Cutaneous squamous cell carcinoma (cSCC) is a relatively indolent malignant tumor compared to other types of cancer and rarely causes metastasis if treated promptly, with a 5-year cure rate > 90%.1 Curative treatment is usually surgery and less frequently radiotherapy, which is of particular interest in frail patients and/or large tumors. Surgery can be conventional or Mohs micrographic surgery (MMS), which achieves lower recurrence rates: 3.1% up to 8% vs 0% up to 4%, respectively.2 Some cases respond poorly to these treatments or may not be the best therapeutic option due to the characteristics associated with the patient or tumor. Alternative treatments—mainly systemic or palliative—are often considered. Immunotherapy with anti-PD-1 has been a therapeutic revolution in the management of advanced and metastatic cSCC. However, approximately 50% of patients will eventually not respond to this therapy, and it is not a good option for transplanted patients.3 New intralesional therapies could represent another therapeutic revolution, potentially solving some situations described in this article.
The objective of this article, resulting from reflection and routine clinical practice, is to identify and analyze the various scenarios in which conventional local treatments such as surgery and radiotherapy are difficult to apply or offer limited curative options. These situations are not always optimally addressed in clinical practice guidelines (National Comprehensive Cancer Network [NCCN] Guidelines4 and European Association of Dermato Oncology [EADO] Guidelines5), or in the staging systems (American Joint Committee on Cancer [AJCC] 8th edition6 and Brigham and Women's Hospital [BWH] system7) that are currently widely used. They are challenging regarding management and treatment and overlap with concepts of high-risk, locally advanced, and metastatic cSCC. These scenarios are (Table 1):
Scenarios in which cutaneous squamous cell carcinoma is difficult to treat using conventional local therapies.
| Invasion of deep structures or cavities |
| Bone involvement and beyond |
| Perineural invasion |
| Complex anatomical locations: nail and penis |
| Hidradenitis suppurativa concomitant with other chronic inflammatory diseases |
| Incomplete excisions or recurrences in flaps |
| Satellite lesions or in-transit metastasis |
| Multiple simultaneous tumors |
| Parotid metastasis |
| Extensive nodal metastasis |
| Very elderly or frail patients |
Some tumors compromise deep structures or cavities such as the orbit8 or the ear.9 Surgery in these cases, beyond posing a higher rate of recurrence due to its complexity, can be so mutilating that it is contraindicated or not feasible. Sometimes, adequate surgical margins are not achieved. Radiotherapy is often contraindicated in these cases. Similarly, tumors in limbs or other locations invading deep structures such as tendons, leading to excessively mutilating surgeries such as amputation, represent a similar scenario.
Discussing these cases with ophthalmologists, otolaryngologists, and other specialists is of paramount importance to give the patient our best clinical judgement. Preoperative imaging modalities and the patient's general condition should be taken into consideration across the entire decision-making process. If surgery is decided upon, MMS should be the treatment of choice.
