its Spanish abbreviation) of the Spanish Academy of Dermatology and Venereology (AEDV) were published in February 2018. This report covers RELCP data for the first 5 years.
Patients and methodsRELCP data were collected prospectively and included diagnosis, treatments, tests, and the current status of patients. We compiled descriptive statistics of the data registered during the first 5 years.
ResultsInformation on 2020 patients treated at 33 Spanish hospitals had been included in the RELCP by December 2021. Fifty-nine percent of the patients were men; the mean age was 62.2 years. The lymphomas were grouped into 4 large diagnostic categories: mycosis fungoides/Sézary syndrome, 1112 patients (55%); primary B-cell cutaneous lymphoma, 547 patients (27.1%); primary CD30+ lymphoproliferative disorders, 222 patients (11%), and other T-cell lymphomas, 116 patients (5.8%). Nearly 75% of the tumors were registered in stage I. After treatment, 43.5% achieved complete remission and 27% were stable at the time of writing. Treatments prescribed were topical corticosteroids (1369 [67.8%]), phototherapy (890 patients [44.1%]), surgery (412 patients [20.4%]), and radiotherapy (384 patients [19%]).
ConclusionThe characteristics of cutaneous lymphomas in Spain are similar to those reported for other series. The large size of the RELCP registry at 5 years has allowed us to give more precise descriptive statistics than in the first year. This registry facilitates the clinical research of the AEDV's lymphoma interest group, which has already published articles based on the RELCP data.
Los linfomas cutáneos primarios (LCP) son un conjunto de entidades poco frecuentes. En febrero del 2018 se describieron los resultados del primer año de funcionamiento del Registro de linfomas cutáneos primarios de la AEDV. En el presente trabajo actualizamos los resultados tras 5 años de funcionamiento.
Pacientes y métodosRegistro de enfermedad de pacientes con LCP. Se recogieron datos prospectivamente de los pacientes, incluyendo diagnóstico, tratamientos, pruebas realizadas y estado actual del paciente. Se realizó un análisis descriptivo.
ResultadosEn diciembre del 2021 se había incluido a un total de 2.020 pacientes en el Registro, pertenecientes a 33 hospitales españoles. El 59% fueron hombres, y la edad media fue de 62,2 años. Se agruparon en 4 grandes grupos diagnósticos: micosis fungoide/síndrome de Sézary (1.112 [55%]), LCP de células B (547 [27,1%]), trastornos linfoproliferativos de células T CD30+ (222 [11%]) y otros linfomas T (116 [5,8%]). La mayoría presentó estadio T1, encontrándose actualmente casi el 75% en remisión completa (43,5%) o enfermedad estable (EE: 27%). Los tratamientos más usados fueron corticoides tópicos (1.369 [67,8%]), fototerapia (890 [44,1%]), cirugía (412 [20,4%]) y radioterapia (384 [19%]).
ConclusiónLas características del paciente con LCP en España no difieren de otras series. El mayor tamaño del registro permite precisar mejor los datos con respecto a los resultados del primer año. Este registro facilita al grupo de linfomas de la AEDV realizar investigación clínica, surgiendo ya trabajos publicados de dicho registro.
Primary cutaneous lymphomas (PCLs) are a heterogeneous group of entities characterized by the primary proliferation of different types of lymphocytes (T cells, B cells, and natural killer cells) in the skin, from where they can spread to the peripheral blood, lymph nodes, and even other organs. They may follow a progressive course and can affect quality of life and have serious consequences.1
Clinical registries are a very useful tool for uncommon, frequently difficult-to-manage, entities, such as PCL. In December 2016, the Spanish Academy of Dermatology and Venereology (AEDV) started a multicenter registry (RELCP, in its Spanish abbreviation) to collect clinical data on PCL.2 The report summarizing the data collected in the first year of the registry was published in February 2018.3 The aim of this study was to summarize observations for the first 5 years of the registry, with a focus on the clinical characteristics of the patients seen at the participating hospitals and the treatments used.
