The use of disease-modifying therapies (DMT) has led to a paradigm shift in the management of multiple sclerosis. A comprehensive narrative review was conducted through an extensive literature search including Medline and Google Scholar to elucidate the link between DMT and the propensity of cutaneous malignancies. Sphingosine-1-phosphate receptor modulators, such as fingolimod and siponimod are associated with a higher risk of basal cell carcinoma (BCC), but not squamous cell carcinoma, or melanoma. The associated physiopathological mechanisms are not fully understood. Alemtuzumab and cladribine show isolated associations with skin cancer. Regarding other DMT, no increased risk has ever been found. Given the evidence currently available, it is of paramount importance to advocate for necessary dermatological assessments that should be individualized to the risk profile of each patient. Nonetheless, additional prospective studies are still needed to establish efficient dermatological follow-up protocols.
Los fármacos modificadores de la esclerosis múltiple (FAME) han supuesto un cambio en el manejo de esta enfermedad. Algunos estudios sugieren un incremento en la incidencia de cáncer cutáneo (CC) asociado a estos medicamentos. Mediante búsquedas bibliográficas en Medline y Google Scholar, hemos realizado una revisión narrativa para esclarecer el riesgo de CC asociado a los FAME. Los moduladores del receptor de la esfingosina 1-fosfato, como el fingolimod y el siponimod, asocian mayor riesgo de carcinoma basocelular (CBC), pero no de carcinoma escamoso cutáneo (CEC) ni de melanoma. Los mecanismos fisiopatogénicos no se comprenden por completo. El alemtuzumab y la cladribina presentan asociaciones aisladas con el CC. En el resto de FAME, no hemos encontrado un incremento del riesgo. Con base en la evidencia disponible, es crucial promover las evaluaciones dermatológicas necesarias adaptadas al perfil de riesgo de cada paciente. No obstante, se requieren estudios prospectivos adicionales para establecer protocolos de seguimiento dermatológico eficientes.
Multiple sclerosis (MS) is an inflammatory, demyelinating, degenerative, and progressive autoimmune disease that affects the central nervous system (CNS). MS is the most common neurological disease in young adults and causes devastating consequences1–5. Since there is no curative treatment for MS, the therapeutic goal is to control inflammatory activity, reduce the frequency of relapses, and slow the progression of the disease and its growing disability4,5. To achieve this goal, it is necessary to use drugs capable of modifying the natural course of the disease. These agents called “disease-modifying therapies” (DMTs) have revolutionized the management of MS3–8. The number of available drugs in this group has grown in recent years with the approval of new sphingosine 1-phosphate receptor (S1PR) modulators and new monoclonal antibodies (Table 1)3–5,8–10.
Disease-modifying therapies (DMTs) approved by the European Medicines Agency (EMA) for the management of multiple sclerosis and their mechanism of action.