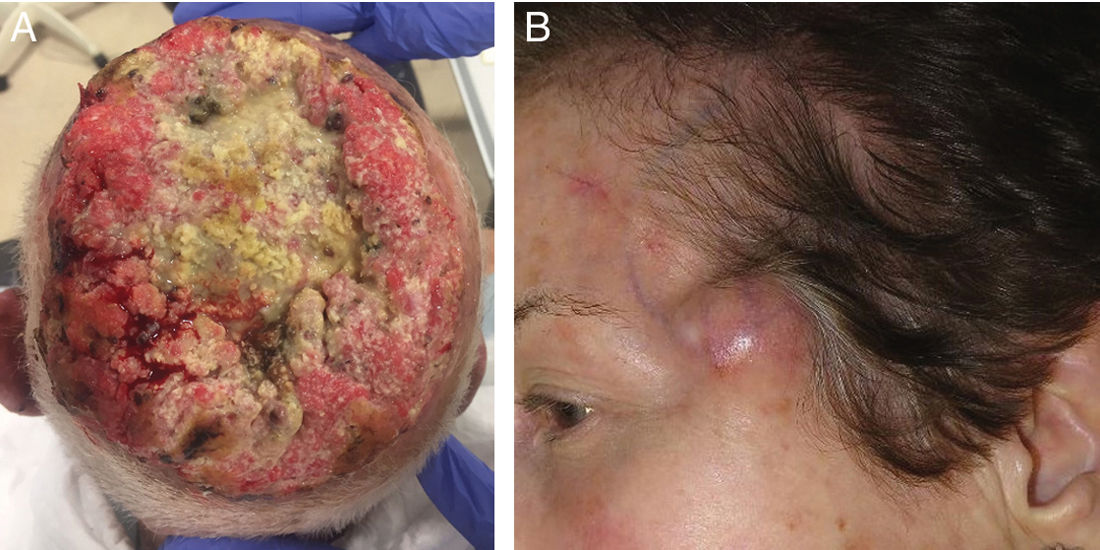
Bone involvement and beyondBone involvement is a recognized poor prognostic factor in major staging systems, being a risk factor for recurrence, disease progression, and mortality. Bone involvement alone is a T4 stage in the AJCC6 and a T3 in the BWH.7 Beyond this, bone resection or irradiation is sometimes not possible. As a matter of fact, in some cases—such as scalp tumors—they can be invasive and lead to intracranial invasion, making curative surgical or radiotherapeutic treatment very difficult or nearly impossible.10 In these cases, curative options are very limited, and a multidisciplinary approach is required (Fig. 1).
a) 98-year-old male with a large cutaneous squamous cell carcinoma, affecting the entire scalp with bone invasion involving the entire cranial vault.
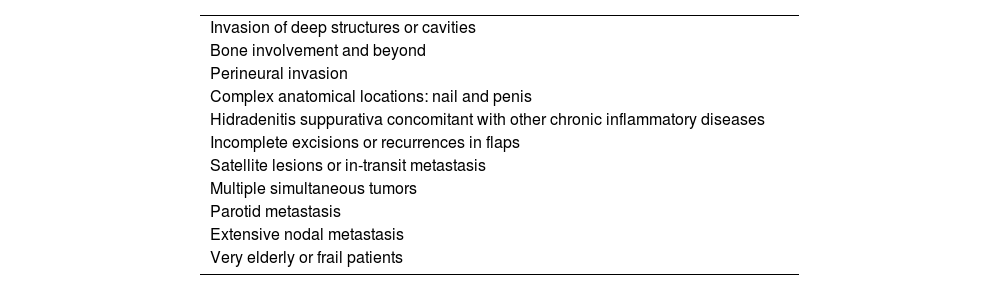
b) Woman with cutaneous squamous cell carcinoma operated on the left forehead (see the scar) who developed satellite lesions in the form of a dermal tumor nodule on the left temple a few weeks after surgery.
Perineural invasion is a known poor prognostic factor for recurrence and mortality, especially when it invades large-diameter nerves (> 0.1mm) or nerves that run deeper than the dermis. Perineural invasion is not a type of lymphatic or hematogenous invasion but a direct spread of the primary tumor. It is often subclinical, though it sometimes causes neurological symptoms such as paresthesias, pain, paralysis... In such cases, performing a preoperative magnetic resonance imaging is advised. The trigeminal nerve, the facial nerve, and their branches are most frequently affected. Despite being considered in staging systems, it often goes unnoticed and is therapeutically challenging. Due to perineural spread, resection is often incomplete, either because surgery cannot proceed to a certain depth or because of undetected spread. Therefore, options such as adding a surgical safety margin despite negative margins in MMS or administering adjuvant radiotherapy are often discussed in the management of this entity.11
Complex anatomical locationsThe anatomical location of the tumor is a risk factor for recurrence and metastasis. Low-risk areas include the trunk and extremities. In contrast, high-risk areas include the head and neck (especially the H-zone of the face), genitals, mucous membranes, ears, pre-tibial region, hands, and feet.4 Although MMS can reduce the rate of recurrence there are locations in which achieving a cure with a single surgical treatment remains challenging. An example is the nail. In this location, the rates of recurrence described—despite treated with MMS—exceed 20%12 (much higher than the 0%-4% reported in other locations). This high rate of recurrence could be explained by 2 non-exclusive hypotheses: 1) the anatomical difficulty of this specific area, 2) the etiology behind these tumors being human papillomavirus infection and the persistence of non-tumor cells infected by this virus causing their recurrence.12 Therefore, studies are needed to confirm the possible role of human papillomavirus and propose new prevention and treatment strategies. Penile squamous cell carcinoma also presents particular etiological features such as phimosis, smoking, human papillomavirus, chronic inflammatory diseases such as lichen sclerosus,... impacting its recurrence and determining the therapeutic and prophylactic strategies that should be used. This location has a high rate of regional recurrence and progression. MMS would avoid mutilating surgeries without affecting the outcomes; however, a joint approach with urology is essential.13
