We review novel technologies with diagnostic and therapeutic applications in dermatology. Among the diagnostic techniques that promise to become part of dermatologic practice in the future are optical coherence tomography, multiphoton laser scanning microscopy, Raman spectroscopy, thermography, and 7-T magnetic resonance imaging. Advances in therapy include novel light-based treatments, such as those applying lasers to new targets and in new wavelengths. Devices for home therapy are also appearing. We comment on the therapeutic uses of plasma, ultrasound, radiofrequency energy, total reflection amplification of spontaneous emission of radiation, light stimulation, and transepidermal drug delivery. Finally, we mention some basic developments in nanotechnology with prospects for future application in dermatology.
Presentamos una revisión actualizada en materia de avances en la aplicación de la tecnología al proceso diagnóstico y terapéutico en dermatología. Respecto a las técnicas de diagnóstico, realizamos una breve reseña sobre la tomografía de coherencia óptica, la microscopia láser multifotónica, la espectroscopia, la termografía y la resonancia magnética nuclear de 7tesla, siendo que estas últimas también prometen incorporarse al arsenal diagnóstico en el futuro. En cuanto a los avances en terapéutica, la aplicación de tecnologías basadas en la luz, como el láser, ven ampliar sus aplicaciones con nuevas dianas y longitudes de onda, además de desarrollarse dispositivos de uso casero. Comentamos también el uso de plasma, ultrasonidos, radiofrecuencia, traser, fotoestimulación y administración transepidérmica de fármacos con fines terapéuticos. Finalmente, mencionamos los aspectos básicos de la nanotecnología y su proyección futura en el campo de la dermatología.
The diagnostic process was traditionally based on information acquired through a meticulous medical history and physical examination. Doctors would use their senses to examine patients and obtain objective information about the state of their health. The stethoscope—the first instrument designed to improve the physician's diagnostic abilities—was invented in 1816 by Dr René Laënnec.1 With the development of anatomic pathology in the 19th century, biopsy of diseased organs became a widespread practice. In 1895, the German physicist Wilhelm Röntgen discovered x-rays and their potential in medicine after accidentally taking a radiograph of his wife's hand. By the end of the 19th century, with Marie and Pierre Curie's discovery of radium in 1898, ionizing radiation started to be used in the treatment of various diseases.2 Since then, the application of the latest discoveries in physics, chemistry, and molecular biology has shaped the technological development of Western medicine. In dermatology, the introduction of histopathology improved the diagnostic process considerably. Although the skin is an organ that is easily accessible for visual inspection and for the collection of biopsy specimens, in recent years there has been a growing demand for new real-time in vivo diagnostic techniques that can be coupled with minimally invasive therapeutic processes and which have acceptable toxicity. The role of dermoscopy as such a technique is now well-established, and confocal microscopy is making its way into routine practice. In the future, the diagnosis and treatment of dermatology patients will require a multidisciplinary approach and the application of advances in physics, chemistry, and molecular biology, which will complement and even surpass techniques that are currently very useful, such as pulsed light and photodynamic therapy (PDT). In this review, we discuss diagnostic and therapeutic techniques that are not yet routinely applied in health care practice, as well as devices for home-based cosmetic treatments, a trend that we believe is worth highlighting (Table 1).
Technologies in Development for Diagnosis and Treatment in Dermatology.
| Diagnostic techniques |
| Optical coherence tomography |
| Multiphoton laser scanning microscopy |
| Raman spectroscopy |
| Thermography |
| 7-T magnetic resonance imaging |
| Therapeutic techniques |
| New targets in laser therapy |
| Sebaceous glands |
| Lipids |
| Sweat glands |
| Exogenous pigments and tattoos |
| Bacteria and fungi |
| Immunotherapy |
| Nonmelanoma skin cancer |
| New wavelengths in laser therapy |
| 1565nm |
| 1940nm thulium laser |
| Other energy-administration techniques |
| Plasma |
| Ultrasound |
| Fractional radiofrequency |
| TRASER |
| Light stimulation |
Abbreviation: TRASER, total reflection amplification of spontaneous emission of radiation.