| **DMT (Approval year)**1,11,39 | Mechanism of action |
|---|---|
| Dimethyl fumarate (2014) | The mechanism is not entirely elucidated. It is an ester of fumaric acid that promotes the transcription of the nuclear factor Nrf2. This triggers the activation of a cellular defense system against toxic stimuli such as inflammatory states and oxidative stress, both present in MS. Although it is believed no to have an immunosuppressive effect, it can modulate the immune system response both peripherally and centrally. |
| Teriflunomide (2013) | This drug selectively and reversibly inhibits the mitochondrial enzyme dihydroorotate dehydrogenase, inhibiting pyrimidine synthesis. As a result, it blocks the activation and proliferation of lymphocytes. |
| Glatiramer acetate (2004) | The mechanism is not entirely elucidated. Its proposed effects include binding to major histocompatibility complex molecules, inhibiting the activation of T-cells against myelin antigens, and inducing specific suppressor T Helper 2 lymphocytes. |
| Interferons ββ-1a (1997)β-1b (1995)Pegylated β-1a (2014) | Mechanism not fully understood. Among its proposed effects are: control of pro-inflammatory and anti-inflammatory cytokine secretion, suppression of T-cell activation, induction of neural stem cell differentiation into oligodendrocytes, and prevention of activated immune cell migration to peripheral blood. |
| Cladribine (2017) | A synthetic purine analog that is cytotoxic to lymphocytes, monocytes, and hematopoietic cells. |
| Mitoxantrone (1996) | Topoisomerase II inhibitor. Disrupts DNA synthesis and repair. |
| Fingolimod (2011)Siponimod (2020)Ozanimod (2020)Ponesimod (2021) | Sphingosine 1-phosphate receptor modulators. The molecules are similar to sphingosine 1-phosphate and compete to occupy the receptor. They act as functional antagonists. This effect prevents lymphocyte migration from the lymph nodes to the central nervous system. |
| Alemtuzumab (2013) | Humanized anti-CD52 monoclonal antibody. Causes a decrease in circulating T and B lymphocyte counts. |
| Ocrelizumab | Humanized anti-CD20 monoclonal antibody. Has an immunomodulatory effect by reducing the number and function of CD20 lymphocytes. |
| Ofatumumab (2021) | |
| Natalizumab (2006) | Humanized monoclonal antibody that selectively inhibits the alpha-4-beta-1 subunit of human integrins. This mechanism prevents the migration of inflammatory cells, mainly monocytes, out of the bloodstream. |
DMT, disease-modifying therapy; MS, multiple sclerosis.
Currently, first-line DMTs approved for relapsing forms include interferon β-1a, β-1b, pegylated β-1a, glatiramer acetate, and dimethyl fumarate. Second-line therapies include immunosuppressants such as alemtuzumab, ocrelizumab, natalizumab, cladribine, and S1PR modulators such as fingolimod as the main representatives4,5.
S1PR modulators are oral drugs used in relapsing-remitting MS (RRMS). Their main function is to prevent lymphocyte migration from the lymph nodes to the CNS. Consequently, lymphocytes—capable of causing lymphopenia—remain confined to the lymph node10–12. In 2010, the use of fingolimod, the first drug in this group, was approved by the U.S. Food and Drug Administration (FDA). In 2011, the European Medicines Agency (EMA) authorized its use in Europe. Since then, new drugs from this same group have gained approval10. S1PRs are distributed across multiple tissues, which explains the diversity of adverse effects associated with these drugs (Tables 2 and 3)13. These receptors are expressed in keratinocytes, with S1PR5 being the most relevant receptor subtype14–16. In animal models, the importance of the sphingosine 1-phosphate pathway in dermatoses such as psoriasis or atopic dermatitis has been demonstrated17. As a matter of fact, there is a clinical trial (CT) underway to evaluate the efficacy of oral ponesimod in the management of plaque psoriasis18. On the other hand, it has been hypothesized that S1PR modulators-induced lymphopenia could increase the risk of skin cancer (SC) by hindering the identification of malignant cells11,19. However, there are probably other mechanisms involved that could explain the theoretical increased risk of SC associated with these drugs12,19. The increased risk of SC does not seem to depend on the receptor subtype they act upon but rather is a class effect of S1PR modulators20–28.
Pharmacological target of sphingosine 1-phosphate receptor modulators approved by the FDA and the EMA and their main sites of expression.
| Pharmacological target (S1PR subtype) | Site of expression | Receptor modulator drug |
|---|---|---|
| S1PR1 | B cells, T cells, dendritic cells, cardiac tissue, neurons, endothelium | Fingolimod, Siponimod, Ozanimod, Ponesimod |
| S1PR2 | Endothelium, cardiac tissue, smooth muscle, lung, fibroblasts | No FDA/EMA approved drug |
| S1PR3 | Smooth muscle, endothelium, cardiac tissue, fibroblasts | Fingolimod |
| S1PR4 | T-cells, dendritic cells | Fingolimod |
| S1PR5 | NK cells, endothelial cells, oligodendrocytes, keratinocytes | Fingolimod, Siponimod, Ozanimod |
EMA, European Medicines Agency; FDA, U.S. Food and Drug Administration; NK, natural killers; S1PR, sphingosine 1-phosphate receptor.
