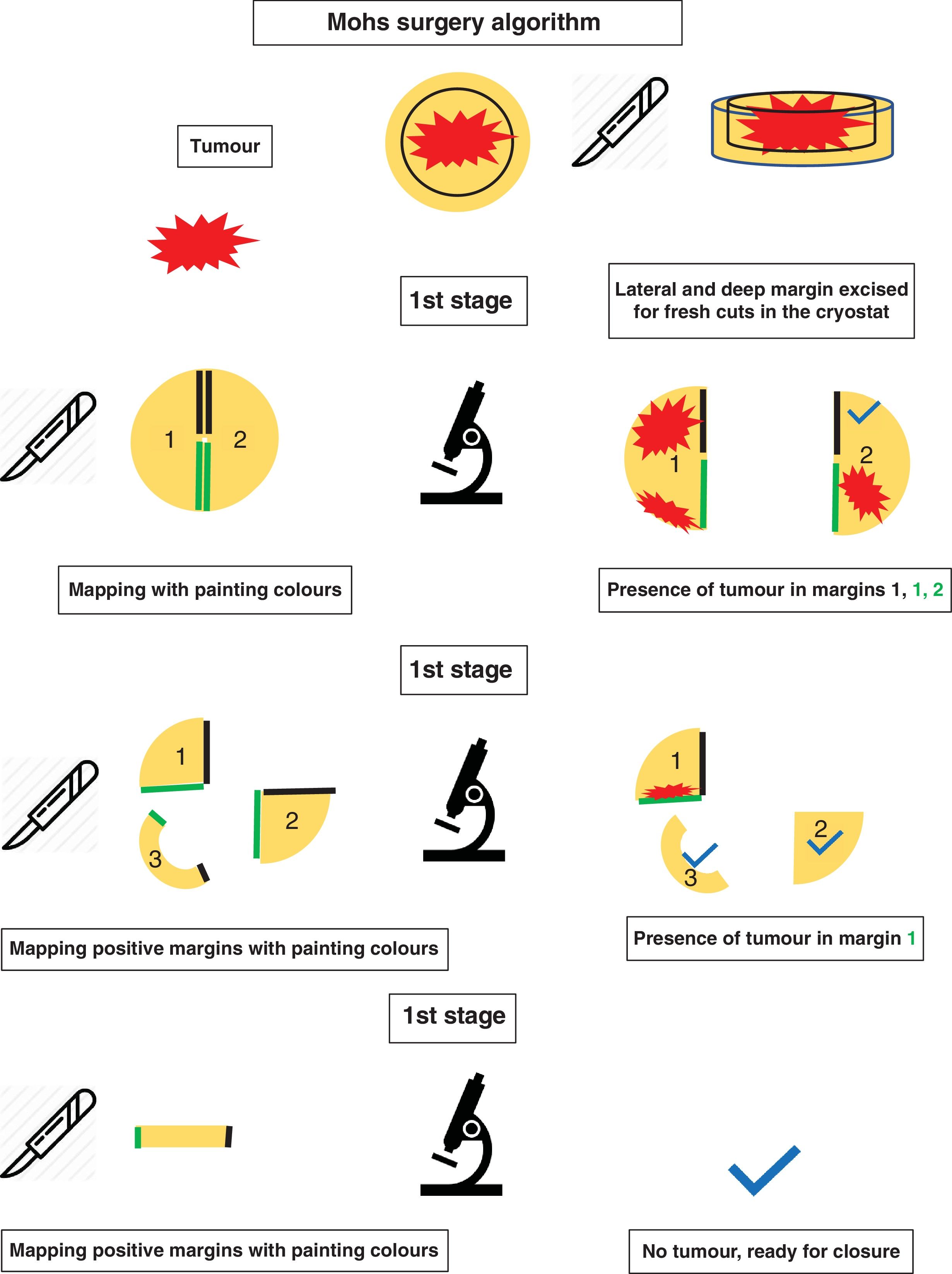
Let us take a look at the state of Mohs micrographic surgery (MMS), almost 100 years after it was first described. It is a technique used mostly in the treatment of non-melanoma skin cancers, which was first conceived by Frederick Mohs (United States) back in the 1930s. At that time, he noticed that the injection of 20% zinc chloride solution caused tissue necrosis, which could be fixed for microscopic observation. From the chemical fixation on animal skin (chemosurgery), it moved to human skin and then to fresh fixation by cryo-freezing in a cryostat. Chemosurgery then gave way to micrographic surgery, which would later be called Mohs micrographic surgery (1970s). Other surgeons contributed to this path, such as Tromovich and Perry Robins. Finally, the European Society for Micrographic Surgery was founded in Portugal in 1992 with the presence of Dr. António Picoto, among other European dermatologists.1 What is the advantage of this technique that combines dermatological surgery and dermatopathology? There are several advantages. Basal cell carcinoma (BCC) grows three-dimensionally through the so-called “silent extensions” that are not palpable or visible to the naked eye and, therefore, go unnoticed by the surgeon. Even in conventional surgery, it will always be a “blind margin.” A total of 5% of small and well-demarcated BCCs extend>4mm of the apparent clinical margin. The histological subtypes micronodular, morpheaform, infiltrative, and basosquamous have a worse prognosis due to their invasive capacity and destruction, which makes them especially aggressive, same as perivascular and perineural invasion. We are mainly referring to tumors in the so-called high-risk areas located in the periorificial areas of the face (>6mm) or in the intermediate risk areas of the head and neck (>1cm). Mohs surgery allows us to control this lateral and deep margin because, unlike conventional paraffin cuts, cryofreezing cuts are horizontal and inverted, placed in the same plane. They are immediate because fresh fixation is a rapid procedure. This is followed by mounting on a slide and staining with haematoxylin–eosin (toluidine blue can be used; it stains cells blue and stroma magenta). This allows re-excising positive margins on the same surgical act (Fig. 1). Innovative for its time? Yes. In addition to basal cell carcinoma other tumors have joined the indications: spindle cell carcinoma (SCC), Merkel carcinoma, dermatofibrosarcoma protuberans for example,2 which has not been the case with malignant melanoma whose atypical melanocytes are difficult to identify fresh.3 In lentigo maligna melanoma—a tumor with undefined clinical margins—it is preferable to use conventional Mohs variants that have emerged like the spaghetti technique: phased excision of the margin for vertical paraffin cuts with prior mapping by the surgeon. Delayed Mohs surgery is one of these variants in which the only similarity to conventional Mohs surgery is to make margin mapping, but not a horizontal margin. It happens, however, that some do not have the latest Mohs technique available due to lack of equipment, technical expertise, or time. Of note that if the margin is positive, the patient will have to return days or weeks later to undergo a new blind excision. What