Material and MethodsThe AEDV's RELCP is a prospective multicenter registry to which any hospital with a dedicated or specialized cutaneous lymphoma unit can contribute. All the patients included in the first 5 years of the registry were diagnosed according to the criteria proposed by the World Health Organization and the European Organization for Research and Treatment of Cancer (WHO-EORTC).1 The participating hospitals included all patients with a diagnosis of PCL seen at their hospital. The only exclusion criterion was patient refusal to participate in the study. Data were entered into an online system provided by the Research Unit of the AEDV Foundation (OpenClinica Open Source software, version 3.1) following a standard protocol. Statistical analyses were performed in Stata (version 17 Statacorp). The study was classified by the Spanish Agency of Medicines and Medical Devices as a non-postauthorization study and approved by the ethics committee at Hospital 12 de Octubre (16/175) and by all participating hospitals. It complied with the principles of the Declaration of Helsinki and current legislation. All patients included in the registry provided written informed consent.
The RECLP includes information collected at inclusion and follow-up visits. At the inclusion visit, a note was made of the following demographic and diagnostic data: date; type of lymphoma according to the WHO classification; stage according to the revised classification system for TNM (or TNMB in the case of mycosis fungoides/Sézary syndrome [MF/SS]) proposed by the International Society for Cutaneous Lymphomas and the Cutaneous Task Force of the EORTC4–6; and diagnostic tests and treatments. The information recorded at the follow-up visits included date of last visit; disease status classified as complete remission (100% clearance since last visit), partial remission (50%–99% clearance since last visit), stable disease (< 25% to < 50% clearance since last visit), disease progression (≥ 25% progression since last visit), death, or recurrence; and presence of cutaneous, lymph node, visceral organ, or blood involvement at the time of the visit.
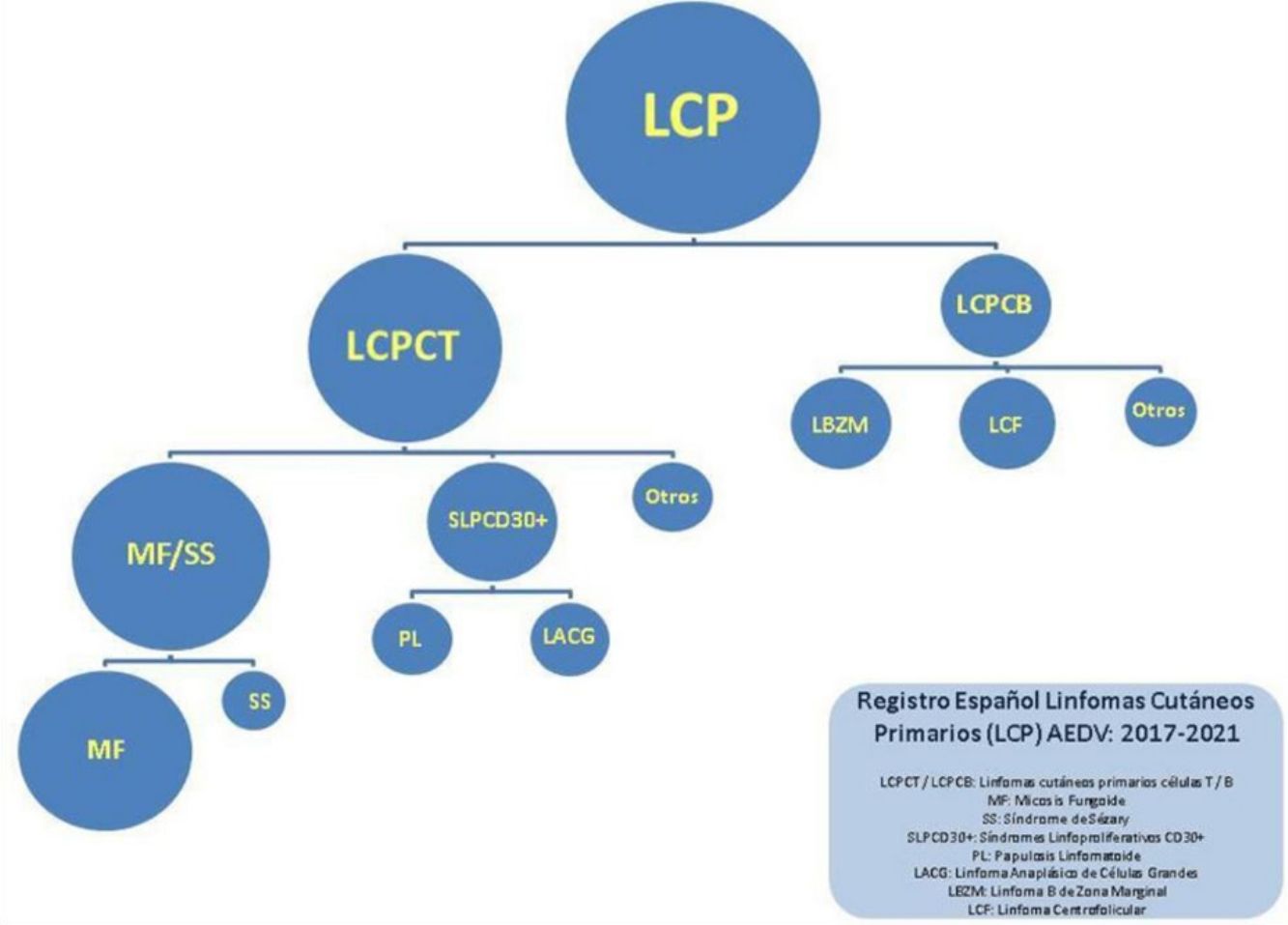
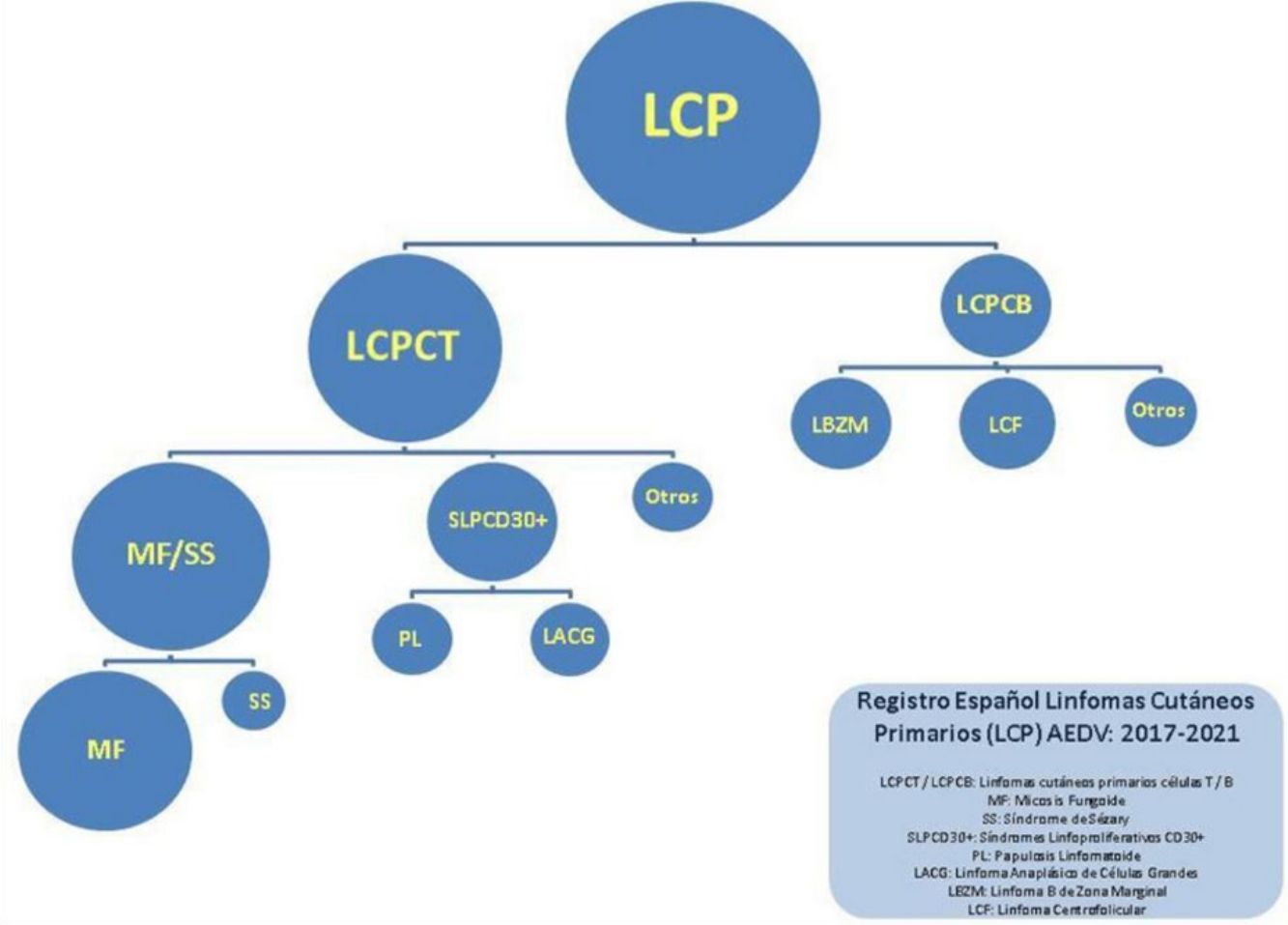
For the purpose of this study, lymphomas were separated into 4 large categories: MF/SS; primary cutaneous CD30+ T-cell lymphoproliferative disorders (CD30+ LPDs), which included lymphomatoid papulosis [LyP] and anaplastic large cell lymphoma [ALCL]); other T-cell lymphomas (TCLs); and B-cell lymphomas (BCLs). The results are reported using absolute numbers and percentages for qualitative variables, mean (SD) for normally distributed continuous variables, and median (range) for nonnormally distributed continuous variables.
