Despite representing only 4% of skin cancers, cutaneous melanoma (CM) accounts for more than 80% of skin cancer-related deaths.1 Prognosis is impacted by Breslow thickness (BT), which determines the T category in the AJCC TNM classification.2 CM can be detected during a routine skin examination.3 However, many of them are still diagnosed with a high BT.
This study aims to compare BT based on the individual who initially detects the CM (patient, relative, general practitioner (GP), dermatologist, or other medical specialists). Associations between detection groups and clinical, epidemiological, and histological features were analyzed as well.
We conducted a cross-sectional multicenter study in Galicia (Spain) predominantly including a white population. CMs diagnosed from 2021 through 2022 were included. Data were drawn from the Galician Melanoma Registry, including demographic, clinical, histological, and genetic variables. Evaluations were conducted by specially trained dermatologists using a detailed questionnaire (Appendix A). The study was approved by the Pontevedra-Vigo-Ourense ethics committee with Code No. 2023/023.
Data analysis was performed using SPSS software (29.0.2.0 version). P values=0.05 were considered statistically significant (Supplementary data).
A total of 928 CMs were reported from 2021 through 2022, with their characteristics being shown in Table 1. The individual detecting the melanoma was recorded in 685 cases: most CMs were detected by the patient (255; 37.2%), followed by the dermatologist (232; 33.9%), relatives (114; 16.6%), the patient's GP (63; 9.2%), and other medical specialists (21; 3.1%).
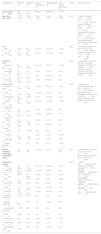
Characteristics of patients and melanomas in the entire study group (n=928).
| Characteristics | ResultsN (%) |
|---|---|
| No. of patients | 928 (100.0) |
| Age, mean (SD), years | 66.2 (±16.5) |
| Sex | |
| Male | 372 (40.1) |
| Female | 556 (59.9) |
| Education level | |
| Primary or less | 293 (49.5) |
| Secondary | 80 (13.5) |
| PE | 70 (11.8) |
| University | 149 (25.2) |
| Total | 592 (100) |
| LV* | 336 |
| Anatomic site | |
| Face and neck | 179 (21) |
| Scalp | 13 (1.5) |
| Anterior trunk | 76 (8.9) |
| Posterior trunk | 236 (27.6) |
| Upper limbs | |
| Right | 57 (6.7) |
| Left | 76 (8.9) |
| Lower limbs | |
| Right | 67 (7.8) |
| Left | 105 (12.3) |
| Acral | |
| Palmar | 3 (0.4) |
| Plantar | 23 (2.7) |
| Finger nails | 14 (1.6) |
| External genitalia | 2 (0.2) |
| Mucosae | 3 (0.4) |
| Total | 854 (100) |
| LV* | 74 |
| Breslow thickness, mm | |
| Median | 0.9 |
| Percentiles | |
| 25 | 0.4 |
| 50 | 0.9 |
| 75 | 2.5 |
| Total | 626 |
| LV* | 302 |
| Histological type | |
| Infiltrating melanoma | 615 (69) |
| Melanoma in situ | 278 (31) |
| Total | 893 (100) |
| LV* | 35 |
| Histological subtype | |
| SSM | 390 (44.5) |
| SSM in situ | 111 (12.7) |
| NM | 85 (9.7) |
| ALM | 33 (3.8) |
| ALM in situ | 9 (1) |
| LMM | 70 (8) |
| LM in situ | 114 (13) |
| Spitzoid melanoma | 1 (0.1) |
| Nevoid melanoma | 4 (0.5) |
| Desmoplastic melanoma | 2 (0.2) |
| Other | 9 (1) |
| Other in situ | 13 (1.5) |
| Not classified | 13 (1.5) |
| Not classified in situ | 23 (2.6) |
| Total | 877 (100) |
| LV* | 51 |
PE: professional education; SSM: superficial spreading melanoma; NM: nodular melanoma; ALM: acral lentiginous melanoma; LMM: lentigo maligna melanoma; SD: standard deviation.
