Lichen planus belongs to the spectrum of interface dermatitis or lichenoid reactions, characterized by a “band-like” inflammatory infiltrate located at the dermo-epidermal junction. The usual clinical pattern of these common entities is the presence of pruritic polygonal papules of various sizes.1
So far, the etiology of lichenoid reactions is not clear, however, the hypothesis of cross-reactivity between exo-antigens and endo-antigens has been suggested, which consequently generates the activation of CD4+ and CD8+ T lymphocytes against keratinocytes of the basement membrane.1,2 There can be multiple triggers, including infections, medications, radiotherapy, dental amalgams, emotional stress, and vaccines such as the hepatitis B vaccine.2
Recently, with the creation and massive dissemination of vaccines for SARS-CoV-2, the appearance of de novo or reactivation of lichenoid-type skin diseases has been reported in literature. Diseases of the spectrum such as lichen planus, lichen planus pigmentosus inversus, lichenoid eruptions, and even lichen planopilaris, have been reported days after the application of the first or second dose of the vaccine.2,3
The vaccines involved so far are those manufactured with RNA technology (Pfizer/Moderna), viral vectors (Oxford-Astrazeneca/Janssen) and inactive viruses (Sinopharm/Sinovac).2,4 It is believed that the pathophysiological mechanism is explained by the increase in cytokines such as IL-2, TNFα and IFNγ, which trigger a TH-1 type response.5 Treatments used in case reports include topical and systemic corticosteroids, JAK inhibitors, metronidazole, acitretin, antimalarials, and antihistamines.2–4
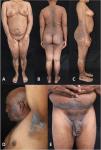
The first case is a 32-year-old female patient with a history of morphea who presented with a generalized skin rash (Fig. 1A–C) 5 days after the application of the first dose of the Vero Cell® (Sinopharm) vaccine. The second is a 71-year-old male patient with a history of arterial hypertension who presented with a skin rash predominantly in folds two weeks after the application of the second dose of the ChAdOx1-S® vaccine (Oxford-Astrazeneca) (Fig. 1D and E). Infectious serologies for both patients were negative.
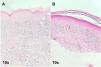
The result of the biopsies of both patients confirmed the diagnosis of pigmented lichen planus (Fig. 2A and B). Both patients were treated with high-potency topical corticosteroids (clobetasol propionate 0.05%) and topical calcineurin inhibitor (tacrolimus 0.1%) once a day with partial clearance of the lesions after 3 months of treatment.
Early recognition as well as diagnosis of the lesions is important in patients with dark phototypes due to the inherent risk of residual pigmentation after an inflammatory process and so that long-term sequelae can be avoided. The inclusion of these cases in the literature is a step forward in the pursuit to eliminate the racial bias that exists when learning dermatology. This would broaden the exposure and access to adequate and timely healthcare in darker phototype patients.
Conflicts of interestThe authors declare that they have no conflicts of interest.