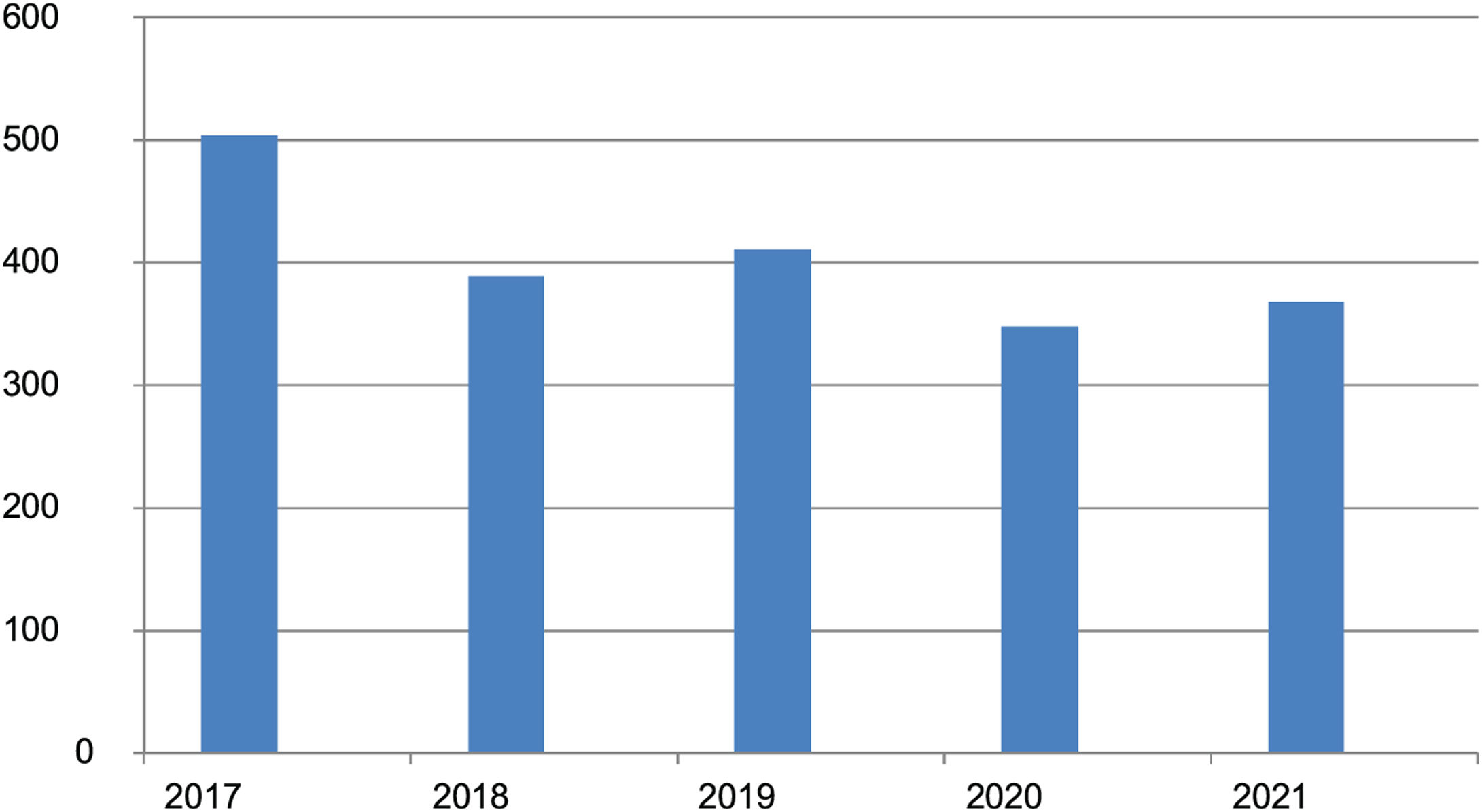
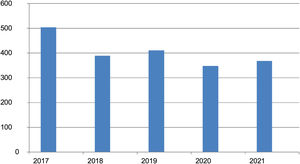
ResultsAt the time of the analysis, December 2021, the registry included data on 2020 patients from 33 Spanish hospitals. There were 830 women and 1190 men with a mean (SD) age of 62.2 (15.6) years and a mean age at inclusion of 55.7 (15.9) years. Age at disease onset ranged from 10 to 97 years. The mean duration of disease was 5.1 (5.8) years. The numbers of patients added annually to the registry over the first 5 years are shown in Fig. 1.
