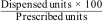Patient-reported outcomes (PROs) are outcomes evaluated by patients based on their perception of their disease and treatment.
ObjectivesDetermine antipsoriatic treatment-related adherence, quality of life (QoL) and satisfaction.
Materials and methodsWe conducted an observational cross-sectional, prospective, and single-center study in which PROs surveys were conducted on adherence (Morisky–Green [MG] test), treatment satisfaction (Spanish Questionnaire of Treatment Satisfaction in Psoriasis [CESTEP]) and QoL (Skindex-29 and DLQI). Additional variables include: PASI, BSA. Statistical analysis: Jamovi®2.3.26.
ResultsA total of 100 surveys were conducted. Based on the MG questionnaire, we found that 75% (75/100) of patients were adherent vs 94% (94/100) from the dispensation records. Regarding CESTEP, a mean score of 7.4±7.7 (close to maximum satisfaction 0) was obtained, while DLQI yielded a score of 2.6±4.6 (indicating a small effect on QoL), and SKINDEX-29 a score of 14.6±15.4 (68% indicating mild (<5) or very mild (6–17) impact according to Nijsten et al.).
Based on CESTEP a p.Rho Spearman value of 0.338 (p=0.004) was obtained in relation to PASI when the study was conducted with a BSA of 0.255 (p=0.050), DLQI results of 0.508 (p<0.001) and Skindex-29 results of 0.397(p<0.001). At the time of the study, the correlation matrix between DLQI result and PASI was 0.365 (p=0.002) with a BSA of 0.347 (p=0.007). Skindex-29 results with PASI were 0.380 (p=0.001) and with BSA, 0.295 (p=0.022).
ConclusionsPatients on therapy exhibit a good QoL, high adherence and satisfaction with their treatment. A significant correlation was seen among satisfaction, QoL, and PASI-BSA at the time of the study.
Resultados comunicados por el paciente (PRO) hace referencia al resultado evaluado por el paciente y basado en la percepción de su enfermedad y tratamiento.
ObjetivosDeterminar la adherencia, calidad de vida (CV) y satisfacción con el tratamiento antipsoriásico.
Materiales y métodosEstudio observacional-transversal, prospectivo y unicéntrico en el que se realizaron encuestas de PRO sobre adherencia (test de Morisky-Green [MG]), satisfacción con el tratamiento (Cuestionario Español de Satisfacción de Tratamiento en Psoriasis [CESTEP]) y CV (Skindex-29 e índice de CV en Dermatología [DLQI]). Otras variables: Psoriasis Area and Severity Index (PASI), Body-Surface Area (BSA). Análisis estadístico: Jamovi®2.3.26 (The Jamovi Project 2024).
ResultadosSe realizaron 100 encuestas. Del cuestionario MG se obtuvo que el 75% (75/100) eran pacientes adherentes y del registro de dispensaciones el 94% (94/100), del CESTEP se obtuvo una media de 7,4±7,7 (resultado cercano a la máxima satisfacción 0), del DLQI de 2,6±4,6 (pequeño efecto en la CV) y de SKINDEX-29 14,6±15,4 (68% afectación leve (<5) o muy leve (6-17) según interpretación de Nijsten et al.).
De CESTEP se obtuvo un valor de p.Rho Spearman en relación con el PASI en el momento del estudio de 0,338 (p=0,004), con el BSA del 0,255 (p=0,050), con el resultado del DLQI de 0,508 (p<0,001) y del Skindex-29 0,397 (p<0,001). De la matriz de correlación entre el resultado del DLQI y el PASI en el momento del estudio de 0,365 (p=0,002) y de BSA de 0,347 (p=0,007). El resultado de Skindex-29 con PASI fue de 0,380 (p=0,001) y con BSA de 0,295 (p=0,022).
ConclusionesLos pacientes tratados presentan una buena CV, una alta adherencia y satisfacción al tratamiento. Existe una correlación significativa entre satisfacción, CV y PASI-BSA en el momento del estudio.
In recent years, the concept of Patient-Reported Outcomes (PRO) has been defined, a term that refers to the outcomes evaluated by the patient and based on their perception of their disease and treatment. It includes symptom assessment, health-related quality of life (HRQoL), health status, adherence, and satisfaction with treatment.
