Ruxolitinib is a JAK1 and JAK2 inhibitor approved in 2011 to treat myelofibrosis (MF), polycythemia vera (PV) (in 2014), and graft-versus-host disease (GVHD) (in 2019).1,2 Since the initial clinical trials with ruxolitinib, a possible increase in the incidence of non-melanoma skin cancer (NMSC) has been observed.2,3 Of note that this drug is used in patients who have a higher risk in relation to the overall population of developing NMSC due to their hematological neoplasms and previous treatments (e.g., hydroxyurea).4 In addition to this increased incidence, cases of exceptionally aggressive squamous cell carcinoma (SCC) have been reported in patients on ruxolitinib.5–7
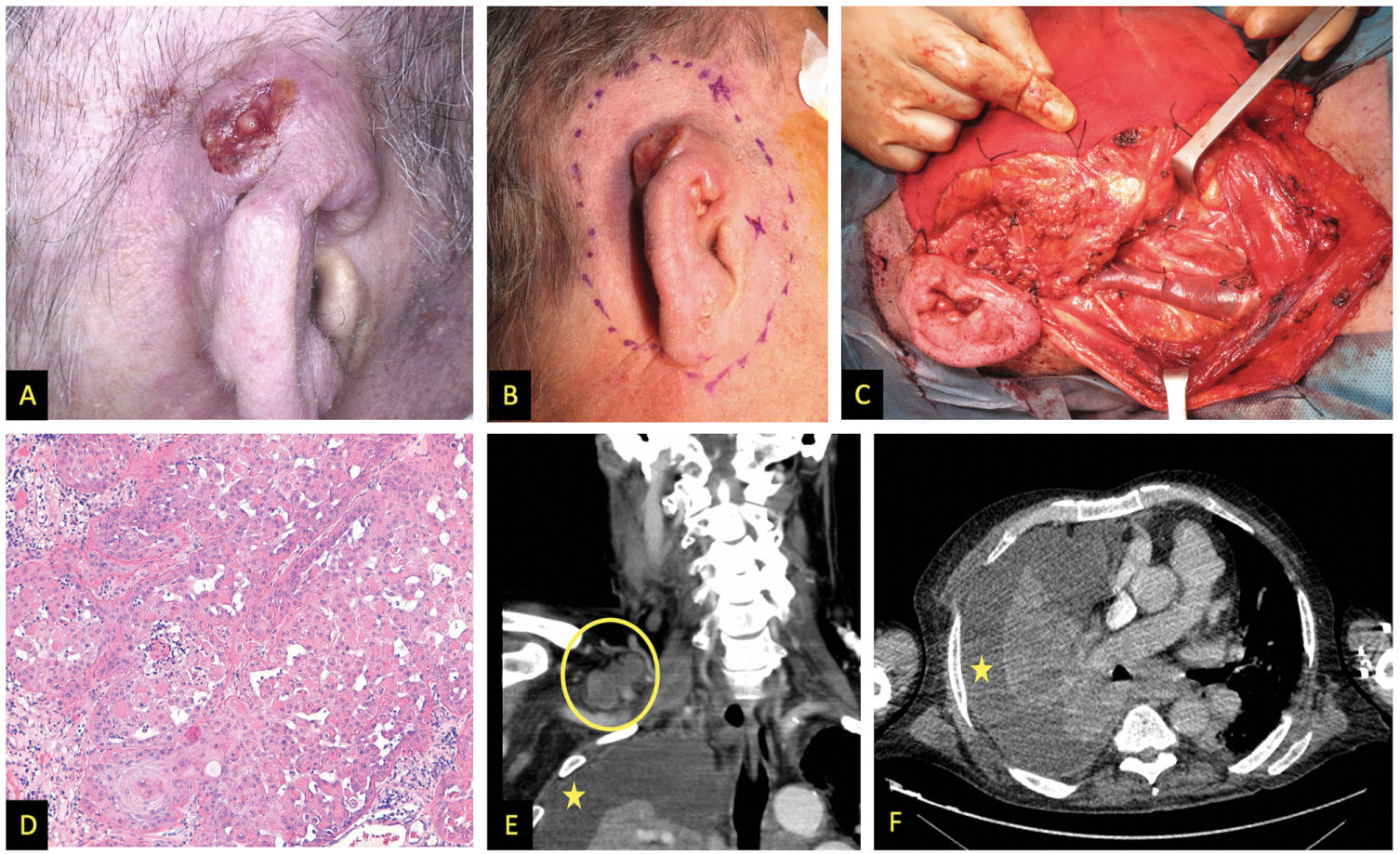
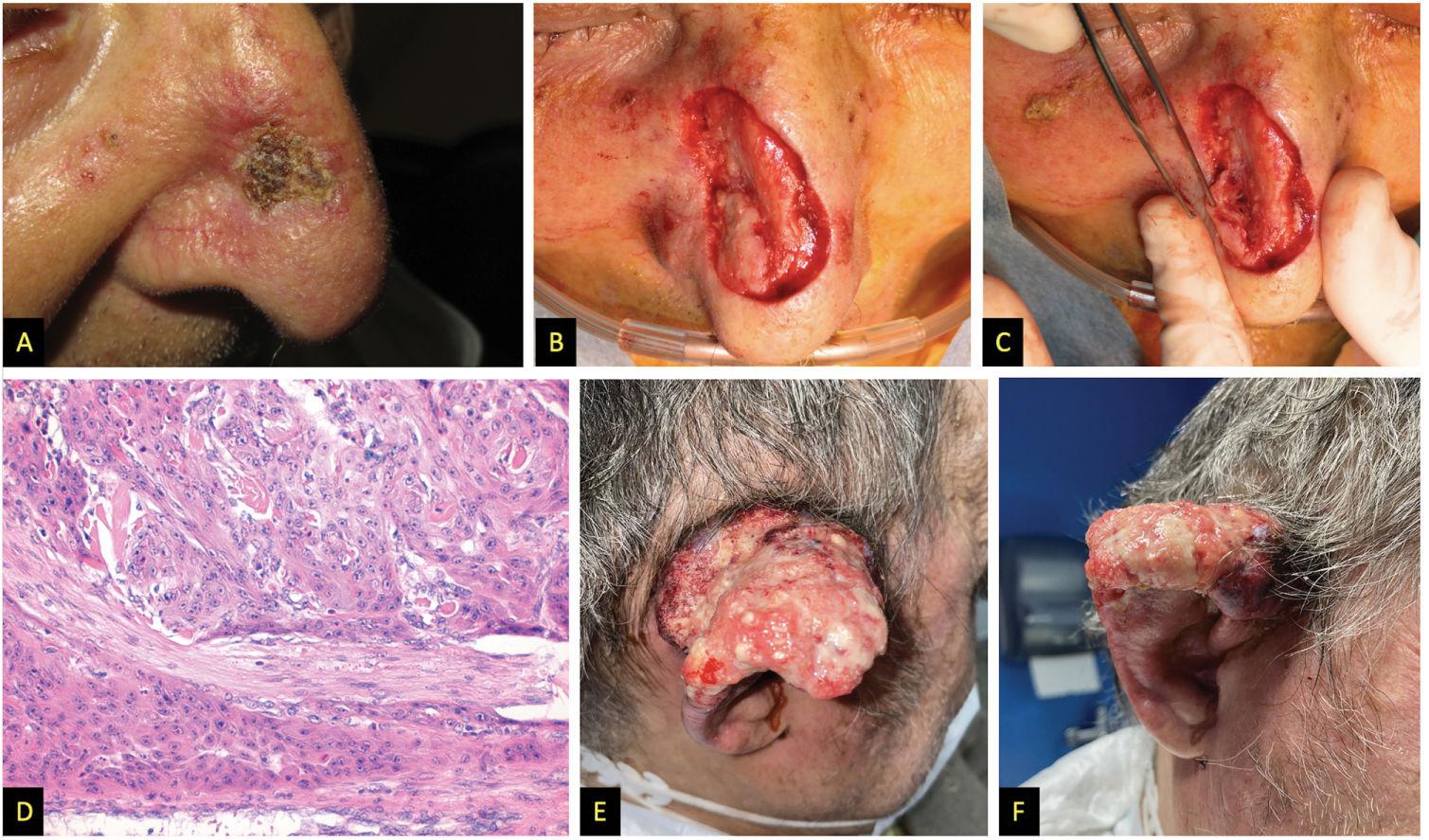
We describe the cases of 3 men who developed high-risk SCC while on ruxolitinib administered for hematological conditions. Table 1 shows the patients’ clinical and demographic characteristics. Patient #1 and patient #3 died due to the progression of their skin neoplasms, initially located in both patients in the pinna (figs. 1 and 2)1. Although patient #2 died shortly after the initial intervention of an unrelated medical problem, the presence of poor prognostic tumor factors, such as perineural invasion or nasal cartilage infiltration, is significant (fig. 2).