Optical coherence tomography (OCT) is an imaging technique that was first used to examine the retina. OCT measures the intensity of the light reflected by tissue by means of a process called low-coherence interferometry. This technique uses infrared light (1300nm). Unlike reflectance-mode confocal laser scanning microscopy (RCLSM), OCT creates virtual images of vertical cross-sections in the plane defined by the incident beam, and it also has higher imaging depth (up to 2mm). However, OCT has lower lateral resolution (10-15μm) than RCLSM, which prevents the technique from distinguishing cellular structures and cytologic details.3–8
Multiphoton Laser Scanning MicroscopyMultiphoton laser scanning microscopy (MPLSM) is based on the autofluorescent properties of the skin. MPLSM excites the endogenous fluorophores in the dermis and epidermis (melanin, keratin, and nicotinamide adenine dinucleotide phosphate), which are normally excited by wavelengths in the UV spectral range. However, this technique uses a femtosecond pulsed laser (1 fs = 1 × 10−15 s) with near-infrared (IR) light, which is less prone to scattering than visible light. Because the laser emits such a large number of photons in such a short amount of time, 2 photons of infrared light occasionally strike the same fluorophore of the skin practically simultaneously. Behaving as though they had been struck by a single photon of UV light, these fluorophores become excited and emit visible fluorescence, which is detected by the optical system of the microscope.
Like RCLSM, MPLSM creates high-resolution images at the cellular and intracellular level in planes horizontal to the skin surface. In dermatology, most studies involving this technique have been performed ex vivo, but systems for in vivo skin studies are now available on the market.9–11
Raman SpectroscopyRaman spectroscopy (RS) is a method for identifying the chemical composition of a sample using a laser. RS makes it possible to classify skin lesions by molecular composition. This technique has been shown to differentiate malignant skin lesions with a sensitivity of > 90%, although its specificity is lower.12–14 RS provides information about the biochemical composition of the analyzed tissue but offers little information about its structure and anatomic site. Nevertheless, by combining RS with OCT—which provides complementary spatial information—it is possible to conduct a combined biochemical and morphological analysis. Given that neoplastic cells have a spectroscopic signature different from that of healthy tissue, RS could be useful in defining surgical margins in Mohs surgery.15,16
ThermographyThermography provides infrared images of the human body that have been used in medicine for diagnostic purposes. This technique can identify areas of the body that have irregular blood flow (either increased or decreased) because these irregularities translate into changes in temperature. Thermography has been used, for example, in herpes zoster and in the monitoring of hemangiomas.17–19
7-T Magnetic Resonance ImagingMagnetic resonance imaging (MRI) is a noninvasive technique for obtaining high-resolution anatomic images. The image resolution of MRI makes it possible to differentiate the stratum corneum, the epidermis, and the dermis.20 The use of MRI in dermatology remains experimental, although its potential for use has been noted in many publications since the 1990s. However, conventional MRI machines cannot produce magnetic fields greater than 3T; because the cells in the stratum corneum and the dermis have low signal intensity, this limitation translates into poor image resolution.21 Some current research has focused on the use of various contrast methods, such as magnetization transfer contrast (MTC). This method is especially useful for evaluating how the various layers of skin tissue interact with the interstitial fluids.22
Recently, MRI scanners with higher-powered magnets that create ultra-high-field (UHF) magnetic fields (greater than 3T) have been developed. The resolution of these scanners is similar to or greater than that of histologic images. The current limitations of this technique are mainly related to cost and infrastructure, because machines capable of generating such strong magnetic fields must be housed in very large and extremely expensive facilities.
Therapeutic TechniquesLaser Devices and New TargetsFor years, dermatologic laser therapy has targeted 3 classic chromophores—hemoglobin, melanin, and water—that have allowed the removal of hair follicles as well as the successful treatment of acne scars, some signs of skin aging, and various vascular and pigmented lesions. However, it could be possible to add new therapeutic targets and to act on subcellular structures and cytoplasmic organelles in addition to macroscopic structures such as hair follicles and blood vessels.23
Sebaceous GlandsRecent studies have attempted to identify more precisely the most effective wavelengths for the treatment of sebaceous glands, which are directly involved in the pathophysiology of acne. With a free electron laser, which can be tuned to any wavelength on the electromagnetic spectrum, we find absorption peaks in the sebaceous glands at 1210, 1728, 1760, 2306, and 2346nm. With pulses of 100-125ms in the range of 1700nm, a free electron laser can induce selective thermal damage to sebaceous glands.