There were 1112 patients (55% of all patients) in the MF/SS category, 222 (11%) in the CD30+ LPD category, 116 (5.8%) in the other TCL category, and 574 (27.1%) in the BCL category. The remaining 23 patients (1.2%) were not classified. The full breakdown by type of lymphoma is shown in Table 1.
Total Number (%) of Lymphomas Included in the Spanish Primary Cutaneous Lymphoma Registry According to Eortc Diagnostic Criteria, Ordered by Frequency.
| EORTC diagnosis | No. | % |
|---|---|---|
| Mycosis fungoides without further specification | 882 | 43.7 |
| Marginal zone B-cell lymphoma | 280 | 13.9 |
| Follicle-center B-cell lymphoma | 229 | 11.3 |
| Folliculotropic mycosis fungoides | 171 | 8.5 |
| Lymphomatoid papulosis | 152 | 7.5 |
| CD4+ small/medium T-cell lymphoproliferative disorder | 76 | 3.8 |
| Anaplastic large-cell lymphoma | 70 | 3.5 |
| Sézary syndrome | 56 | 2.8 |
| Diffuse large B-cell lymphoma, leg type | 31 | 1.5 |
| Other lymphomas | 22 | 1.1 |
| Nonspecified peripheral T-cell lymphoma | 20 | 1.0 |
| Subcutaneous panniculitis-like T-cell lymphoma | 7 | 0.3 |
| Gamma-delta T-cell lymphoma | 3 | 0.1 |
| Acral CD8+ T-cell lymphoma | 3 | 0.1 |
| Pagetoid reticulosis | 2 | 0.1 |
| CD8+ epidermotropic cytotoxic T-cell lymphoma | 2 | 0.1 |
| Extranodal nasal-type natural killer/T-cell lymphoma | 2 | 0.1 |
| Intravascular B-cell lymphoma | 2 | 0.1 |
| EBV+ diffuse large B-cell lymphoma | 2 | 0.1 |
| Granulomatous slack skin | 1 | 0.0 |
| Follicular T-cell lymphoma | 1 | 0.0 |
| Hydroa vacciniforme-like lymphoproliferative disease | 1 | 0.0 |
| EBV+ mucocutaneous ulcer | 1 | 0.0 |
| Angioimmunoblastic T-cell lymphoma | 1 | 0.0 |
| Plasmacytoid dendritic cell neoplasm | 1 | 0.0 |
| Hodgkin lymphoma | 1 | 0.0 |
| Posttransplant lymphoproliferative disorder | 1 | 0.0 |
| Total | 2020 | 100 |
Abbreviations: EORTC, European Organization for Research and Treatment of Cancer; EBV, Epstein–Barr virus.
The most common diagnoses in the MF/SS category were classic MF (882, 79.3% of patients in this category and 43.7% of all patients); 171 patients had folliculotropic MF and 55 had SS (15.3% and 5% of all patients in the MF/SS category, respectively).
BCLs were the second largest category. The most common diagnoses were marginal zone B-cell lymphoma (MZL) and follicle-center B-cell lymphoma (FCL), with 280 (51.2%) and 229 (41.9%) cases, respectively.
In the CD30+ LPD category, lymphomatoid papulosis accounted for approximately twice as many cases as CD30+ anaplastic large cell lymphoma (152 [68.5%] vs 70 [31.5%]).
