Alopecia areata is a common autoimmune disease that considerably affects quality of life and very frequently leads to depression and anxiety. Standard therapy includes corticosteroids administered via several routes, immunosuppressants, and contact immunotherapy. The response to therapy is very variable. Pathophysiology involves CD8+ T lymphocytes, interferon γ, interleukin 15, and the Janus kinase (JAK) signaling pathway. The JAK family comprises tyrosine kinase proteins that mediate transmission of signals and modulate gene expression via their intracellular signaling ligand, signal transducer and activator of transcription.1
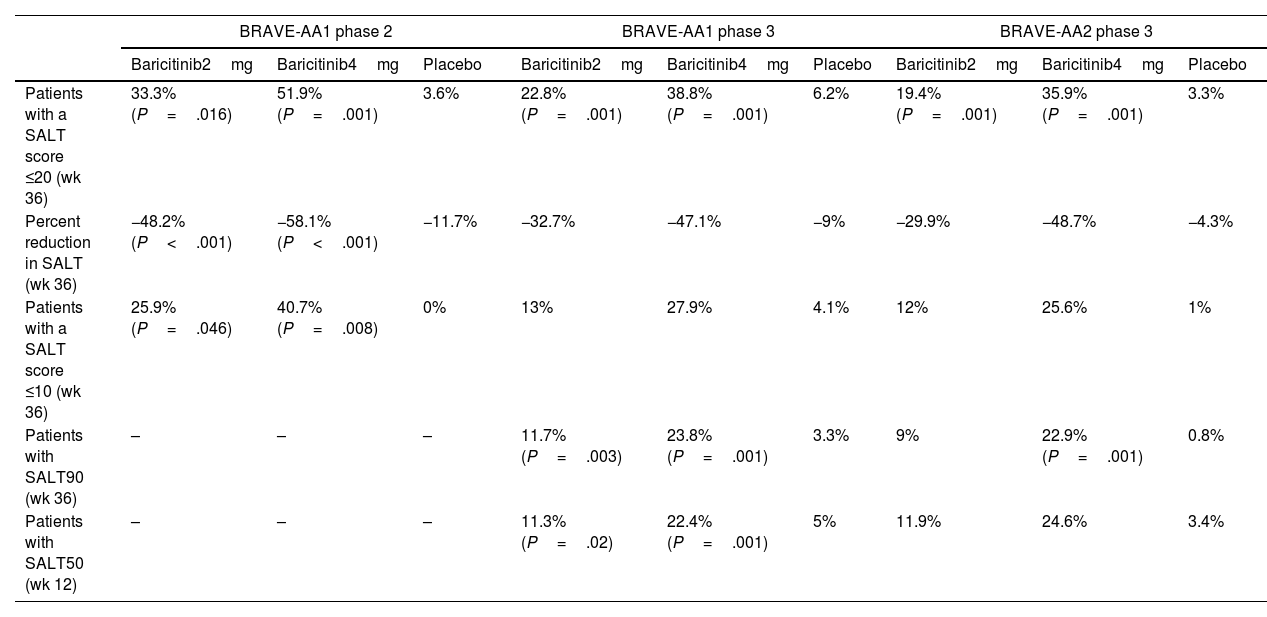
The United States Food and Drug Administration and the European Committee for Medicinal Products for Human Use recently approved a new oral drug, the reversible JAK1 and JAK2 inhibitor baricitinib, for severe alopecia areata. These decisions are supported by the results of 2 phase 3 randomized clinical trials, BRAVE-AA1 and BRAVE-AA2, which recruited 1200 patients with severe alopecia areata, defined as a score of ≥50 on the Severity of Alopecia Tool (SALT). The authors evaluated the percentage of persons who reached SALT ≤20 at 36 weeks. The score was reached by 38.8% and 35.9% of the study population with baricitinib 4mg, 22.8% and 19.4% with baricitinib 2mg, and 6.2% and 3.3% with placebo (BRAVE-AA1 and BRAVE-AA2, respectively). A complete or almost complete response (SALT90) was reached with baricitinib 4mg by 22.9%–23.8% of patients. Adverse events led to discontinuation of baricitinib 2mg in only 3 and 4 patients and of baricitinib 4mg in 5 and 6 patients (BRAVE-AA1 and BRAVE-AA2, respectively). Depending on the dose and series, the events included acne (4.7%–5.8%), increased serum creatine kinase (1.6%–5.7%) and low-density lipoproteins (20.5%–30.3%), urinary infections (1.1%–7.7%), and cytopenia.2 Safety and efficacy outcomes were similar to those of the phase 2 randomized clinical trial BRAVE-AA1 (Table 1). No long-term safety data for alopecia areata are available, although a study on rheumatoid arthritis (median follow-up, 4.6 years) showed a relative risk for severe infection and herpes zoster infection of 2.6 and 3.0, respectively, with no greater incidence of cancer or severe cardiovascular events.4
Comparison of Efficacy Results From Randomized Clinical Trials of Baricitinib in Alopecia Areata.2,3
