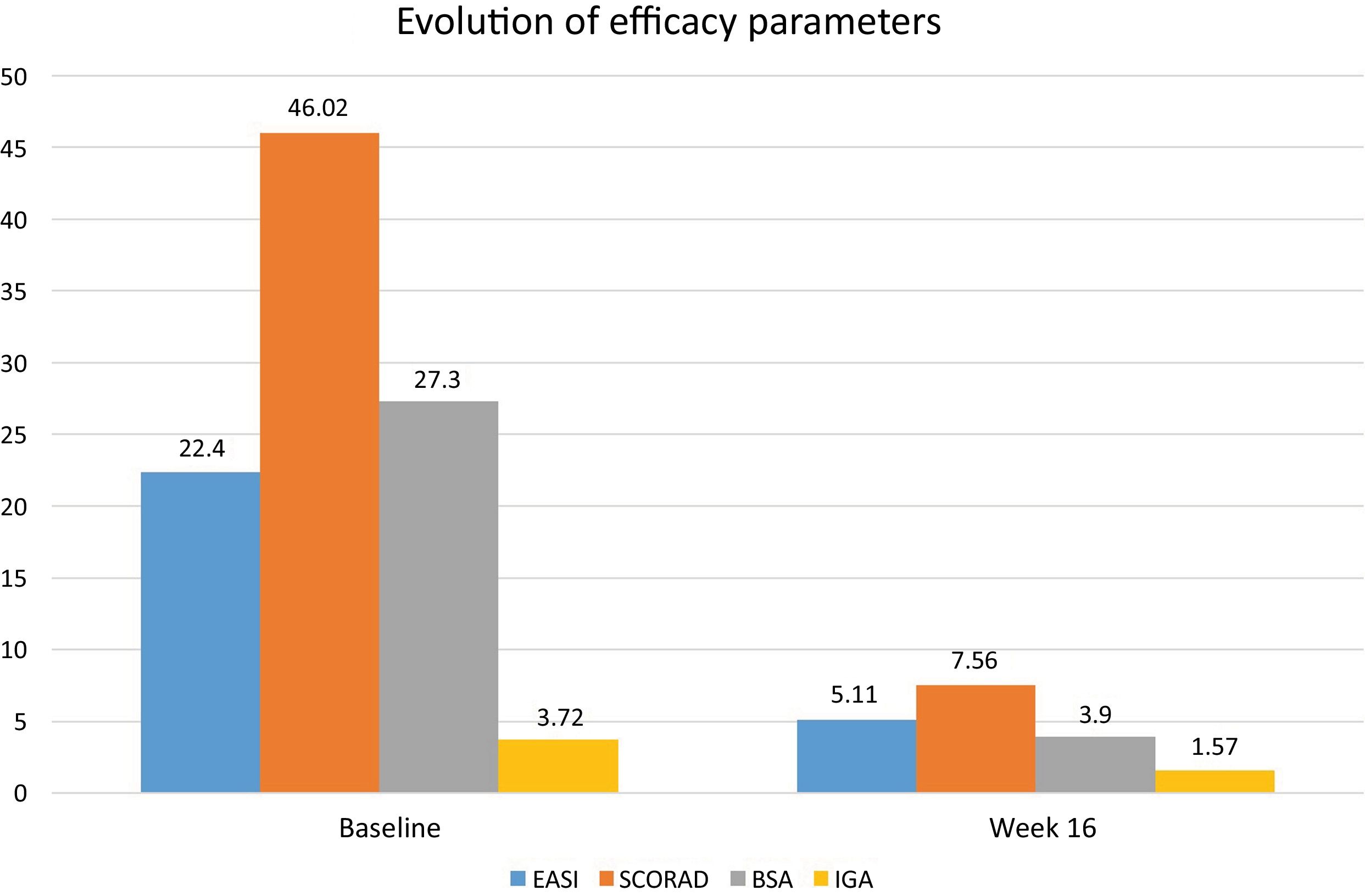
After the publication of the results from the BREEZE clinical trials on baricitinib, this drug was approved for the treatment of moderate-to-severe atopic dermatitis (AD). We describe a series of 13 patients diagnosed with moderate-to-severe AD and treated with baricitinib 4mg/day with a 16-week follow-up. The study was approved by the hospital Ethics Committee (DER-HUSC-2022-009). Statistical analysis was performed using GraphPad software v.9.2 (San Diego, CA, United States). Demographic data are shown in Table 1. A total of 3 permanent discontinuations were reported due to a lack of therapeutic response (no changes in Eczema Area and Severity Index [EASI] vs baseline) 8 weeks after starting baricitinib. The mean baseline EASI score was 22.4 (±2.4) and 5.1 (±6.6) at 16 weeks (with a mean EASI reduction of -17.3); Body Surface Area (BSA) decreased from 27.3 (±15.6) down to 3.9 (±4.1); Scoring Atopic Dermatitis (SCORAD) from 46 (±13.2) down to 7.6 (±9.8); and Investigator Global Assessment (IGA) from 3.7 (±0.4) down to 1.6 (±1.6), all of which were statistically significant results (p<0.001) at 16 weeks (Figure 1). There was an improvement in the baseline mean of the Dermatology Life Quality Index (DLQI) from 17.4 (±2.5) down to 2.6 (±5.5) at 16 weeks, and pruritus was also reduced, with a baseline mean reduction in the Pruritus-Numerical Rating Scale (P-NRS) from 6.8 (±2.3) down to 0.9 (±1.3) at 16 weeks, with a mean reduction of -5.95 at week 16 (p<0.001). Evaluating the 13 patients who received treatment, at week 16, 7 (53.8%) achieved EASI 75, 5 (38.5%) achieved EASI 90, and 3 (23.1%) achieved EASI 100. Three out of the 5 patients who had previously used dupilumab—with a mean treatment duration of 12 months—did not respond to baricitinib.