The smallest category was other TCLs, the most common of which was CD4+ small/medium T-cell lymphoproliferative disorder (76, 65.5% of all cases in this category).
StagesTNM/TNMB stages for the full sample are shown in Table 2. In terms of cutaneous involvement, 1065 patients (52.7%) had stage T1 disease at diagnosis, and of these 604 (29.9% of all patients) were stage T1a. Five-hundred patients had stage T2 disease (24.8%), 273 (13.5%) T3 disease, and 92 (4.6%) T4 disease. The degree of cutaneous involvement in the remaining 90 cases was recorded as unknown or not applicable.
TNM Stages (TNMB Stages for MF/SS) (% of Total Sample).
| MF/SS | Non-MF/SS lymphomas | |||||
|---|---|---|---|---|---|---|
| Stage | No. | % | Stage | No. | % | |
| T stage (skin) | T1 | 110 | 9.9 | T1 | 125 | 13.8 |
| T1a | 286 | 25.7 | T1a | 318 | 35 | |
| T1b | 172 | 15.5 | T1b | 54 | 5.9 | |
| T2 | 95 | 8.5 | T2 | 27 | 3 | |
| T2a | 98 | 8.8 | T2a | 92 | 10.1 | |
| T2b | 128 | 11.5 | T2b | 43 | 4.7 | |
| T3 | 98 | 8.8 | T2c | 17 | 1.9 | |
| T4 | 89 | 8 | T3 | 36 | 4.0 | |
| Unknown | 36 | 3.2 | T3a | 49 | 5.4 | |
| T3b | 90 | 9.9 | ||||
| T4 | 3 | 0.3 | ||||
| Unknown | 54 | 5.9 | ||||
| N stage (lymph nodes) | N0 | 988 | 88.8 | N0 | 823 | 90.6 |
| N1 | 44 | 3 | N1 | 20 | 2.2 | |
| N2 | 3 | 1.3 | N2 | 6 | 0.7 | |
| N3 | 6 | 0.5 | N3 | 4 | 0.4 | |
| Nx | 25 | 2.2 | Nx | 2 | 0.2 | |
| Unknown | 46 | 4.1 | Unknown | 59 | 6.5 | |
| M stage (organs) | M0 | 1062 | 95.5 | M0 | 844 | 93 |
| M1 | 2 | 0.2 | M1 | 4 | 0.4 | |
| Unknown | 48 | 4.3 | Unknown | 60 | 6.6 | |
| B stage (peripheral blood)* MF/SS | B0 | 970 | 87.2 | |||
| B1 | 32 | 2.9 | ||||
| B2 | 43 | 3.9 | ||||
| Unknown | 67 | 6 | ||||
Abbreviation: MF/SS, mycosis fungoides/Sézary syndrome.
The immense majority of patients (1811, 89.7%) did not have lymph node involvement at the time of this study. In 132 patients (6.5%), lymph node status was recorded as unknown, not evaluated, or not applicable. Just 77 patients (3.8%) had lymph node involvement. Visceral organ involvement at diagnosis was very uncommon (6 patients, 0.3%).
The breakdown and distribution of disease stages according to a diagnosis of MF/SS vs. a non-MF/SS lymphoma are shown in Table 2. Just 6.8% of patients with MF/SS had peripheral blood involvement.
Overall clinical stages for patients with MF/SS are shown in Table 3. Almost three-quarters of the patients (826/1112, 74.3%) had stage I disease, and within this category, the majority (518, 46.6%) were stage IA; 184 patients (16.6%) had advanced disease (stage IIB or higher). Stage was unknown for 6.3% of patients.
Diagnostic ProceduresHistologic examination was performed in all patients, and laboratory tests in the vast majority (1980/2020, 98%). Immunohistochemical studies were performed in 1909 patients (94.5%) and molecular studies in 1358 (67.2%).
Imaging studies were performed in 1491 patients (73.8%), and additional radiological tests in 1677 (83%).
TreatmentsThe treatments used to manage PCL are shown in Table 4. Topical corticosteroids were by far the most widely prescribed treatment (1369 patients, 67.8% of total). These were followed by different forms of phototherapy (890 patients, 44.1%), surgery (412, 20.4%), and radiotherapy (384, 19%).