Adverse effects associated with sphingosine 1-phosphate receptor modulators.
| Adverse effect* | Incidence rate |
|---|---|
| Very Common (≥1/10) | |
| Headache | 24.5% |
| Asymptomatic elevation of liver enzymes | 15.2% |
| Diarrhea | 12.6% |
| Cough | 12.3% |
| Influenza | 11.4% |
| Sinusitis | 10.9% |
| Back pain | 10% |
| Common (≥1/100 to < 1/10) | |
| Dyspnea | 9.1% |
| Dizziness | 8.8% |
| Bronchitis | 8.2% |
| Hypertension | 8.0% |
| Lymphopenia | 6.8% |
| Migraine | 5.7% |
| Atrioventricular blocks | 4.0% |
| Eczema | 2.7% |
| Pruritus | 2.7% |
| Bradycardia | 2.6% |
| Leukopenia | 2.2% |
| Herpes Zoster | 2.0% |
| Increased triglycerides | 2.0% |
| Asthenia | 1.9% |
| Pityriasis versicolor | 1.8% |
| Basal cell carcinoma | 1.6% |
| Uncommon (≥1/1000 to <1/100) | |
| Pneumonia | 0.9% |
| Melanoma | 0.8% |
| Seizure | 0.9% |
| Thrombocytopenia | 0.3% |
| Macular edema | 0.5% |
| Rare and very rare (< 1/10,000) | |
| Posterior reversible encephalopathy syndrome (PRES) | Unknown |
| Lymphomas (mainly non-Hodgkin) | Unknown |
| Progressive multifocal leukoencephalopathy (PML) | Unknown |
| Cryptococcal infections | Unknown |
The incidence rates reported are for fingolimod, based on results from the FREEDOMS and FREEDOMS II clinical trials.
Source: European Medicines Agency13.
Alemtuzumab is a humanized anti-CD52 monoclonal antibody that depletes circulating B and T lymphocytes. It has been approved by the FDA and the EMA for the management of RRMS9,29. Mild nasopharyngeal infections and headaches, and skin disorders such as rash, urticaria, and pruritus are among its most common adverse reactions9,27.
Cladribine is a purine analog that is cytotoxic to lymphocytes and, to a lesser extent, to monocytes and hematopoietic cells9. In 2013, the EMA rejected its approval on suspicion of increased malignant neoplasms9. In 2015, Pakpoor et al. published a meta-analysis of 11 CTs in which no increased neoplastic risk was reported compared to other DMTs30. In 2017, the EMA approved the drug. Rashes, pruritus, alopecia, nummular eczema, and cases of mucositis are some of its skin-related adverse effects31–33. Additionally, cladribine use has been associated with an increased risk of more severe herpetic infections32.
MS does not seem to be associated with an increased risk of neoplasms. However, since DMTs act directly on the immune system and are generally long-term therapies, whether these drugs are associated with a higher risk of cancer has been a major concern9,34. The main DMTs we should take into consideration regarding the risk of SC are S1PR modulators, which are particularly associated with basal cell carcinoma (BCC)10,20–24,27,28,35–37. This risk does not seem to be present in other DMTs9,10,38–41. However, there is controversy surrounding the use of alemtuzumab and cladribine10,29,42–46. Our objective is to review the existing evidence on the risk of DMT-related SC and provide recommendations on the dermatological follow-up of these patients.