Specifically, the term HRQoL refers to the outcomes perceived by the patient regarding the impact on their well-being and the way they perform in their daily lives.1 Psoriasis likely has a greater negative influence on quality of life (QoL) than most skin diseases. Multiple studies conclude that psoriatic patients, HRQoL even shows degrees of impairment comparable to those found in patients with ischemic heart disease, diabetes, asthma, or epilepsy.2
To assess the severity of psoriasis and the effectiveness of treatment, clinical measures are often used, such as the Psoriasis Area and Severity Index (PASI), and Body Surface Area (BSA), or Physician Global Assessment (PGA). However, since these do not fully capture the patient's perspective regarding the impact of symptoms on daily life,3 it is necessary to incorporate PRO.4
HRQoL is used to study the evaluation and prognosis of chronic diseases. In recent years, generic and specific questionnaires for different populations have been developed. However, studies using specific questionnaires in psoriasis are scarce.5
The prevalence of HRQoL in Spain is 2%.6 It is a chronic inflammatory and relapsing disease that presents challenges for therapeutic management,7 potentially leading to physical, psychological, or social disability.
The most widely used scale to assess its severity is the intensity and severity index: PASI.8 The main objective, due to the greater efficacy and potency of the new targeted biological therapies (TBT), is to achieve PASI 90,9 meaning that patients reach a 90% improvement in their PASI score.
On the other hand, the available data from studies evaluating treatment adherence are concerning. A doctoral thesis presented in 2013 showed an adherence rate of 18%, being higher for systemic treatments (30%) and lower for topical treatments (12%).10 In principle, new treatments with TBT seem to improve expectations in terms of effectiveness, tolerability, and adherence. However, the available literature with real-world data is still limited and presents contradictory results.
Thus, the main objective of this study is to determine adherence, satisfaction, and QoL of patients undergoing treatment with anti-psoriatic drugs.
MethodsStudy designWe conducted an observational, cross-sectional, prospective, and unicentric study in which PRO surveys were conducted regarding adherence, treatment satisfaction, and QoL.
Study population and sampleIn October 2022, a total of 147 psoriatic patients visited the Pharmacy Service of Hospital Universitario Miguel Servet (Zaragoza, Spain) to collect their drugs. All of them were offered to participate in the study. Participants were selected consecutively at the time of dispensing.
All patients who signed the informed consent form and completed the surveys were included in the study.
Study variablesThe outcome variables of the study were:
- -
Adherence variable: To determine current adherence there is no method of choice. The most widely used questionnaire is the Morisky–Green test (MG)11 (Annex 1), consisting of 4 questions. In addition, adherence was recorded using Equation #12:
Equation #1: Calculation of adherence according to the dispensations over the last 3 months:
- -
Treatment satisfaction variable: This was determined using the Spanish Treatment Satisfaction Questionnaire in Psoriasis (CESTEP) (Appendix A, Annex 2). This 12-item questionnaire is a useful, simple, and reliable instrument for evaluating the impact of treatment,13 where 0 is the maximum possible value of satisfaction and 48 is the maximum possible value of dissatisfaction. After the 12 items, patients must rate their overall satisfaction with treatment from 0 (worst possible score) to 100 (best possible score).
- -
QoL variable: The specific dermatology questionnaires Skindex-29 and Dermatology Life Quality Index (DLQI) were used.
The Skindex-29 questionnaire (Annex 3) consists of 29 items. The score for each dimension is obtained by transforming the sum of the responses into a linear scale of 100, from 0 (absence) to 100 (maximum impact on HRQoL). According to Nijsten et al.,14 the results obtained are interpreted as very mild (<5), mild (6–17), moderate (18–36), and severe impairment (>37).
The DLQI (Appendix A, Annex 4) consists of 10 questions. The score for each question is summed. The results obtained are interpreted as: no effect on QoL (0–1), small effect (2–5), moderate (6–10), very large (11–20), and extremely large (21–30).
From the electronic health records, the following variables were collected: sex, age, diagnosis, time since diagnosis, baseline PASI/BSA value, and number of previous disease-modifying treatments.