PDT has also been shown to cause some degree of inhibition of the sebaceous glands, which has a beneficial effect on inflammatory acne lesions. To avoid excessive damage to the keratinocytes, exposure to low-power red light (635nm) or blue light (420nm) has been used successfully during incubation with aminolevulinic acid (ALA), thereby preventing the massive accumulation of protoporphyrinIX. Protoporphyrin IX causes cytotoxic damage to keratinocytes on exposure to high-energy red light. The use of PDT with low-power red or blue light has been called inhibition photodynamic therapy (I-PDT).24
LipidsAmong the absorption peaks for the various cutaneous lipids, the 1210nm wavelength stands out for its ability to cause selective thermal damage to subcutaneous tissue when used in combination with epidermal cooling systems. At this wavelength, a new therapeutic concept called selective photothermostimulation (SPS) occurs. SPS could have the capacity to stimulate the adipocytes and mesenchymal cells in the subcutaneous tissue, making it useful for treating cellulite and even large lipomas. Satisfactory results have also been obtained with fibers placed laterally underneath the skin surface that deliver 1440nm laser pulses to the dermal-hypodermal interface.25
Sweat GlandsVarious wavelengths (Nd:YAG, 924/927nm diode, 800nm diode) have been used to destroy eccrine sweat glands in axillary hyperhidrosis. Radiofrequency and ultrasound systems have also been used to induce thermal damage in these glands. In 2011, the US Food and Drug Administration approved a microwave-based device for the treatment of axillary hyperhidrosis.26–31
Exogenous Pigments and TattoosUltra-short-pulse (picosecond) lasers appear to be superior to nanosecond-pulse lasers in pigment removal, especially for colors—such as blue and green—that are resistant to removal with current systems.32–36 These devices operate on familiar wavelengths. Alexandrite and Nd:YAG lasers are used to remove black, blue, and green pigments, while 532nm Nd:YAG lasers are used to remove red, yellow, and orange pigments.
Bacteria, Fungi, and Other Cutaneous PathogensResearch has shown that exposing Trichophytonrubrum to 42.5°C for 2minutes can inactivate the fungus. However, the conclusions of this in vitro study cannot be extrapolated to the conditions of clinical practice. Q-switched pulsed Nd:YAG lasers and 1320, 870, and 930nm lasers have been used in the treatment of onychomycosis.37–41 Studies of PDT have demonstrated that this technique is useful even in onychomycosis caused by nondermatophyte fungi, which tend to respond very poorly to conventional pharmacotherapies.42,43
Lasers in ImmunotherapyThe efficacy demonstrated in some studies by vascular laser treatment in diseases such as lupus erythematosus, psoriasis, and inflammatory rosacea may be due not only to the effect of the lasers on the vessels but also to the suspected involvement of certain immune modulation mechanisms.44,45
Nonmelanoma Skin CancerRecent studies have demonstrated the efficacy of vascular laser treatment against basal cell carcinoma.46–50 However, it has been suggested that this technique could increase the risk of multifocal growth in recurrent tumors, as occurs in recurrences following cryotherapy.
Laser Devices and New WavelengthsAmong the most recent advances is the discovery of the potential application of “old” wavelengths for the treatment of new targets. For example, long-pulse alexandrite lasers, used primarily in hair removal, can be used in the treatment of vascular lesions.51 The combination of lasers and antiangiogenic agents has also been investigated as a treatment that holds promise in the short term. One example is the use of pulsed dye lasers in combination with topical rapamycin in capillary malformations.
New Wavelengths- •
1565nm. A nonablative fractional laser that emits 1565nm infrared light with greater penetration than 1540 or 1550nm light has been approved, but it is still too soon to know whether it is more effective than previous lasers.
- •
Thulium 1940nm laser. Nonablative fractional 1940nm lasers operate at a new wavelength with a water absorption coefficient that is higher than that found at other nonablative wavelengths (1410-1550nm) but lower than that of ablative lasers.
Plasma—defined as the fourth state of matter—consists of highly ionized gas that transforms into a radiating material. In fact, plasma accounts for 99% of the observable matter in the universe and is found, for example, in stars, the aurora borealis, and lightning.
Plasma can be classified as either cold or hot. Cold plasmas can be used in rejuvenation treatments to induce controlled thermal damage in the skin, which results in new collagen production and the restructuring of the dermal architecture. Cold plasma devices have also been used to improve wound healing, to induce hemostasis, and to treat infectious dermatoses, atopic dermatitis, and other conditions. Today, cold plasmas are mainly used routinely for skin rejuvenation in cosmetic dermatology.52,53
UltrasoundIn addition to its diagnostic applications, ultrasound may have new therapeutic uses. In recent years, ultrasound cavitation has been developed for ultrasonic body contouring54 and the selective destruction of sweat glands as treatment for axillary hyperhidrosis.55,56
Several articles have underscored the utility of ultrasound as a means of enhancing the transcutaneous penetration—and therefore increasing the bioavailability—of various drugs by means of the sonophoresis effect.57–65 Other therapeutic uses of this technique involve focusing the ultrasonic waves at different depths in the skin tissue. In rejuvenation therapies, for example, due to the effect of ultrasonic waves on collagen, this technique is used to heat the dermis and cause dermal tightening.