Basic characteristics of the series. Body mass index (BMI).
| Basic Characteristics of the Series | Values |
|---|---|
| Sex (n, %) | |
| Male | 9 (69.2%) |
| Female | 4 (30.8%) |
| Age (mean, years) (range) | 42 years (20-61) |
| Duration of AD (mean, years) (range) | 26 years (15-55) |
| Previous Treatments (n, %) | |
| Systemic corticosteroids | 13 (100%) |
| Ciclosporin | 12 (90.9%) |
| UVB-NB Phototherapy | 6 (45.4%) |
| Dupilumab | 5 (45.4%) |
| Methotrexate | 9 (72.7%) |
| Azathioprine | 9 (72.7%) |
| IV Immunoglobulin | 2 (18.1%) |
| Mycophenolate mofetil | 3 (27.2%) |
| Baseline Severity Data (mean, SD) | |
| EASI | 22.5±2.4 |
| BSA | 27.3±15.6 |
| DLQI | 17.9±2.5 |
| P-NRS | 7.5±2.0 |
| IGA | 3.7±0.4 |
| SCORAD | 46.0±13.2 |
| Comorbidities | |
| Atopic | |
| Asthma | 6 (45.4%) |
| Rhinitis | 6 (45.4%) |
| Conjunctivitis | 3 (27.2%) |
| Nasal polyposis | 1 (9%) |
| Food allergy | 1 (9%) |
| Non-atopic | |
| Hypertension | 1 (9%) |
| Obesity (BMI>30) | 3 (27.2%) |
BSA: body surface area; AD: atopic dermatitis; SD: standard deviation; DLQI: Dermatology Life Quality Index; EASI: Eczema Area and Severity Index; IGA: Investigator Global Assessment; BMI: body mass index; P-NRS: Pruritus-Numerical Rating Scale; SCORAD: Scoring Atopic Dermatitis; UVB-NB: narrowband ultraviolet B.
The patient who experienced conjunctivitis from dupilumab (EASI prior to drug change was 19) and was subsequently switched to baricitinib showed a complete response at week 16 (EASI 100). Baricitinib-related adverse effects at the 16-week follow-up included 1 episode of perioral herpes simplex virus and 1 acneiform eruption—both mild and transient—resolving completely without sequelae.