Statistical analysisQualitative variables were analyzed by calculating absolute frequencies and percentages. For quantitative variables, the mean with standard deviation and median with range were determined. The non-parametric Fisher test, Spearman's Rho, and Mann–Whitney U test were used. p values<0.05 were considered statistically significant. The analysis was performed using Jamovi® 2.3.26.
Ethical aspectsThe study was approved by the Research Ethics Committee of Aragón (EPA22/047).
ResultsAlthough a total of 108 informed consents were collected, only 100 surveys were conducted (n=100) (final participation rate, 68% (100/147)). The demographic characteristics, treatments, type, and severity are shown in Table A.1 (supplementary data).
The MG questionnaire revealed that 75% of respondents (75/100) were adherent patients. However, the mean adherence obtained from the dispensing records of Farmatools® was 94%. The results from the CESTEP, DLQI, and SKINDEX-29 surveys are shown in Table A.2 (supplementary data).
Breaking down the results based on the value obtained in the DLQI questionnaire, it was found that 59% of patients reported no effect (0–1); 25%, a small effect (2–5); 12%, a moderate effect (6–10); 2%, a very large effect (11–20); and 2%, an extremely large effect on QoL (21–30).
On the other hand, the interpretation by Nijsten et al. of the SKINDEX-29 questionnaire revealed that 34% of respondents had very mild impairment (<5); 34%, mild impairment (6–17); 23%, moderate impairment (18–36); and 9%, severe impairment (>37).
Statistically significant differences were found when comparing adherence from the MG questionnaire with the route of administration, the dispensing records obtained for subcutaneous treatment with regimens longer and shorter than 14 days, and when establishing the correlation matrix between the PASI at the time of the study and the CESTEP results (Table A.3, see supplementary data), meaning that patients on subcutaneous treatment with a >14-day regimen are more adherent, and more satisfied with therapy.
Table A.4 (supplementary data) shows the adherence obtained from the MG questionnaire. The Farmatools® software program is presented based on the therapy received and categorized by the mechanism of action (p=0.034 vs p=0.346; Kappa index<0.20). When comparing the satisfaction results from CESTEP and the overall rating provided by the patient in the survey with the PASI and BSA at the time of the study, and the QoL results from the DLQI and SKINDEX-29 surveys (Table A.5, supplementary data), statistically significant results were obtained. Lower PASI, lower BSA, and higher QoL correlate with greater satisfaction with therapy.
On the other hand, when analyzing the results obtained from CESTEP and the overall rating provided, p values=0.550 and 0.539 were obtained, respectively. When analyzed based on the mechanism of action, p values=0.441 and p=0.938 were obtained, respectively.
Regarding the QoL variable, the results obtained are shown in Table A.6 (supplementary data). Lower PASI and BSA correspond to greater QoL.
Finally, when analyzing the results obtained from the DLQI based on treatment, a p=0.634 was obtained (p=0.262 for the SKINDEX-29). When analyzed based on the mechanism of action, p values=0.884 and 0.569 were obtained, respectively
DiscussionIn the present study, patients on subcutaneous treatment with >14-day regimens were more adherent. Additionally, greater satisfaction with treatment was associated with higher adherence, lower PASI, lower BSA, and greater QoL. It is noteworthy that a greater QoL was obtained with oral rather than subcutaneous therapy; however, the standard deviation of these means is large.
The median age obtained is slightly higher vs other studies (56 vs 42–47 years).15,16 A slight predominance of the male sex was observed (1.1 men/woman ratio), when the usual ratio is 1.7–2 men/woman.16
Regarding the severity measured by the PASI scale, 23.7% had severe psoriasis before starting the current treatment; and 47.5%, moderate psoriasis. This result varies from those reported by Hernánz et al.,16 where approximately 76.2% of the patients had moderate disease.16 However, as in other studies, the most common diagnosis in our population was plaque psoriasis (79%).17
Patient distribution across different treatments is a data point that often does not align across publications. In this study, 41% of patients were on anti-TNF treatment; 82.5% of these on adalimumab. The percentage of patients on etanercept (7%) is notable since this result is not consistent with other articles where TBT are among the most prescribed ones.16,18 The publication date of the studies should be considered, as some TBT are of recent commercialization.