Radiofrequency EnergyRadiofrequency equipment is less expensive to manufacture—and therefore more accessible—than laser devices. One development in this area is the use of fractional radiofrequency devices that induce microlesions on the skin surface similar to those caused by fractional CO2 lasers. The radiofrequency energy is delivered by a roller tip that is moved across the surface of the skin. Some fractional radiofrequency devices use a system of needles to deliver the energy to deeper tissue planes.66–70
Total Reflection Amplification of Spontaneous Emission of RadiationTotal reflection amplification of spontaneous emission of radiation (TRASER) is a concept introduced by Morgan Gustavsson. In this technique, fluorescent dyes are excited by the sudden, flash-like release of energy, generally from a light source. After absorbing light of a precise wavelength, the fluorescent dye molecules become excited and reemit a narrow band (peak) of fluorescence at a nearby wavelength (Stokes shift phenomenon). This peak of intensity varies depending on the fluorescent dye used. TRASER is neither a laser (it lacks an optical resonator and induces non-coherent, non-collimated light) nor a pulsed light (it has no filters). TRASER is simpler than those systems and therefore less expensive to manufacture. Because it uses cheap, nontoxic, long-lasting dyes, TRASER is a very attractive technology for future development.
Through the use of different dyes, TRASER can be tuned to different wavelengths of pulsed light ranging from UV-A to near-infrared. Experiments have been carried out with various dyes, including sulforhodamine 640, which emits a peak wavelength of 658nm and has been shown to be effective in hair removal, and pyrromethene 556, which emits fluorescence with a peak of 543nm and is useful for vascular treatments. The utility of TRASER in these 2 applications has been investigated in clinical and histologic studies. This technique could have medical, domestic, and industrial uses. It will soon be seen whether TRASER is capable of replacing laser technology in a single device.71
Home DevicesMost of the techniques mentioned in the previous sections remain under development and are not yet applied in routine health care practice. In this section, however, we will briefly review the home devices for the treatment of cosmetic problems that have invaded the market in recent years. Most of these devices target 5 therapeutic areas:
- 1)
Hair removal (Epila, Silkin, Gillette, Philips, Boots, Palomar, Tria Beauty, etc.).
- 2)
Rejuvenation (PaloVia, Solta, Syneron, etc.).
- 3)
Acne (Blue Light, Omnilux, etc.).
- 4)
Hair stimulation.
- 5)
Cellulite treatment.
Although these devices are affordable for the general public, they are much less effective than professional equipment and are therefore no substitute for it. Nevertheless, they can be useful in many cases. The safety profile of these devices is not fully established. Moreover, the safety profile is largely determined by the patient's level of instruction in the use of the device.72–75