Breakdown of Treatments Reported in the Spanish Primary Cutaneous Lymphoma Registry.
| Treatment | No. | % |
|---|---|---|
| Topical corticosteroids | 1369 | 67.8 |
| Topical nitrogen mustard | 23 | 1.1 |
| Topical carmustine (BCNU) | 24 | 1.2 |
| Topical bexarotene | 31 | 1.5 |
| PUVA | 484 | 24.0 |
| Re-PUVA | 34 | 1.7 |
| Narrow-band UV-B | 372 | 18.4 |
| Electron beam therapy | 66 | 3.3 |
| Radiotherapy | 384 | 19.0 |
| Systemic chemotherapy | 246 | 12.2 |
| Surgery | 412 | 20.4 |
| Systemic retinoids | 92 | 4.6 |
| Interferon | 163 | 8.1 |
| Fusion antibodies with toxins | 6 | 0.3 |
| Histone deacetylase inhibitors | 11 | 0.5 |
| Intravenous anti-CD20 antibodies | 107 | 5.3 |
| Subcutaneous anti-CD20 antibodies | 71 | 3.5 |
| Bone marrow transplant | 25 | 1.2 |
Abbreviations: PUVA, psoralen plus UV-A therapy; Re-PUVA, PUVA combined with retinoids.
Approximately two-thirds of patients received 1 (713, 35.3%) or 2 (623, 30.9%) treatments; 327 (16.2%) received 3 treatments and 316 (15.7%) 4 or more. Just 41 patients (2.0%) did not receive any treatment for the management of their PCL.
Clinical CourseTreatment responses and disease status are shown in Tables 5 and 6. Just over half of the patients (1134, 56.1%) responded to treatment, with most achieving a complete response. Stable disease was recorded for 546 patients (27%) at the time of this study. Approximately 10% had progressive disease or had died.
Treatment Responses in MF/SS and non-MF/SS Groups.
| Patient status at time of study | MF/SS | Non-MF/SS | Total | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Complete remission | 307 | 27.6 | 574 | 63.2 | 881 | 43.6 |
| Partial remission | 197 | 17.7 | 59 | 6.5 | 256 | 12.7 |
| Stable disease | 413 | 37.1 | 133 | 14.6 | 546 | 27.0 |
| Progressive disease | 78 | 7.0 | 32 | 3.5 | 110 | 5.4 |
| Loss to follow-up | 54 | 4.9 | 63 | 6.9 | 117 | 5.8 |
| Deceased | 55 | 4.9 | 31 | 3.4 | 86 | 4.3 |
| Relapse | 7 | 0.6 | 15 | 1.7 | 22 | 1.1 |
| Unknown | 1 | 0.1 | 1 | 0.1 | 2 | 0.1 |
Abbreviation: MF/SS, mycosis fungoides/Sézary syndrome.
Disease Course by Compartment in MF/SS and non-MF/SS Groups.
| Current status | MF/SS | Non-MF/SS (rest) | Total | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Cutaneous disease at time of study | ||||||
| No | 344 | 30.9 | 622 | 68.5 | 966 | 47.8 |
| Yes | 711 | 63.9 | 238 | 26.2 | 949 | 47.0 |
| Unknown | 57 | 5.1 | 48 | 5.3 | 105 | 5.2 |
| Lymph node involvement at time of study | ||||||
| No | 937 | 84.3 | 810 | 89.2 | 1747 | 86.5 |
| Yes | 64 | 5.8 | 20 | 2.2 | 84 | 4.2 |
| Unknown | 111 | 10.0 | 78 | 8.6 | 189 | 9.4 |
| Visceral organ involvement at time of study | ||||||
| No | 962 | 86.5 | 802 | 88.3 | 1764 | 87.3 |
| Yes | 16 | 1.4 | 8 | 0.9 | 24 | 1.2 |
| Unknown | 134 | 12.1 | 98 | 10.8 | 232 | 11.5 |
| Blood involvement at time of study | ||||||
| No | 896 | 80.6 | 713 | 79.0 | 1609 | 79.9 |
| Yes | 64 | 5.8 | 8 | 0.9 | 72 | 3.6 |
| Unknown | 152 | 13.7 | 182 | 20.2 | 334 | 16.6 |
Abbreviation: MF/SS, mycosis fungoides/Sézary syndrome.
At the time of our analysis, 962 patients (47.6%) had cutaneous involvement versus 950 (47.0%) who did not. No data were available for the remaining 5.4% of patients. The respective figures for lymph node, visceral organ, and peripheral blood involvement were 4.2% (84), 1.2% (24), and 3.6% (72).