Table 2 includes the main articles on the clinical practice experience with baricitinib in AD. From the published series, the authors have observed the following information. Most patients treated with baricitinib were male. The Asian population showed better results in terms of efficacy vs those recorded during clinical trials. Patients naïve to dupilumab showed a greater reduction in EASI vs those who switched from the biologic drug to baricitinib due to a lack of response. Those whose switch was due to the appearance of dupilumab-related conjunctivitis achieved good control of AD with the drug. Baricitinib showed a good therapeutic response in the head and neck pattern. Regarding pruritus, a significant and rapid reduction was observed within the first 4 weeks, both in those in whom baricitinib was used as a first-line therapy and those who had previously been treated with dupilumab. In general, the rate of permanent discontinuations in the published series is relatively high, mostly due to a lack of response. It would be interesting to consider the possibility of combining baricitinib with phototherapy or methotrexate to reduce this situation and increase the EASI reduction within the first few weeks. Patients who respond to baricitinib do so within the first 8 weeks in most cases, maintaining the response at week 16. The safety of Janus kinase (JAK) inhibitors has been a concern since their approval. The reality is that serious adverse effects have a low incidence. However, herpes infections have shown a high incidence rate,1 prompting recommendations for varicella-zoster virus vaccination before starting treatment. Perhaps the most interesting aspect is identifying patients at risk of developing a herpes infection (personal history of herpetic virus infection, AD with Validated Investigator Global Assessment [vIGA] ≥3, periocular herpes, etc.), or herpes zoster (unvaccinated patient, diabetic, older than 50 years, absence of specific immunoglobulin G [IgG], etc.). Prophylaxis with antivirals does not seem to make much sense but educating patients to detect prodromal symptoms of herpetic infection, along with appropriate instructions for early management (initiation of antiviral treatment, temporary suspension of baricitinib as indicated in the package insert, etc.) could be an interesting strategy to avoid major complications. Results of the extension phase of BREEZE-AD32 have shown a recovery of therapeutic response within the first 4 weeks when the dose of 4mg is down titrated to 2mg or to placebo. Although situation could reflect the therapeutic approach in clinical practice, it has not been published nor are there data available from the different published series in this context. None of our patients received the 2mg dose of baricitinib.
Reports on the treatment of atopic dermatitis with baricitinib in real-world clinical practice.
| Author/Year | No. of Patients | Follow-up | Clinical parameters | Previous dupilumab | Key highlights |
|---|---|---|---|---|---|
| Rogner et al.3 (2022) | 12 (11 men; 1 woman) | 12 weeks | EASI 75: 90.1% 65% reduction in pruritus NRS at week 4 | 6 patients | Better EASI 75 results vs clinical trials. Best response in bio-naïve patients or switch due to conjunctivitis. |
| Boesjes et al.4 (2022) | 51 (34 men; 17 women) | 16 weeks | EASI <7 in 19/36 patients vIGA <1 in 13/36 patients | 38 patients | 22 discontinuations (17 due to lack of efficacy). Therapeutic plateau in responders at week 8. Good response if switching from dupilumab to baricitinib due to conjunctivitis. Lower mean reduction in pruritus. |
| Vanlerberghe et al.5 (2023) | 34 (not available) | 12 weeks | vIGA 0-1 (or -2): 41.2% Mean pruritus NRS reduction: -2 (-3 to 0) | Not specified (78.8% out of the 100 patients) | Better vIGA results vs clinical trials. Lower mean pruritus reduction vs other series. Discontinuations almost equal to upadacitinib 15 mg. |
| Uchiyama et al.6 (2022) | 14 (12 men; 2 women) | 12 weeks | EASI 75: 64.2% EASI 90: 35.7% | 0 patients | Better efficacy results vs clinical trials. Asian population. Good response in head and neck pattern. |
| Hagino et al.7 (2023) | 36 (28 men; 8 women) | 12 weeks | EASI 75 head and neck: 27.8% EASI 75 upper extremity: 41.7% EASI 75 lower extremity: 66.7% EASI 75 trunk: 30.6% | 3 patients | Lower extremities showed better response vs other locations. |
| Vittrup et al.8 (2023) | 44 (31 men; 13 women) | 16 weeks | Mean EASI decrease of 5.4 [2.6-9.6] at week 16 EASI 75: 33% at week 16 | 17 patients | Lower baseline EASI in the group previously on dupilumab vs the naïve group. Statistically significant results regarding pruritus reduction. |
EASI: Eczema Area and Severity Index; NRS: Numerical Rating Scale; vIGA: Validated Investigator Global Assessment.
Based on our experience along with the analysis of published clinical practice series, it is suggested that the response of AD to baricitinib treatment is observed within the first weeks in those who respond to it. Additionally, patients with significant pruritus, lesions with a head and neck pattern, of Asian ethnicity, or those who developed dupilumab-related ocular involvement may respond adequately to the drug.
FundingNone declared.
Conflicts of interestNone declared.
We wish to thank Dr. José Carlos Armario Hita and Dr. Pilar Font for their contribution to the present work by providing patients and conducting statistical analysis.