Major statistical differences were reported among melanoma detection groups: dermatologists identified melanomas with the lowest BT. In the self-detection group patients were younger, with a higher percentage of women, and a higher level of education. The most common location was the lower limbs and the most common subtype was nodular melanoma. Results are shown in Table 2, including a post-hoc analysis.
Analysis of clinical, histologic, and prognostic variables based on the individual who detected the melanoma.
| Characteristics | PatientN (%) | RelativeN (%) | General practitionerN (%) | DermatologistN (%) | Other medical specialistsN (%) | P value | Post-hoc analysis |
|---|---|---|---|---|---|---|---|
| No. of patients (n=685) | 255 (37.2) | 114 (16.6) | 63 (9.2) | 232 (33.9) | 21 (3.1) | ||
| Age, mean (SD), years | 61.74 (16.02) | 72.18 (16.10) | 70.84 (14.75) | 64.93 (17.13) | 69.05 (16.50) | <0.001 | - Patient vs. relative (61.74±16.02 vs. 72.18±16.10; P<0.001).- Patient vs. general practitioner (61.74±16.02 vs. 70.84±14.75; P=0.001).- Dermatologist vs. relative (64.93±17.13 vs. 72.18±16.10; P=0.001).- Breslow thickness increased significantly with patient age (r=0.241). |
| Sex | 0.023 | - Self-detection, women vs. men (41.6% vs. 31%; P=0.023).- Breslow thickness, women vs. men (1.81±2.62 vs. 2.30±3.02; P=0.040). | |||||
| Male (n=281) | 87 (31) | 48 (17.1) | 33 (11.7) | 101 (35.9) | 12 (4.3) | ||
| Female (n=404) | 168 (41.6) | 66 (16.3) | 30 (7.4) | 131 (32.4) | 9 (2.2) | ||
| Education level | <0.001 | - Self-detection, university education vs. primary education or less (44.8% vs. 30.9%; P<0.001).- Relatives, university education vs. primary education or less (5.5% vs. 11.9%; P<0.001). | |||||
| Primary or less (n=285) | 88 (30.9) | 71 (24.9) | 34 (11.9) | 78 (27.4) | 14 (4.9) | ||
| Secondary (n=78) | 25 (32.1) | 14 (17.9) | 3 (3.8) | 32 (41) | 4 (5.1) | ||
| PE (n=67) | 29 (43.3) | 7 (10.4) | 4 (6) | 27 (40.3) | 0 (0) | ||
| University (n=145) | 65 (44.8) | 15 (10.3) | 8 (5.5) | 57 (39.3) | 0 (0) | ||
| Anatomic site | <0.001 | - Posterior trunk, dermatologist vs. patient (34.1% vs. 20.2%; P<0.05).- Posterior trunk, other medical specialists vs. patient (52.6% vs. 20.2%; P<0.05).- Lower limbs, patient vs. relative (17.8% vs. 6.7%; P<0.05). | |||||
| Face and neck (n=112) | 35 (31.2) | 20 (17.8) | 10 (8.9) | 46 (41.1) | 1 (0,1) | ||
| Scalp (n=11) | 3 (27.3) | 2 (18.2) | 3 (27.3) | 2 (18.2) | 0 (0) | ||
| Anterior trunk (n=65) | 32 (49.2) | 6 (9.2) | 5 (7.7) | 20 (30.8) | 2 (3.1) | ||
| Posterior trunk (n=188) | 49 (26.1) | 35 (18.6) | 20 (10.6) | 74 (39.4) | 10 (5.3) | ||
| Upper limbs | |||||||
| Right (n=44) | 15 (34.1) | 8 (18.2) | 2 (4.5) | 19 (43.2) | 0 (0) | ||
| Left (n=59) | 26 (44.1) | 11 (18.6) | 6 (10.2) | 15 (25.4) | 1 (1.7) | ||
| Lower limbs | |||||||
| Right (n=47) | 26 (55.3) | 9 (19.2) | 4 (8.5) | 8 (17) | 0 (0) | ||
| Left (n=85) | 43 (50.6) | 7 (8.2) | 6 (7.1) | 29 (34.1) | 0 (0) | ||