Light StimulationLight-emitting diodes (LEDs) are used to stimulate the dermal fibroblasts, which then increase procollagen synthesis. Although this technique has been widely studied in animals, there have been very few controlled clinical trials that provide evidence of its efficacy. However, LED PDT has been proven effective in dermatology and is used in photorejuvenation and the treatment of erythema, scarring, acne, and alopecia. There have been a few recent studies on the use of LED PDT in alopecia, and various systems for LED emission through hat-, helmet-, comb-, and brush-like devices are now commercially available.76–78
Transepidermal DeliveryAblative fractional CO2 laser treatment makes it possible to open the epidermal barrier by forming dermal-epidermal channels, which can be used to deliver biomolecules (antioxidants, hyaluronic acid, and proteins), drugs (retinoids), photosensitizing agents for PDT, and stem cells for regenerative therapies. Ultrasound compression systems have been developed to accelerate and increase the penetration of drugs and biomolecules. These dermal-epidermal channels could also be used for the extraction of intradermal substances.79–81
Tissue SolderingSeveral studies have demonstrated the possibility of soldering tissues with photomechanical soldering techniques that reach temperatures of around 60°C and use light-activated adhesives. Tissue soldering could be improved through nanotechnology, specifically, the design of nanofibers and nanoparticles. This technique has been used in the skin, nervous tissue, and vascular tissue.82–85
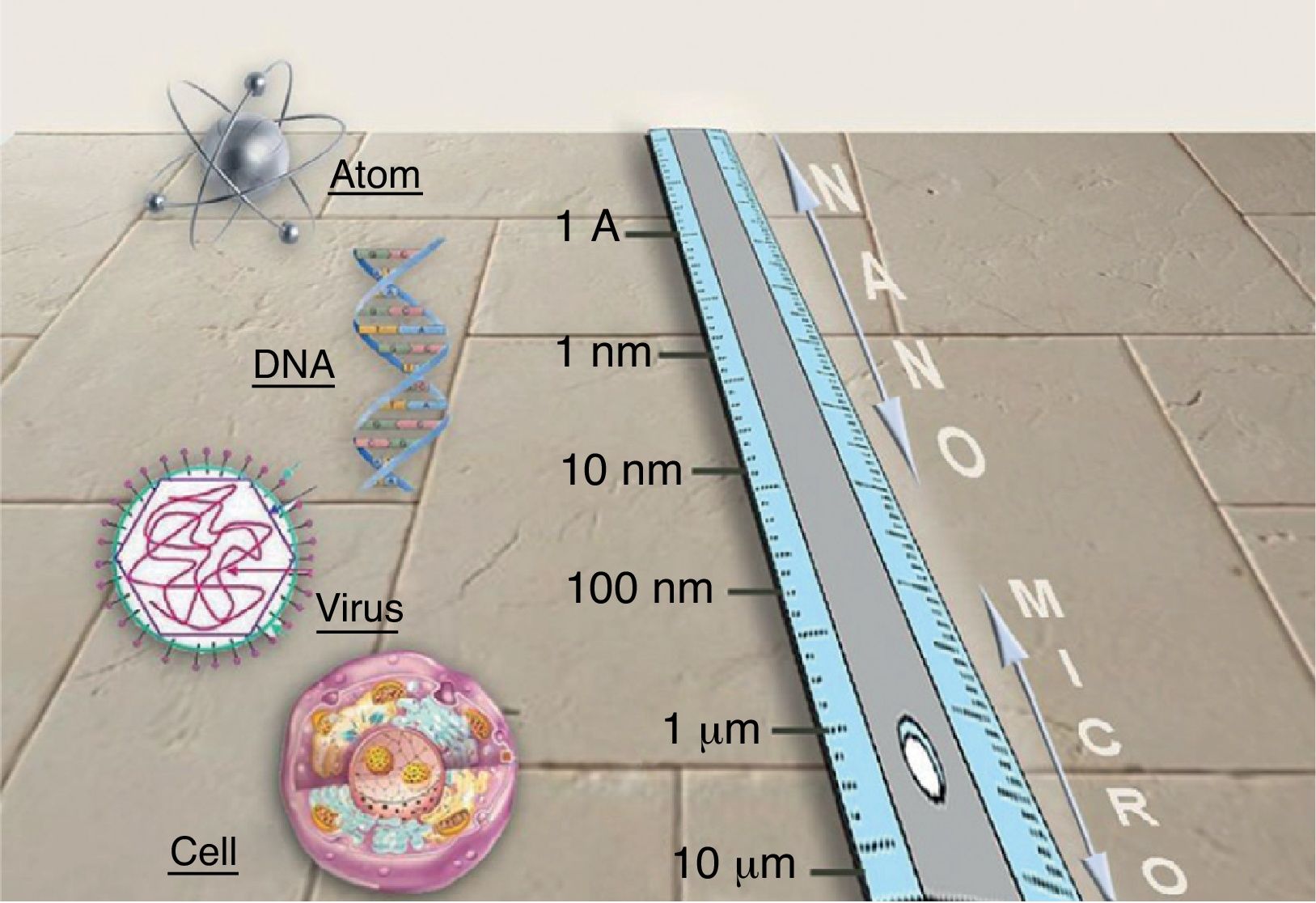
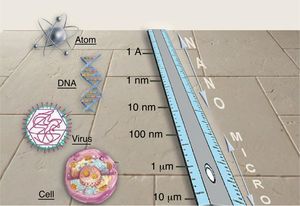
NanotechnologyNanotechnology consists in manipulating matter at the atomic and molecular scales. The conceptual foundation of nanotechnology can be traced back to Richard Feynman, winner of the 1965 Nobel Prize in Physics. In 1959, in a celebrated lecture entitled “Plenty of Room at the Bottom,” Feynman described the possibility of material synthesis processes based on the direct manipulation of atoms. Although there are great expectations surrounding the development and the possible applications of new nanometric technologies, this technical revolution is still in its infancy. Nanotechnology is based on the advantages of manufacturing materials at the nanometric scale (1-100nm) (Fig. 1), the scale of biomolecules. Many of the optical, mechanical, magnetic, electrical, and chemical properties of a material change when it is manipulated at the nanometric scale. This is due to the fact that nanoparticles (NPs) have an exponentially greater surface-area-to-volume ratio than larger particles and therefore interact with the surrounding environment to a greater degree. Consequently, the laws of quantum physics are better able to predict their properties than the laws of classical physics. Nanotechnology will make it possible to create new materials that open up a vast range of applications in medicine and in daily life: earlier diagnoses and new treatments for cancer; faster microprocessors and computers; flexible mobile telephones; harder and more flexible materials; batteries; new industrial fabrics (self-cleaning, invisible to the human eye, extremely strong and elastic); and other industrial applications currently under development.