Materials and methodsWe conducted a narrative literature review in July 2023. The search was conducted across Medline and Google Scholar databases using the terms “multiple sclerosis,” “disease-modifying therapies,” “fingolimod,” “siponimod,” “ozanimod,” “ponesimod,” “sphingosine 1-phosphate receptor modulators,” “natalizumab,” “ocrelizumab,” “teriflunomide,” “alemtuzumab,” “cladribine,” “skin,” “cutaneous cancer,” “basal cell carcinoma,” “squamous cell carcinoma,” “melanoma,” “clinical trial,” “post-authorization safety study,” “pharmacovigilance study,” and “meta-analysis.” We included studies written in Spanish and English. We selected phase 3 CTs with a follow-up of 1 or more years, post-authorization studies (PAS) and pharmacovigilance studies (PVS), meta-analyses, systematic and narrative reviews, and cohort studies. We excluded studies with ≤ 3 patients and those that did not clearly specify their methodology. In the included studies with sufficient data, we estimated the odds ratio (OR) for the development of SC. The 3 authors (MBC, MMP, DMC) conducted the search and selected the articles.
ResultsSphingosine 1-phosphate receptor modulatorsWe found 1 PVS, 1 PAS, 1 meta-analysis, and 9 CTs. The latter (Table 4) included a total of 10,071 patients treated with S1PR modulators, and 126 cases of SC reported during therapy (cumulative incidence of 1.24%)20–28. After calculating the OR based on the results published by the different CTs, only the INFORMS study by Lublin et al.23 obtained a statistically significant OR, suggesting that fingolimod is a risk factor for SC (OR, 3.18; [95% confidence interval [CI], 1.47-6.84]). In absolute numbers, the SC cases described in the CTs were mostly BCC, though there were also reports of melanoma and cutaneous squamous cell carcinoma (cSCC). SC was one of the most frequent serious adverse events in these CTs that led to therapeutic discontinuation in some cases. The LONGTERMS study (n=4083) by Cohen et al. followed patients on fingolimod for up to 14 years and found no clear trend in the increase in the annual incidence (AI) of BCC with the cumulative dose of the drug. In the first year, they detected 5 cases (AI, 0.1%); in the fifth year, 5 more (AI, 0.3%); and on the tenth year, 5 more (AI, 0.3%). No new cases were reported on years 12+ or 13+24.
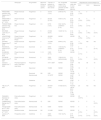
Included studies in the review and relation between drug-No./Subtype of reported skin cancer (basal cell carcinoma [BCC], squamous cell carcinoma [SCC], or melanoma).¶
| Study type | Drug studied | Maximum follow-up duration (years) | Total No. of patients on therapy with skin cancer | No. of reported cases in the control group (placebo or other therapies)* | Calculated odds ratio for skin cancer [95%CI] | Reported skin cancer subtype (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| BCC | SCC | Melanoma | |||||||
| FREEDOMS extension trial., Kappos et al.20, 2014 | Phase III clinical trial | Fingolimod | 4 | 10/773 (1.3%) | 2/300 (0.7%) | 1.95 [0.42-8.97] | 10 (1.3%) | 0 | 0 |
| FREEDOMS II Calabresi et al.21, 2014 | Phase III clinical trial | Fingolimod | 3 | 20/728 (2.74%) | 4/335 (1.2%) | 2.39 [0.79-6.89] | 16 (2.2%) | 4 (0.5%) | 0 |
| TRANSFORMS extension trial. Cohen et al.22, 2016 | Phase III clinical trial | Fingolimod | 4.5 | 8/772 (1.0%) | 3/341 (0.8%) (interferon β-1a) | 1.18 [0.31-4.47] | 7 (0.9%) | 1 (0.1%) | 0 |
| INFORMS Lublin et al.23, 2016 | Phase III clinical trial | Fingolimod | 5 | 21/336 (6.3%) | 10/487 (2.1%) | 3.18 [1.47-6.84] | 14 (4.2%) | 6 (1.8%) | 1 (0.3%) |