Comparisons between patients with MF/SS and non-MF/SS lymphomas are also shown in Tables 5 and 6. The main difference observed was for the percentage of patients who had achieved complete remission, which is reflected in the percentage of those with cutaneous involvement at the time of the study: more than 60% of non-MF/SS patients had achieved a complete response compared with less than 30% of MF/SS patients. In addition, disease progression was almost twice as common in the MF/SS category (7% vs. 3.5%).
DiscussionPCLs are rare, with an estimated annual incidence of approximately 1 case per 100 000 people.7 Several studies in Germany,8 the United Kingdom,9 Norway,10 Denmark,11 and France12 have reported incidence rates of between 2.9 and 4 cases×106 a year. The creation of a national PCL registry 5 years ago was prompted by the low incidence of these diseases. It was designed to facilitate collaborative research and has already led to several publications in international journals over the years.13–15 In addition, the number of patients added to the registry each year has remained stable, within a range of between 348 and 411 patients, following the initial 504 included in year 1. (The higher initial number is to be expected as hospitals will have included nonincident cases.) The similarity between the numbers in the first and following years can largely explained by the notable increase in the number of hospitals contributing to the registry, which has risen from 16 in year 1 to the current number of 33.
The breakdown of diagnoses is similar to that described in the literature,1,16 albeit with slight differences. T-cell lymphomas accounted for 72% of diagnoses, compared with 27% for BCLs. MF together with its variants was the most common entity (52.3%), followed by MZL (13.9%) and FCL (11.3%). Contrasting with reports in the latest update of the WHO-EORTC classification for PCLs,1 we observed a slightly higher proportion of MF and BCL cases. Mean age at diagnosis was 55.6 years, and the ratio of male to female patients was 1.4:1. These findings are similar to those reported for the first year of the registry.3 The proportions of MZL and FCL in the BCL category and LyP and ALCL in the CD30+ LPD category are also very similar to those in the first year.3 Of note in year 1 and now is the practically identical number of MZL and FCL cases. This similarity was not reflected in the latest large-scale revisions,1,16 although it has been described in other studies.8 The profile of CD30+ LPDs is also similar to that described in the literature.1,17,18 LyP, with twice as many cases as ALCL and CD4+ small/medium T-cell LPDs, remains the second most common T-cell LPD (10.5% of cases in this category, 7.5% of total).
Advanced disease accounted for just a small proportion of cases in the RELCP; 16.5% of patients with MF/SS had stage IIB disease or higher, and just 8.2% of these were stage III-IV. Advanced disease was also uncommon in the other categories. Observations from the RELCP registry suggest that MF/SS follows a worse disease course, as patients in this group were almost twice as likely to develop progressive disease as those with a non-MF/SS lymphoma, and these in addition were twice as likely to achieve complete remission.
The RECLP registry has some limitations, including the potential inaccuracy of some diagnoses (procedures are not centralized) and variability between hospitals. Even though clinical guidelines help standardize procedures and treatments, there will be inevitable differences such as greater or lesser access to diagnostic resources, such as genetic tests. The proportion of patients seen by the dermatology department may also vary, as in some hospitals, patients with more advanced disease or severe manifestations will be under the care of the hematology department. Nevertheless, the large number of hospitals that contribute to the registry and the consecutive enrolment of patients by all hospitals should limit the risk of selection bias and ensure a true reflection of PCL in Spain.
ConclusionsOverall, the clinical characteristics of patients with PCL in Spain are similar to those described in other series. The classic breakdown of 75% vs. 25% for MF/SS vs. non-MF/SS lymphomas was maintained. MF was by far the most common entity, followed by MZL and FCL. At the time of this study, most patients had early-stage disease, more than 50% had responded completely or partly to treatment, and 25% had stable disease.
The AEDV's PCL registry facilitates clinical studies on this rare group of diseases and provides easy access to groups of patients for subsequent studies, including those with a prospective design.
Conflicts of InterestThe Spanish Registry of Primary Cutaneous Lymphomas (RELCP) is sponsored by the Healthy Skin Foundation of the Spanish Academy of Dermatology and Venereology. Kyowa Kirin helps with funding to maintain the registry. Collaborating companies have no role in the design or conduct of studies, the writing of manuscripts, or publication decisions.
We thank Marina de Vega (Healthy Skin Foundation of the Spanish Academy of Dermatology and Venereology) for overseeing the registry.