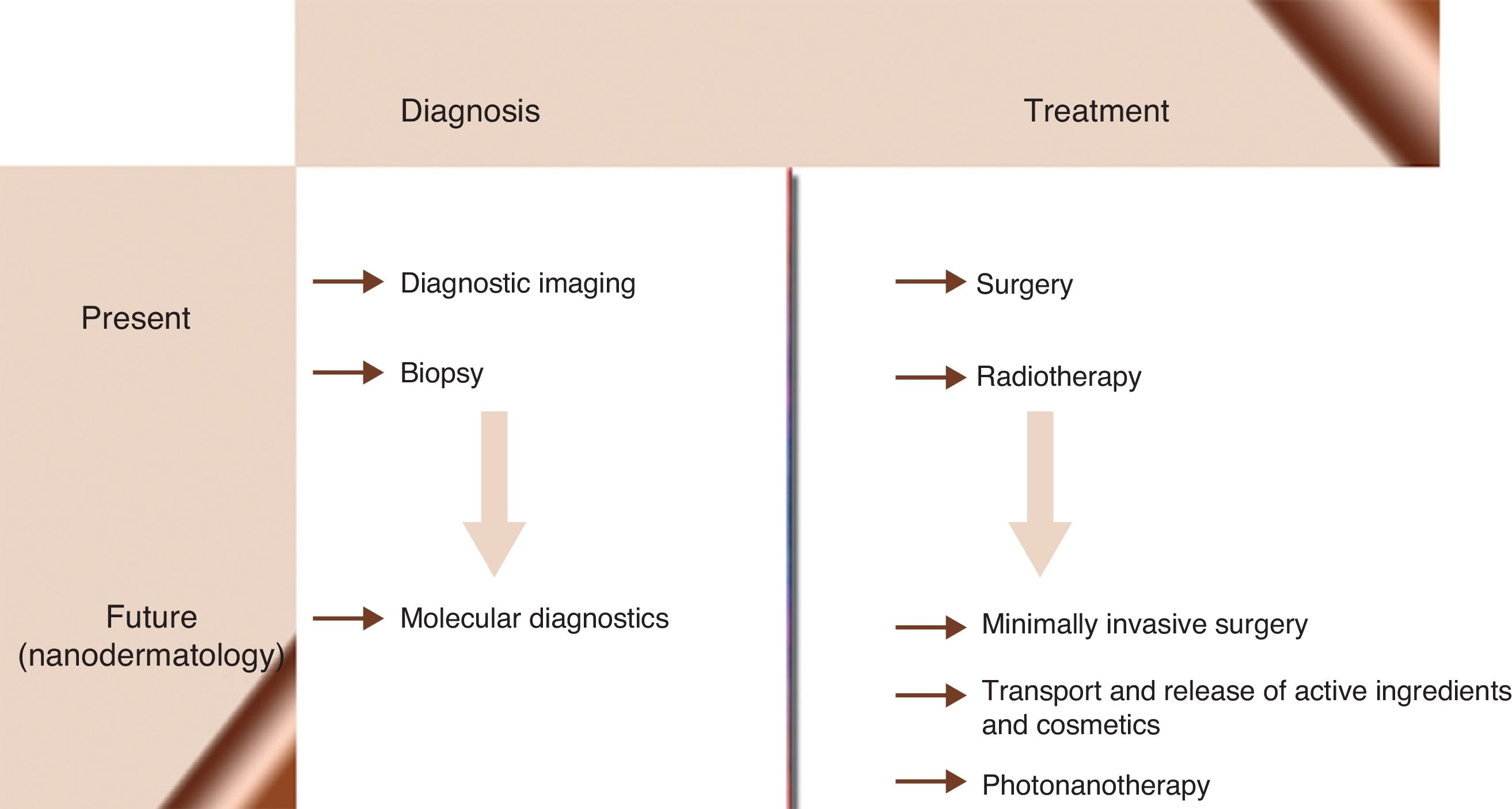
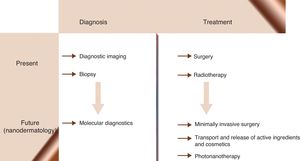
Nanomedicine is the application of nanotechnological knowledge in health care. It is already possible to design NPs capable of functioning in a coordinated manner as biosensors for diagnosing, monitoring, and treating disease in early stages when it is still curable. Nanomedical researchers predict a new age of medicine (Fig. 2) in which diseases such as cancer, diabetes, atherosclerosis, Alzheimer disease, and human immunodeficiency virus infection will be cured or will become chronic conditions. Behind these ideas is the hope of offering a personalized form of medicine in which treatment regimens can be adapted to each patient and each stage