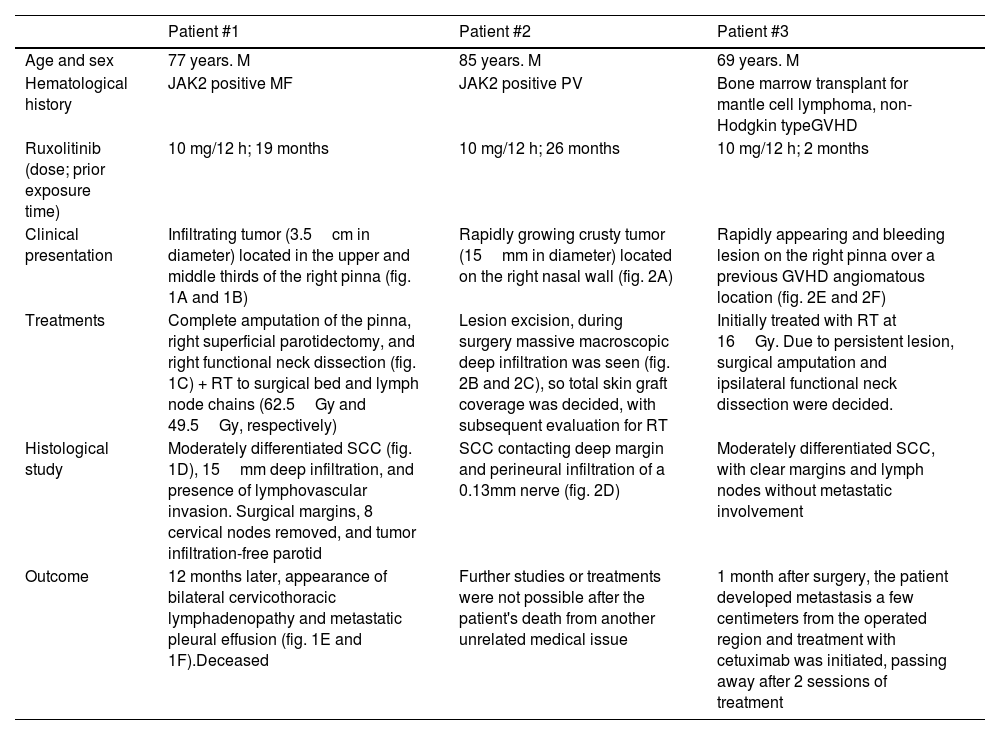
Main characteristics of patients who developed high-risk SCC while on ruxolitinib.
| Patient #1 | Patient #2 | Patient #3 | |
|---|---|---|---|
| Age and sex | 77 years. M | 85 years. M | 69 years. M |
| Hematological history | JAK2 positive MF | JAK2 positive PV | Bone marrow transplant for mantle cell lymphoma, non-Hodgkin typeGVHD |
| Ruxolitinib (dose; prior exposure time) | 10 mg/12 h; 19 months | 10 mg/12 h; 26 months | 10 mg/12 h; 2 months |
| Clinical presentation | Infiltrating tumor (3.5cm in diameter) located in the upper and middle thirds of the right pinna (fig. 1A and 1B) | Rapidly growing crusty tumor (15mm in diameter) located on the right nasal wall (fig. 2A) | Rapidly appearing and bleeding lesion on the right pinna over a previous GVHD angiomatous location (fig. 2E and 2F) |
| Treatments | Complete amputation of the pinna, right superficial parotidectomy, and right functional neck dissection (fig. 1C) + RT to surgical bed and lymph node chains (62.5Gy and 49.5Gy, respectively) | Lesion excision, during surgery massive macroscopic deep infiltration was seen (fig. 2B and 2C), so total skin graft coverage was decided, with subsequent evaluation for RT | Initially treated with RT at 16Gy. Due to persistent lesion, surgical amputation and ipsilateral functional neck dissection were decided. |
| Histological study | Moderately differentiated SCC (fig. 1D), 15mm deep infiltration, and presence of lymphovascular invasion. Surgical margins, 8 cervical nodes removed, and tumor infiltration-free parotid | SCC contacting deep margin and perineural infiltration of a 0.13mm nerve (fig. 2D) | Moderately differentiated SCC, with clear margins and lymph nodes without metastatic involvement |
| Outcome | 12 months later, appearance of bilateral cervicothoracic lymphadenopathy and metastatic pleural effusion (fig. 1E and 1F).Deceased | Further studies or treatments were not possible after the patient's death from another unrelated medical issue | 1 month after surgery, the patient developed metastasis a few centimeters from the operated region and treatment with cetuximab was initiated, passing away after 2 sessions of treatment |
GVHD, graft-versus-host disease; M, male; MF, myelofibrosis; PV, polycythemia vera; RT, radiotherapy; SCC, squamous cell carcinoma.