| Acral | |||||||
| Palmar (n=1) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | ||
| Plantar (n=17) | 8 (47.1) | 3 (17.6) | 1 (5.9) | 3 (17.6) | 2 (11.8) | ||
| Finger nails (n=10) | 5 (50) | 1 (10) | 1 (10) | 1 (10) | 2 (20) | ||
| External genitalia (n=1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | ||
| Breslow thickness, mm(median, interquartile range) | 3 (1–8.75) | 5 (3–9.5) | 1 (1–2.5) | 0 (0–1) | 6 (0.5–6.5) | <0.001 | - Dermatologist vs. patient (0 [0–1] vs. 3 [1–8.75]; P=0.001).- Dermatologist vs. relative (0 [0–1] vs. 3 [3–9.50]; P<0.001). |
| Histological subtype | <0.001 | - Patient: NM>Melanoma in situ (LM, MES, unspecified subtype) (P<0.05).- Relatives: NM/LMM>Melanoma in situ (SSM subtype) (P<0.05).- Dermatologist:∘ Melanoma in situ (any histological subtype)>NM/SSM/ALM (P<0.05).∘ LMM/MES>NM (P<0.05).∘ ALM>NM/SSM/LMM (P<0.05). | |||||
| SSM (n=296) | 121 (40.9) | 51 (17.2) | 33 (11.1) | 84 (28.4) | 7 (2.4) | ||
| SSM in situ (n=87) | 28 (32.2) | 4 (4.6) | 10 (11.5) | 45 (51.7) | 0 (0) | ||
| NM (n=59) | 36 (61) | 15 (25.4) | 2 (3.4) | 4 (6.8) | 2 (3.4) | ||
| ALM (n=21) | 7 (33.3) | 4 (19) | 2 (9.5) | 2 (9.5) | 6 (28.6) | ||
| ALM in situ (n=11) | 2 (40) | 2 (40) | 0 (0) | 1 (20) | 0 (0) | ||
| LMM (n=57) | 18 (31.6) | 13 (22.8) | 7 (12.3) | 18 (31.6) | 1 (1.8) | ||
| LM in situ (n=76) | 20 (26.3) | 9 (11.8) | 3 (3.9) | 44 (57.9) | 0 (0) | ||
| Spitzoid melanoma (n=1) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Nevoid melanoma (n=3) | 2 (66.7) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | ||
| Desmoplastic melanoma (n=1) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Other (n=5) | 4 (80) | 1 (20) | 0 (0) | 0 (0) | 0 (0) | ||
| Other in situ (n=11) | 0 (0) | 0 (0) | 1 (9.1) | 9 (81.8) | 1 (9.1) | ||
| Not classified (n=10) | 2 (20) | 4 (40) | 2 (20) | 1 (10) | 1 (10) | ||
| Not classified in situ (n=22) | 4 (18.2) | 2 (9.1) | 0 (0) | 14 (63.6) | 2 (9.1) | ||
PE: professional education; SSM: superficial spreading melanoma; NM: nodular melanoma; ALM: acral lentiginous melanoma; LMM: lentigo maligna melanoma; SD: standard deviation.
Variations in total number of patients in each category are due to missing data.
In our study, 37.2% of melanomas were self-detected, which is consistent with a recent work reporting a 30.4% self-detection rate.4 In contrast, former studies, such as the one conducted Avilés-Izquierdo et al. reported a 53% self-detection rate.5 The lower rate in our study may reflect the older mean age of our sample, underscoring the importance of promoting early detection in this high-risk group.
Dermatologists identified melanomas with the lowest BT, while those detected by patients or relatives were thicker. Former studies reported thinner BT in melanomas identified by dermatologists vs. other professionals,5–7 though most were published 20 years ago. Our study, being more recent, may better represent current dermatological clinical practice.