of disease evolution. From the beginning, nanomedicine has aimed to revolutionize therapeutics by using nanostructures as vehicles for transporting drugs to molecular targets, thereby increasing the efficacy of the drugs while decreasing their toxicity.86–90
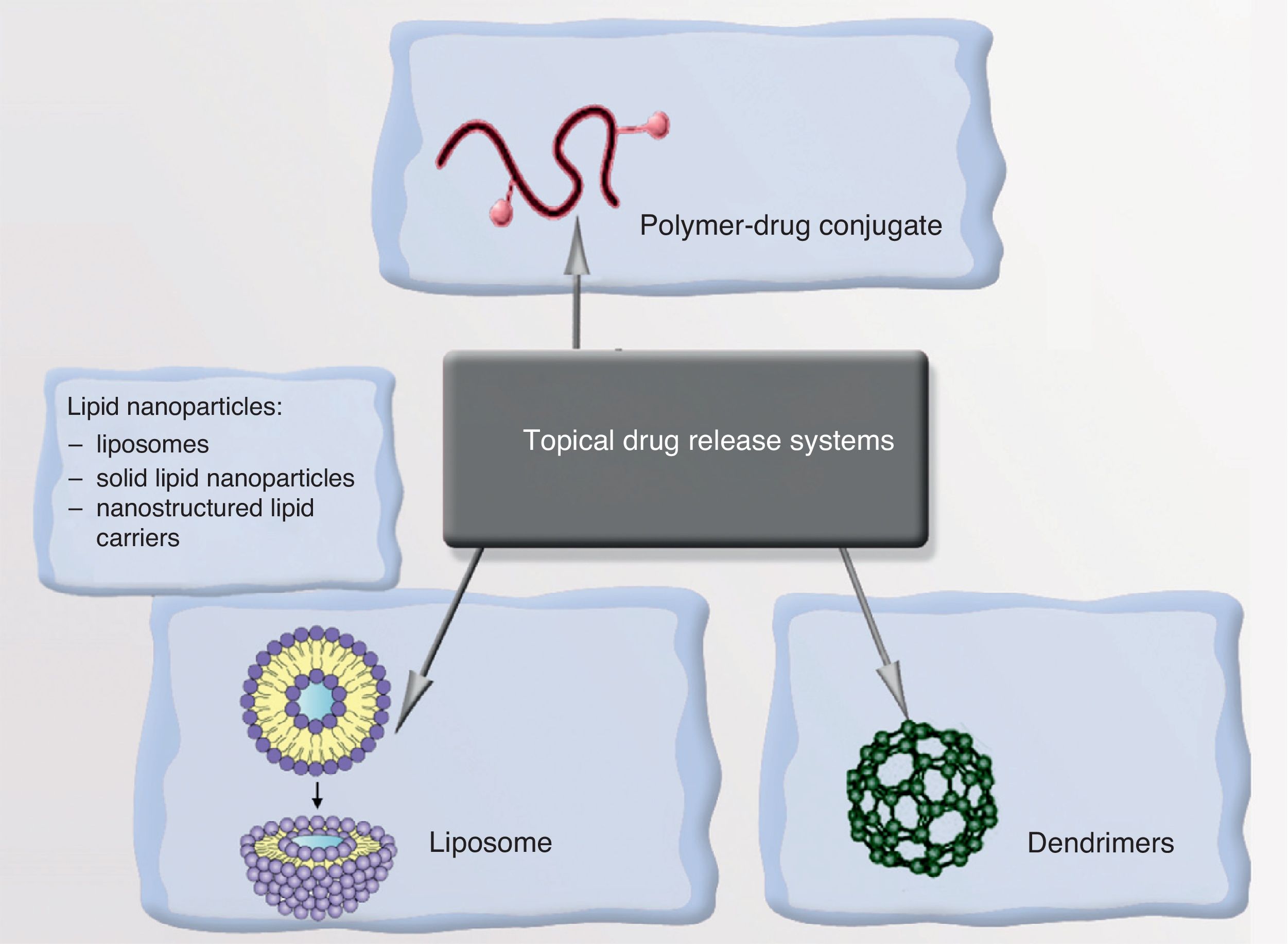
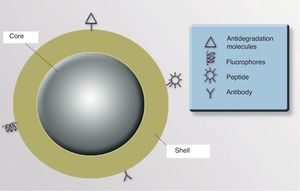
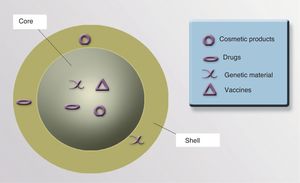
NanoparticlesNPs can be either organic (liposomes and dendrimers) or inorganic (quantum dots, nanospheres, carbon nanotubes, and metallic NPs). The basic structure of an inorganic NP consists of a core and a shell. Each of these components offers various possibilities for designing contrast media, drug carriers, and selective drug release systems.