Images from patient #1. A and B) Clinical appearance of SCC affecting the upper and middle thirds of the right pinna. C) Functional neck dissection. The image shows the external jugular vein, the parotid gland, and the facial nerve. D) Hematoxylin and eosin stain, 200x. Moderately differentiated keratinizing tumor forming keratin pearls. E) Coronal image, contrast-enhanced computed tomography (CT) showing right supraclavicular lymphadenopathy (circle); asterisk indicates pleural effusion. F) Cross-sectional image, contrast-enhanced CT, asterisk indicates pleural effusion.
A) Patient #2, squamous tumor on the nasal wall. B and C) Intraoperative appearance suggesting deep infiltration of nasal cartilages. D) Hematoxylin and eosin stain, 200x, showing tumor with perineural infiltration. E and F) Patient #3, large fleshy tumor with spontaneous bleeding affecting almost the entire pinna.
Ruxolitinib is one of the most widely used drugs to treat patients with myeloproliferative syndromes and corticoid-refractory GVHD as it is one of the very few treatments that has proven capable of improving overall survival (in both MF and PV) and disease-free survival (in GVHD).2,3,8 The 5-year assessment of the COMFORT-II clinical trial—which ultimately led to the approval of the indication for MF treatment—confirmed a 17.1% incidence rate of NMSC (25 out of 146) in patients on ruxolitinib vs a 2.7% incidence rate (2 out of 73) in the control group on the optimal medical therapy.3 However, after adjusting for exposure (patient-years), this difference did not reach statistical significance.
Recently, clinical practice data from the largest cohort to date of 188 patients on ruxolitinib for MF or PV and controls adjusted for age, sex, race, time from diagnosis, and previous treatments—including hydroxyurea—have been published1. In this study, a hazard ratio (HR) for the development of SCC in patients on ruxolitinib of 3.2 (95% confidence interval [CI], 1.5-7) was found. In patients without the JAK2 mutation, this HR went up to 7.4 (95%CI, 2.5-21.6).1 The authors hypothesized that this difference could be explained by the absence of the JAK2 mutation, which may have conditioned a more intense ruxolitinib inhibition of the tissue lymphocytes responsible for preventing the development of skin neoplasms1. Unexpectedly, no significant differences were found in the development of SCC in patients on hydroxyurea vs those who were treatment-naïve (HR, 1.1). However, having received a > 2-week regimen of cyclosporine, azathioprine, or systemic corticosteroids was associated with a higher risk of SCC. Most SCCs appeared between the 1st and 2nd year after starting ruxolitinib, with a median of 66.5 weeks (range, 11-245).
Regarding GVHD, long-term data on the development of skin neoplasms in patients on ruxolitinib are still lacking.8 Patient #3 is the only case we could find in the published literature of a SCC in a patient with GVHD while on ruxolitinib. Given the fatal outcome, it seems advisable to be aware of the possible appearance of aggressive SCC in this group of patients.
For the topical use of ruxolitinib, no cases of SCC have ever been reported in patients on this topical therapy.9 Furthermore, in a review of the pharmacokinetics of ruxolitinib cream for atopic dermatitis—which included 1139 patients from clinical trials—no blood concentrations capable of having a systemic effect or causing hematological changes were found.10 This makes it very likely that the safety profile of the topical presentation is very different from that of the systemic drug.
In conclusion, we recommend the dermatological follow-up of patients on ruxolitinib for hematological problems, a recommendation that is currently included in the drug technical sheet.1 We should be aware of the aggressive and lethal course of the disease these patients may present. Future post-marketing studies, with longer follow-up periods than those collected in clinical trials, may shed more light on the risk of patients with GVHD on ruxolitinib.
FundingNone declared.
Conflicts of interestNone declared.