Patients who self-detected melanoma were younger vs. those identified by a relative or their GP. Additionally, patients whose melanoma was detected by dermatologists were also younger than those identified by GPs. As far as we know, this relationship has not been previously studied. Moreover, older patients tend to exhibit thicker melanomas,5,8 which reinforces the importance of educating this population on self-examination and promoting regular checks by relatives and GPs.
Women self-detected melanoma more frequently than men did, as previously reported.5 Additionally, male sex has been associated with thicker melanomas at diagnosis.5,8,9 These data highlight the need to raise awareness among men about regular self-examination.
College education was associated with increased self-detection, whereas those with lower educational levels relied more on their relatives or GPs. Although this relationship has not been previously studied, it is consistent with findings of thicker BT in patients with lower educational levels.8 This underscores the need for accessible dermatological care across all socioeconomic groups to ensure timely detection and intervention. However, the association between education and self-detection should be interpreted with caution, as younger individuals are usually better educated, which may act as a confounding factor.
Melanomas detected by dermatologists and relatives were more commonly located on the posterior trunk, whereas self-detected melanomas were more common on the lower limbs, which is consistent with former studies.5,9 It has been demonstrated that melanomas in less visible areas tend to have greater BT,5 highlighting the importance of thorough skin examinations by dermatologists and general practitioners, and educating patients on checking less visible areas.
Histologic subtype also influenced detection, with nodular melanomas more likely to be self-diagnosed, while melanoma in situ, lentigo maligna, and superficial spreading melanoma were predominantly identified by dermatologists. This is consistent with former studies,6,8,9 and may be due to the more rapid growth and symptoms of nodular melanoma, which make it easier for patients to detect these.10
The strengths of our study include its multicenter design and prospective data collection. Limitations include its retrospective statistical analysis, reduced precision due to the weighted mean for BT, non-mandatory reporting in the public health registry, and potential data collection challenges during the COVID-19 pandemic.
In conclusion, our study provides novel insights into melanoma detection, revealing that younger patients and those with higher educational levels are more proactive in self-detection, which happen to be findings not previously reported. While dermatologists detect melanomas with the lowest BT, they assess only a small percentage of the population. It is crucial to ensure GPs are trained to identify suspicious lesions and have proper referral pathways to dermatologists. Educational campaigns targeting high-risk groups – such as men, older adults, and individuals with lower educational levels – focusing on promoting regular self-examination can enhance outcomes as an effective strategy for secondary prevention.
Conflict of interestThe authors declare that they have no conflict of interest.
The authors wish to thank all participants and patients included in this study for their collaboration and involvement.
Ángeles Flórez1,2, Hae Jin Suh-Oh1,2, Beatriz Fernández Jorge3, Francisca Piñeyro Molina3, Olalla Figueroa Silva4, Celia Posada García5, Ánder Zulaica Gárate5, Laura Sainz Gaspar5, Mª Luisa Fernández Díaz6, Romina Rodríguez Lojo6, Ignacio Suárez Conde7, Pilar Gómez Centeno7, and Lucía Vilanova-Trillo1,2.
1Dermatology Department, Complexo Hospitalario Universitario de Pontevedra, Pontevedra, Spain
2Grupo de Investigación DIPO, Instituto de Investigación Sanitaria Galicia Sur (IIS Galicia Sur), SERGAS-UVIGO, Pontevedra, Spain
3Dermatology Department, Complexo Hospitalario Universitario de A Coruña, A Coruña, Spain
4Dermatology Department, Complexo Hospitalario Universitario de Ferrol, A Coruña, Spain
5Dermatology Department, Complexo Hospitalario Universitario de Vigo, Pontevedra, Spain
6Dermatology Department, Hospital Universitario Lucus Augusti, Lugo, Spain
7Dermatology Department, Complexo Hospitalario Universitario de Ourense, Ourense, Spain