Regarding adherence, the most appropriate method for calculating it has not been established yet, complicating result comparison.19 The MG questionnaire indicated an adherence rate of 75%, which falls within the upper margin of the range observed in other studies (40% up to 77%).20 However, the adherence value obtained from the dispensing program was 94%. According to the kappa index, the agreement between results is weak (0.01–0.20).
The highest percentage of non-adherent patients was found in the PDE-4 inhibitor group. Statistically significant differences were seen between adherence obtained with oral treatment and with subcutaneous treatments according to the MG survey. This is likely due to the oral regimen of apremilast vs other subcutaneous treatments with more convenient dosing schedules.
On the other hand, according to the dispensing registry adherence, we found that patients with a SC>14-day regimen had greater adherence vs those with shorter regimens. However, no statistically significant differences were found when analyzing the “ 30-day regimen”. Currently, there are numerous studies showing a direct relationship between greater treatment complexity and non-adherence. Simplification is an effective strategy that translates to greater adherence.21
Similarly, statistically significant results were obtained when relating the PASI at the time of the study with the results obtained from the dispensing registry and the PASI-BSA with those obtained from the CESTEP, DLQI, and SKINDEX-29 surveys, as well as when analyzing the results obtained from the different surveys among themselves (CESTEP-DLQI, SKINDEX-29-DLQI results). These associations undoubtedly help to support the internal validity of our study. On the other hand, lower PASI values are obtained when patients are more adherent, and lower PASI-BSA values correspond to greater satisfaction among patients with their therapy and higher QoL (considering the results obtained from both questionnaires). This finding differs from the study conducted by Guavita et al., which reported a weak correlation between DLQI and PASI results, suggesting that visible signs and symptoms do not reflect the patient's real perspective.22
Moreover, satisfaction with treatment is higher with greater adherence recorded in the dispensations. This finding is consistent with the conclusions on rheumatoid arthritis—treated with TBT—drawn by Ahijón, where it was specified that the overall satisfaction with the drugs was “greater among adherent patients”.23
When analyzing CESTEP, a median score of 7.41 [0–48] was obtained, which a result close to the maximum satisfaction value. These values do not match the preliminary study published by Delgado et al., which states that more than 70% of their patients are dissatisfied with treatment. Additionally, it claims that, according to other authors, there is often no association among disease impact, perception of well-being, and estimated severity measured by PASI.24 In contrast, this study found statistically significant results. Lower PASI corresponds to greater satisfaction.
The occurrence of adverse effects and the awareness of more convenient regimens influence the satisfaction obtained. Patients on PDE-4 inhibitors reported lower satisfaction.25
The DLQI questionnaire found that treated psoriasis does not excessively impact QoL (1 [0–14]). The median score from the SKINDEX-29 was 13 [0–71]. The results from Hernandez et al.26 indicate that treated psoriasis has a moderate impact on QoL; however, in the present study, the interpretation by Nijsten et al.14 suggests that treated psoriasis has a mild impact. Additionally, it is noteworthy that the mean QoL obtained with subcutaneous treatment is approximately half of that obtained through oral treatment, although the value of the standard deviation is high.
Finally, it is important to consider the limitation of sample selection, as it was not estimated to guarantee that the number was representative of the population of psoriatic patients who are dispensed drugs at our hospital. Another limitation is the small number of patients in some treatment groups, meaning that the population was not homogeneous regarding the drug in treatment, with 54% of patients being on anti-TNF (41%) or PDE-4 inhibitors (13%).
ConclusionsPsoriasis is a disease that can lead to significant changes in habits and QoL, potentially resulting in physical, psychological, or social disability. Our study demonstrates that psoriatic patients have high adherence to systemic therapy dispensed at the hospital, with greater adherence observed in drugs administered>14 days and lower adherence in PDE-4 inhibitors.
The importance of using more than 1 method to determine adherence and the lack of utility for comparing oral and subcutaneous treatments with such disparate frequencies is confirmed. Patient satisfaction with their therapy is high, and it increases with greater adherence recorded in the dispensations. The patients’ QoL is not significantly affected by their psoriasis in a high percentage of treated patients.
A significant correlation exists between satisfaction, QoL, and PASI-BSA at the time of the study, and adherence and the dispensing registry, satisfaction, and PASI.
FundingNone declared.
Conflicts of interestNone declared.