Intradermal melanoma on a pre-existing intradermal nevus is an exceptional finding. Since 1961, a total of 11 cases have been published1–8; none of these were in the Spanish medical literature (PubMed, December 27, 2014).
We report the case of a 42-year-old man who underwent treatment at his health center for a lesion in the center of his forehead. In the clinical history, the lesion was described as a fibroelastic nodule measuring 7mm in diameter. The patient reported that the lesion had been present since childhood and that it had grown progressively during the last year. The patient's personal history contained no record of relevant disease, previous removal of cutaneous lesions, or family history of neoplasm.
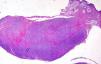
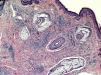
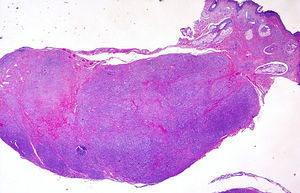
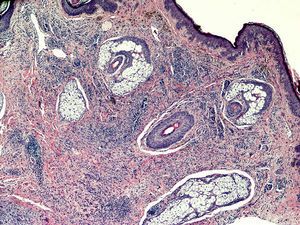
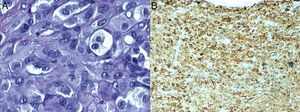
Histopathology revealed a nodular lesion (Fig. 1) composed of an intradermal melanocytic nevus in contact with the surgical margins (Fig. 2) at the center of which was a nodule whose larger diameter measured 5mm. The nodule was composed of atypical melanocytes. Additional histopathologic findings included cell pleomorphism, aberrant nucleoli, absence of maturation, isolated cellular necrosis, and 3 mitotic figures per square millimeter (Fig. 3A). No significant lymphocytic or junctional components were observed. The atypical cells showed intense cytoplasmic expression of S-100 and MelanA (diffuse) and HMB-45 (focal) (Fig. 3B).
Physical examination revealed no further atypical pigmented lesions or enlarged lymph nodes. Extending the margins with a lateral 1-cm excision that reached the fascia only revealed scar fibrosis. Selective biopsy of the sentinel node was performed, and staining of several sections with hematoxylin-eosin, S-100, and HMB-45 revealed absence of tumor cells.
Finally, the extension study ruled out metastasis of primary melanoma from another site. No significant findings were revealed by ultrasound scan (eyes, neck, and abdomen) or positron emission tomography with computed tomography. The results of the laboratory analysis were also unremarkable, with a normal S-100 protein concentration (0.05μg/L). The clinical, laboratory, and histopathology findings confirmed the diagnosis of melanoma developing from an intradermal nevus.
The risk of progression to malignancy in benign melanocytic lesions was recently studied in a meta-analysis,9 which revealed a 2% incidence for melanoma, especially for congenital nevi >40cm located on the trunk.
However, very few epidemiological data are available on intradermal melanoma arising from an intradermal nevus, which constitutes both a diagnostic and a therapeutic challenge. The 11 published cases reveal common characteristics: onset in adulthood, nodular appearance, nodular histopathologic pattern, and variable degrees of deep invasion. In more than half of the cases, melanoma was located in deeper planes of the nevus. In the case we present, the melanoma was situated within the nevus. No intraepidermal or junctional melanocytic activity was detected in any of the above-mentioned patients.
Cytology facilitates diagnosis. Melanoma cells often contain mitotic figures and nuclear atypia. The immunohistochemistry study showed that positivity for S-100 was more intense in atypical melanocytes. In addition, intradermal nevi are usually negative for immunostaining with HMB-45, which is generally positive in junctional nevi and in melanoma. All of the above findings were observed in the case we present.
The differential diagnosis should be with long-standing melanocytic nevus, malignant blue nevus, and primary intradermal melanoma. Long-standing nevus appears as a nodular lesion on the face of patients older than 60 years. It contains small, monomorphic melanocytes exhibiting maturation, together with large solitary melanocytes with a hyperchromatic nucleus but no pleomorphism or mitosis. Occasionally, it is associated with degenerative signs such as thrombosis, hemorrhage, fibrosis, and sclerosis. Malignant blue nevus develops over a pre-existing blue nevus, it is easily distinguished in histopathology, and its prognosis is generally poor. Primary intradermal melanoma contains no nevus cells in histopathology and is a diagnosis of exclusion after primary disease has been ruled out at other levels.10
The histopathologic differential diagnosis includes other nodular melanocytic lesions. Noticeably common is melanocytic nevus with large nests at the junctional and intradermal levels, with no cellular atypia, pleomorphism, mitosis, or necrosis. Intradermal expansive nodules and proliferative nodules (more often the large cell variety) can present in the setting of a conventional melanoma. Congenital nevus contains large but isomorphic undifferentiated melanocytes exhibiting maturation and few or no mitotic figures.
Finally, the prognosis of this disease is unclear, and the few published cases prevent a suitable prognosis from being proposed. Distance metastasis was observed in only 1 case.2 Progression was associated mainly with the intradermal location and the diagnostic delay.
Given that primary intradermal melanoma associated with intradermal nevus is an exceptional finding, we must bear in mind the possibility of metastasis from a melanoma at another anatomic level. The first step is a detailed history, which should include previous excisions and regression of previous lesions. This should be followed by a meticulous physical examination including the mucosa and eyeballs. Finally, additional tests should include a complete blood count, biochemistry with lactate dehydrogenase, sentinel node study, and positron emission tomography with computed tomography or whole-body computed tomography. Histopathology of the lesion removed is also important and should take into account the differential diagnoses mentioned above. These data enable the tumor to be staged and treatment tailored to the individual patient.
We are grateful to Dr. Andrés Sanz Trellez, dermatologist, for his help with the case report.
Please cite this article as: Arjona-Aguilera C, Gil-Jassogne C, Jiménez-Gallo D, Albarrán-Planelles C. Melanoma intradérmico asociado a nevo melanocítico intradérmico. Actas Dermosifiliogr. 2015;106:776–777.