| LONGTERMS Cohen et al.24, 2019 | Phase III clinical trial extension Phase | Fingolimod | 14 | 45/4083 (1.1%) | NS/ND | NS/ND | 36 (0.9%) | 9 (0.2%) | 0 |
| EXPAND Kappos et al.25, 2018 | Phase III clinical trial | Siponimod | 3 | 14/1099 (1.3%) | 8/546 (1.5%) | 0.87 [0.36-2.08] | 11 (1.0%) | NS | NS |
| RADIANCE Cohen et al.26, 2019 | Phase III clinical trial | Ozanimod | 2 | 3/872 (0.3%) | 1/440 (0.2%) (interferon β-1a) | 1.17 [0.12-11.29] | 2 (0.1%) | 0 | 1 (0.1%) |
| SUNBEAM Comi et al.27, 2019 | Phase III clinical trial | Ozanimod | 1-1.5 | 1/843 (0.1%) | 0/412 | NS/ND | 1 (0.1%) | 0 | 0 |
| OPTIMUM Kappos et al.28, 2021 | Phase III clinical trial | Ponesimod | 2 | 3/565 (0.5%) | 1/566 (0.2%) (teriflunomide) | 3.01 [0.31-29.08] | 2 (0.4%) | 0 | 1 (0.2%) |
| Vasileios-Periklis et al.10, 2022 | Pharmacovigilance study, Case-Non-case study | Fingolimod | 197/11,855† | NS/ND | 4.54ψ [3.86-5.32] | 92 | 48 | 70 | |
| Siponimod | 15/288 | NS/ND | 9.68ψ [5.48-15.79] | 13 | 2 | 1 | |||
| Ozanimod | NS | 0/58 | NS/ND | NS/ND | 0 | 0 | 0 | ||
| Alemtuzumab | 31/1678† | NS/ND | 4.40ψ [2.98–6.25] | 15 | 5 | 12 | |||
| Cladribine | 5/351 | NS/ND | 3.28ψ [1.17–7.13] | 2 | 1 | 2 | |||
| ωWu et al.36, 2021 | Meta-analysis | Fingolimod | 2 | 20/1557 (1.3%) | 4/1528 (0.3%) | Relative risk for BCC: 4.40 [1.58-12.24] | 20 | NS | NS |
| PANGAEA Ziemssen et al.37, 2022 | Post-authorization study | Fingolimod | 5 | 25/4067 | NS/ND | NS/ND | 25 (0.61%) | 0 | 0 |
| CAMMS03409 Steingo et al.42, 2020 | Post-authorization study | Alemtuzumab | 12 | 2/60 | NS/ND | NS/ND | 0 | 0 | 2 (3.3%) |
| Theodorsdottir et al.29, 2021 | Post-authorization study | Alemtuzumab | 10 | 0/209 | NS/ND | NS/ND | 0 | 0 | 0 |
| Guarnera et al.43, 2017 | Narrative review | Alemtuzumab | NS | 10/1486 | NS/ND | NS/ND | 6 (0.4%) | 0 | 4 (0.3%) |
| Leist et al.45, 2020 | Prospective cohort | Cladribine | 8 | 4/923 (0.4%) | 1/641 (0.2%) | 2.79 [0.31-24.98] | 1 (0.1%) | 1 (0.1%) | 2 (0.2%) |
CI, confidence interval; SCC, squamous cell carcinoma; NS/ND, not specified or not determined due to absence of a control group.
Studies specifying the subtype and absolute No. of reported skin cancer cases are included. Data from patients who completed the study are collected.
The number of BCC cases is indicated in parentheses if they occurred while on therapies other than the studied drug or placebo.
The total No. of patients with skin cancer is lower than the sum of detected cancer subtypes. This is likely because the study included patients with more than 1 skin cancer.
In the PVS conducted by Vasileios-Periklis et al., all cases of BCC, cSCC, and melanoma were collected from a sample that included all adverse events associated with DMTs for the management of MS from 2004 through 2020 by the FDA Adverse Event Reporting System10. They obtained a total of 203,196 reported adverse events. A total of 944 of these cases were due to SC. The drugs siponimod and fingolimod were associated with a higher risk for developing SC (including BCC, cSCC, and melanoma): siponimod had an OR of 9.68 (5.48-15.7) and fingolimod an OR of 4.54 (3.86-5.32). Similarly, a meta-analysis of 11 CTs (n=7184, 3085 of which were used to assess SC risk) reported a relative risk of BCC in patients on fingolimod of 4.40 (1.58-12.24) and no significant relative risk for melanoma36. However, this meta-analysis only considered fingolimod and no other drugs in the same family, did not include cSCC, and the CT with the longest follow-up period was 2 years.