| BRAVE-AA1 phase 2 | BRAVE-AA1 phase 3 | BRAVE-AA2 phase 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baricitinib2mg | Baricitinib4mg | Placebo | Baricitinib2mg | Baricitinib4mg | Placebo | Baricitinib2mg | Baricitinib4mg | Placebo | |
| Patients with a SALT score ≤20 (wk 36) | 33.3%(P=.016) | 51.9%(P=.001) | 3.6% | 22.8%(P=.001) | 38.8%(P=.001) | 6.2% | 19.4%(P=.001) | 35.9%(P=.001) | 3.3% |
| Percent reduction in SALT (wk 36) | −48.2%(P<.001) | −58.1%(P<.001) | −11.7% | −32.7% | −47.1% | −9% | −29.9% | −48.7% | −4.3% |
| Patients with a SALT score ≤10 (wk 36) | 25.9%(P=.046) | 40.7%(P=.008) | 0% | 13% | 27.9% | 4.1% | 12% | 25.6% | 1% |
| Patients with SALT90 (wk 36) | – | – | – | 11.7%(P=.003) | 23.8%(P=.001) | 3.3% | 9% | 22.9%(P=.001) | 0.8% |
| Patients with SALT50 (wk 12) | – | – | – | 11.3%(P=.02) | 22.4%(P=.001) | 5% | 11.9% | 24.6% | 3.4% |
Abbreviation: SALT, Severity of Alopecia Tool. The SALT score is a measure of alopecia affecting the scalp based on the percentage of hair loss evaluated in different areas separately. In the calculation, the percentage of hair loss is multiplied by 0.4 for the vertex, by 0.24 for the posterior area, and by 0.18 on the right and left profiles. The SALT score is obtained from the sum of the 4 resulting figures.
Other JAK inhibitors, such as tofacitinib and ruxolitinib, are currently being evaluated. While no head-to-head comparisons with other oral JAK inhibitors are available, one meta-analysis did not reveal significant differences in response to treatment, reporting a similar percentage of clinical response with tofacitinib (77.3% [34/44]) and ruxolitinib (82.3% [14/17]).5 Topical administration has a better safety profile but is less efficacious (response of 77.8% with oral treatment vs. 46.4% with topical treatment). A considerable loss of efficacy was observed a few weeks or months after discontinuation for all the JAK inhibitors studied in alopecia areata.5
The approval of baricitinib adds an effective and well-tolerated therapeutic option for patients with severe alopecia areata. However, some areas remain unclear, such as the long-term effects (infections, cancer, and thrombosis, events with other JAK inhibitors in other indications), the minimum effective maintenance dose and its impact on quality of life, and school and work absenteeism. A therapeutic algorithm including baricitinib should be designed for alopecia areata. In addition, the high price of the drug means that cost-effectiveness studies should also be performed.