Hidradenitis suppurativa concomitant with other chronic inflammatory diseasescSCCs developing in areas touches by chronic inflammatory diseases such as hidradenitis suppurativa (other examples include conditions such as lichen planus or tumors arising in irradiated zones, ostomies, or patients with congenital epidermolysis bullosa) often present a therapeutic challenge. Firstly, there is often a diagnostic delay, making it not uncommon to encounter locally advanced tumors. Secondly, they occur on skin damaged by the underlying disease, leading to more frequent postoperative complications such as wound dehiscence. The skin is usually less elastic and may exhibit fibrosis, making closures and flaps difficult. Furthermore, this skin is often unsuitable for irradiation. This inflamed skin acts as a field of cancerization, and recurrences can occur despite proper tumor treatment. Finally, hidradenitis suppurativa causes fistulous tracts where the tumor finds an ideal plane for progression, often resulting in greater local tumor spread than anticipated, complicating its excision. These fistulas can run deep and be associated with anorectal and urogenital structures, which may be compromised by the tumor or surgery.
To address this scenario, it is essential to maintain a high level of suspicion during the screening of these patients for the earliest possible detection and optimize the treatment of the underlying disease to prevent new tumors from appearing. Secondly, once faced with a tumor of this type, it is essential to perform imaging modalities to plan surgery and approach it along with the corresponding specialist (general surgeon, urologist, gynecologist...).14
Incomplete excisions or recurrences in flapsThe occurrence of a recurrence in a flap or the excision with a tumor affected margins whose defect has been reconstructed with a flap remains a relatively frequent and difficult scenario to manage. Firstly, recurrence per se is a poor prognostic factor, and incomplete excision may be due to a tumor of difficult clinical delineation. Secondly, it is challenging to determine where the tumor persists in cases of affected margins, and in cases of recurrence, the tumor often finds a plane of dissemination through the flap scars and/or has a long progression time due to deep recurrences that go clinically unnoticed. Finally, reconstructions can be challenging as we have “used up” other reconstructive options before.
In the authors’ opinion, to prevent this from happening, MMS should be performed to analyze 100% of the tumor margins whenever a flap is to be used to reconstruct a tumor excision defect. Even so, some cases (not many) treated with MMS will present recurrences on flaps. These cases should always be approached with another MMS, preferably delayed.
Satellite lesions or in-transit metastasisSatellite lesions or in-transit metastasis are one of the long-forgotten scenarios in the management of cSCC. These are non-epidermal lesions originating between the primary tumor and the first draining lymph nodes. It has been confirmed that satellite lesions are an independent risk factor for poor prognosis in cSCC and that, in terms of recurrence and disease-specific survival, the clinical outcomes of patients with cSCC-induced satellite lesions are similar to those of nodal metastases.15 Although rare, encountering a patient with satellite lesions in the routine clinical practice is a diagnostic and therapeutic challenge since they are often omitted from the main currently used staging systems and clinical practice guidelines.4–7 Recently, it has been demonstrated that not all satellite lesions are the same and that sizes ≥ 2cm and the presence of > 5 lesions confer an increased risk of tumor recurrence and specific mortality.16
Incorporating satellite lesions into upcoming staging systems and clinical practice guidelines—as it has already been the case with melanoma and Merkel cell carcinoma—would be a first step toward initiating clinical trials and other studies to determine the optimal therapeutic strategy in each case (Fig. 1b).