The PANGAEA study evaluated the safety and efficacy of fingolimod in the routine clinical practice in Germany after 5 years on therapy37. They found a total of 25 cases of BCC in 4067 treated patients, obtaining an AI per person of 0.002 (0.001-0.003), which would correspond to approximately 200 cases/100,000 inhabitants/year. This incidence is higher than the expected BCC incidence in the German population according to one of the models published by Rudolph et al. (76.3 cases/100,000 inhabitants/year) in 201047 and to that reported in Spain in the systematic review by Tejera et al. in 2016 (113.05 cases/100,000 inhabitants/year)48. Additionally, patients starting on these drugs tend to be young. The mean age at the start of the medication in the PANGAEA study was 40 years old. At that age, the expected incidence of BCC is lower: Bielsa et al. reported that the incidence of BCC among the male population of Barcelona, Spain, between 40 and 45 years was 37.12 cases/100,000 inhabitants (63.65 for the female population)49.
AlemtuzumabWe found 1 extension phase of a CT, 1 PAS of the drug, 1 PVS, and 1 narrative review. The extension phase of the CAMMS223 CT included a total of 60 patients and 2 cases of melanoma detected after a 12-year follow-up, with no other cases of SC being reported42. In the PVS by Vasileios-Periklis et al., alemtuzumab had an OR of 4.40 (2.98-6.25) for the risk of SC (including BCC, cSCC, and melanoma)10. Despite this finding, other PASs of alemtuzumab have not found a significant number of SC cases29. The narrative review by Guarnera et al. included 3 CTs of alemtuzumab and PAS on the development of 4 melanomas in a total of 1486 patients (Table 4)43. The retrospective cohort study by Puttarajappa et al. evaluated the risk of growing malignant neoplasms—including melanoma—with induction treatment in the context of kidney transplantation. After a mean follow-up of 4 years, alemtuzumab was not associated with a higher risk of cancer. However, we should mention that cases of non-melanoma SC were excluded from this study50.
CladribineWe found 1 meta-analysis, 1 PAS, 1 narrative review, and 1 prospective cohort. In the meta-analysis by Pakpoor et al., a total of 11 phase 3 CTs were considered. The study included a total of 11,400 patients on various DMTs such as cladribine, dimethyl fumarate, fingolimod, teriflunomide, natalizumab, alemtuzumab, interferon B, or placebo, and concluded that there was no higher neoplastic risk with cladribine compared to the risk observed during the CTs of other DMTs30. The same conclusion was drawn in the narrative review by Rammohan et al.44. The cohort published by Leist et al. included a total of 923 subjects (3754 patient-years) on cladribine vs a control group of 641 (2275 patient-years)45. This study found a higher absolute number of SC cases in the treatment group (n=4: 1 case of BCC, 1 of cSCC, and 2 of melanoma) compared to placebo (1 case of BCC). However, it was concluded that cladribine did not specifically increase the risk of any neoplasm subtype (OR, 2.79; [95%CI, 0.31-24.98]). In the PVS by Vasileios-Periklis et al. (n=203,196, 944 of whom had SC), an OR of 3.28 (1.17-7.13) was found for the risk of SC (including BCC, cSCC, and melanoma) (Table 4)10. Conversely, in vitro studies have shown that cladribine does not facilitate the progression of normal or malignant melanocytic cells but even has an anti-invasive and anti-migratory effect46.
Other DMTsWe found 4 narrative reviews, 1 therapeutic positioning report, and 1 PVS. None of the studies revealed an increase in the expected number of SC cases for the following drugs: dimethyl fumarate, teriflunomide, glatiramer acetate, interferons β (β-1a, β-1b, pegylated β-1a), mitoxantrone, ocrelizumab, ofatumumab, or natalizumab9,10,38–41.