changed in 2023 in dermatology that led us to reconsider a technique that serves, in summary, to completely excise a skin cancer in a single surgical act? Is this infallible and a recurrence-free technique? It is not. There is presence of basal cell carcinomas, especially in the morphemic and infiltrative groups that recur or are impossible to excise due to anatomical limitations with the exclusive approach of the dermatologist or too long surgical times with local anaesthesia.1,4–7 In recent years, therapeutic options have emerged, both topical and systemic such as PDT, imiquimod8 (immunological therapy), and vismodegib (which inhibits the Hedgehog pathway, one of the signalling pathways involved in the formation of basal cell carcinoma). They have shown surprisingly good results as neoadjuvant therapies, not only reducing the size of the tumors but achieving a prolonged cure (although more follow-up studies are needed). Another relevant question is if new non-invasive dermatologic imaging modalities can aid in vivo clinical observation and histological analysis to abbreviate the need for unpredictable number of fresh histological sections (Mohs stages). The point is: can we delimit the excision margin in advance to ensure complete tumour removal? Is tumour persistence at the margins the only variable involved in recurrence? As far as we know, it is not, but it is one of them. Let us list the new imaging modalities available: dermoscopy,8,9 multiphoton tomography, confocal microscopy,10 optical coherence microscopy, intravital microscopy, 2-photon microscopy, fluorescence microscopy associated with digital dermoscopy called polarized and fluorescence light dermoscopy, reflectance spectroscopy, high-frequency ultrasound, Raman spectroscopy or liquid biopsy.11 Although all these are referred to in the dermatological literature, they are far from being widely used due to their high cost and slow learning curve associated with these new technologies. They are cited as useful techniques to reduce the number of Mohs stages, not as a substitute of such technique. In addition, the application of artificial intelligence (AI) to pathology image analysis has shown promising results in the accurate and rapid identification of surgical margins making Mohs surgery even more accurate and efficient. Another potential application of AI in Mohs surgery is through robotic assistance. Robotic systems could potentially help surgeons perform more precise and controlled excisions, reducing the risk of damaging healthy tissue and improving overall surgical outcomes.12 However, of note that AI is still in the early stages of development in the field of Mohs surgery. This means that old MMS remains the gold standard for the treatment of skin tumors with indefinite margins mainly because cutaneous histology has not yet been replaced as the most reliable diagnostic technique in this kind of tumors. No other technique or AI has yet done it. Until it happens, let us continue to use our classic Mohs surgery with a traditional microscope whenever possible.
Authors’ contributionsAll authors contributed to the writing and revision of the article.
Ethical approvalNot applicable.
FundingNo funding received.
Conflict of interestNo conflict of interest.
Availability of data and materialsNot applicable.