Multiple simultaneous tumorsThe presence of multiple simultaneous tumors does not indicate widespread or metastatic disease like satellite lesions but just shares with the fact of exhibiting multiple lesions too. In some cases, due to the number, size, location, or rapid emergence of new tumors, treatment with conventional therapies becomes difficult or nearly impossible.
Parotid metastasisIn countries with a high incidence of skin cancer, such as Australia, cSCC-induced metastasis is the leading cause of malignancy in this salivary gland. However, this situation has not been included in the main staging systems. While gland involvement can occur through local invasion, it is mostly affected by intraparotid nodal metastases. In the most widely used staging systems,6,7 parotid metastasis is often equated with cervical lymph node metastasis, despite several studies propose alternative staging systems that classify this scenario separately due to its unique prognostic characteristics. The most well-known is the study conducted by O’Brien,17 which differentiates parotid metastases from cervical lymph node metastases and establishes 3 levels of prognosis-related severity. Despite a few controversial results, this classification has been corroborated by other studies throughout the years. However, current staging systems still do not provide the distinction and particularity this situation deserves, leading to suboptimal management and treatment of these patients.
Extensive nodal metastasisPatients with nodal metastasis exhibit, by definition, advanced disease. Surgical treatment of nodal metastasis can be curative but often fails when metastases are large, involve numerous nodes, or show extracapsular extension, which is why radiotherapy is usually added in these cases.6 Lymphadenectomies are associated with postoperative complications in > 55% of cases, including infections, seromas, dehiscence, or lymphedema. Despite undergoing surgery and radiotherapy, as recommended by the guidelines, in these cases, the rates of recurrence are generally between 20% and 35%, while the 5-year disease-free survival and disease-specific survival rates are 59%-83% and 63%-83%, respectively.4
Very elderly or frail patientscSCC predominantly occurs in elderly patients, making it a common finding in the routine clinical practice to encounter frail patients or those with comorbidities contraindicating surgery, in whom radiotherapy is considered palliative. Aging is associated with increased frailty, risk of dependence, and reduced autonomy. Frail patients have worse survival and tolerate standard treatments less well. Some geriatric oncology societies recommend that elderly cancer patients should undergo geriatric assessments to detect problems that may go unnoticed in routine physical examinations or in the patient's medical history to predict survival and help in therapeutic decision-making.18 Geriatric assessment is a multidimensional and interdisciplinary tool that identifies functional, nutritional, cognitive, psychological, social support, and comorbidity factors. Although comprehensive geriatric assessment can be useful in oncology, it requires complex and long visits and tests. In this regard, there are rapid geriatric screening tools currently available beyond ECOG, such as the G8 and the Vulnerable Elders Survey-13, which have proven useful in identifying patients requiring further evaluation.19 In cases in which frailty is indicated by the score, comprehensive geriatric assessments evaluating physical, mental, nutritional, comorbidity, and social function are advised. If frailty is confirmed, interventions to revert to non-frail states and consideration of non-surgical or minimally aggressive treatments are advised. Additionally, the Charlson Comorbidity Index can predict short- or long-term mortality based on the patient's comorbidities and has, also, been validated in some cancer populations. Its results should be validated in cSCC patients.20
ConclusionsThe present article identifies and analyzes the main scenarios in which cSCC is difficult to treat with conventional local therapies. Therefore, there is no clear consensus on what their therapeutic management should be. Preoperative imaging modalities, MMS, multidisciplinary committee discussions, and individualized therapies are common elements for the optimal management of various scenarios. The scientific community should focus on including these scenarios in the routine clinical guidelines, conducting studies to optimize their management, and including these patients in clinical trials (especially now that we lieve in the new era of intralesional therapies) to offer the best therapeutic options to these patients.