DiscussionMS is a disease with extremely high morbidity and mortality rates and, until recently, with limited therapeutic options2,3,8. MS patients tend to be young, which highlights the importance of establishing a safe and effective therapeutic plan1. The present review found that S1PR modulators—particularly fingolimod and siponimod—may be associated with an increased risk of SC, especially BCC. This increase could be up to 4 to 9 times higher, though it varies considerably depending on the studies evaluated (Table 4)10,36,37. Conversely, alemtuzumab and cladribine do not seem to be associated with a significant risk10,29,42–46,50. For the remaining DMTs, the development of SC has not been consistently reported9,10,38–41.
Based on the supposed risk of SC, the EMA issued a communication back in 2015 warning about the danger of fingolimod-related BCC, contraindicating its use in the presence of an active neoplasm and recommending a dermatological exam before starting the drug and annually thereafter51. However, we have not found any clinical guidelines on the dermatological follow-up of these subjects, nor prospective studies demonstrating that such follow-up reduces SC-related morbidity and mortality.
The increased risk of SC has already been reported in widely used drugs such as hydrochlorothiazide52, whose chronic use can increase the likelihood of SC, especially cSCC (with up to 4-time higher increases), but also BCC, while, at this point, the association with melanoma remains weak53–55. Other drugs associated with a higher risk of SC include, among many others, calcium channel blockers, angiotensin-converting enzyme inhibitors, TNF inhibitors, Janus kinase (JAK) inhibitors, and classic immunosuppressants55–61. However, in most cases, there is not a single guideline on the regular dermatology follow-up, and the burden on the health care system could make the massive systematic screening and monitoring of these patients not cost-effective. We should also mention that the main studies reviewed on S1PR modulators do not provide any information on the location of the SC, its histological subtype, its progression, or response to treatment. If these drugs were associated with aggressive histological variants or high-risk locations, this would justify the comprehensive screening of these individuals. Dubrall et al. recently published a study on adverse event reporting in Germany on cases of BCC and cSCC related to various drugs, including fingolimod. They did not find any significant differences in the location of cSCC and BCC vs spontaneous occurrences52. We believe that the dermatological follow-up of patients on S1PR modulators such as fingolimod and siponimod should be individualized and based on each subject's individual risk profile, considering, among other, factors such as age, personal and family history of SC, actinic damage, and the presence of other drugs that could increase the risk. Additionally, the role of the general practitioner in monitoring these individuals, especially those at lower risk, should be taken into consideration. Individualized monitoring strategies are recommended for solid organ transplant recipients, who may have up to a 200-fold increased risk of developing SC, particularly cSCC, which tends to be more aggressive and has a higher metastasis rate62–65. These patients are currently recommended to undergo screening and follow-up based on their risk profile. Risk stratification scales such as the Skin and UV Neoplasia Transplant Risk Assessment Calculator (SUNTRAC®)65,66, which includes factors such as age, race, age at transplant, history of SC before transplant, and transplant type, have recently been validated for the European population66.
LimitationsThis study has the limitations of being a narrative rather than a systematic review. Additionally, the follow-up period of most studies is relatively short (<5 years), which limits the assessment of long-term risk of SC, especially for neoplasms with long latency periods.
ConclusionsMS is associated with high morbidity and mortality rates, predominantly affecting young people. More drugs are being approved to fight this disease. For S1PR modulators such as fingolimod and siponimod—associated with an increased risk of SC—it is essential for dermatologists and general practitioners to be well-informed on these agents and conduct necessary dermatological evaluations individualized to meet the risk profile of each patient. However, this review did not find any clinical guidelines or prospective studies demonstrating that the maintained long-term dermatological follow-up of individuals on DMTs has a positive impact on morbidity and mortality. Finally, conducting a meta-analysis/systematic review to evaluate the risk of DMT-related SC would be advisable.
FundingNone declared.
Conflicts of interestNone declared.