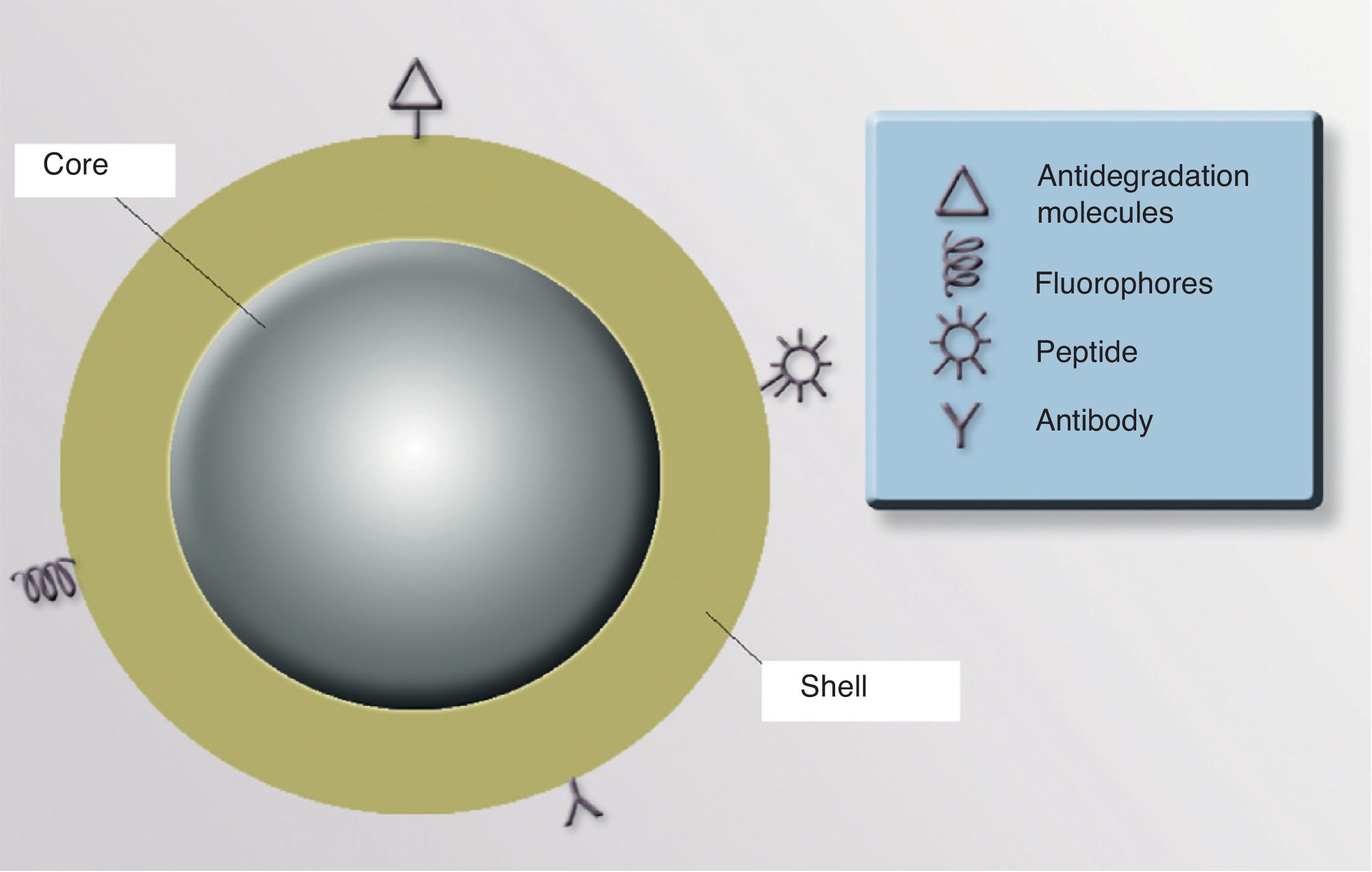
ShellThe optical, magnetic, electronic, and fluorescent characteristics of a NP are determined by its shell. The shell can be coated with fluorescent materials that make it possible to detect the presence of the NP, which is useful for diagnostic and therapeutic purposes. If the shell is coated with certain monoclonal antibodies that have an affinity for 1 or more specific cell receptors, it could be possible to direct the NP precisely to an organ or tissue, or even to a type of cellular target (neoplastic cells, cells infected by viruses or intracellular bacteria, populations of specific inflammatory cells, etc.). It is also possible to coat the NP surface with molecules that increase the stability of the particle and prevent degradation and uptake by macrophages, thereby increasing the half-life and bioavailability of the drugs associated with the NP91 (Fig. 3).
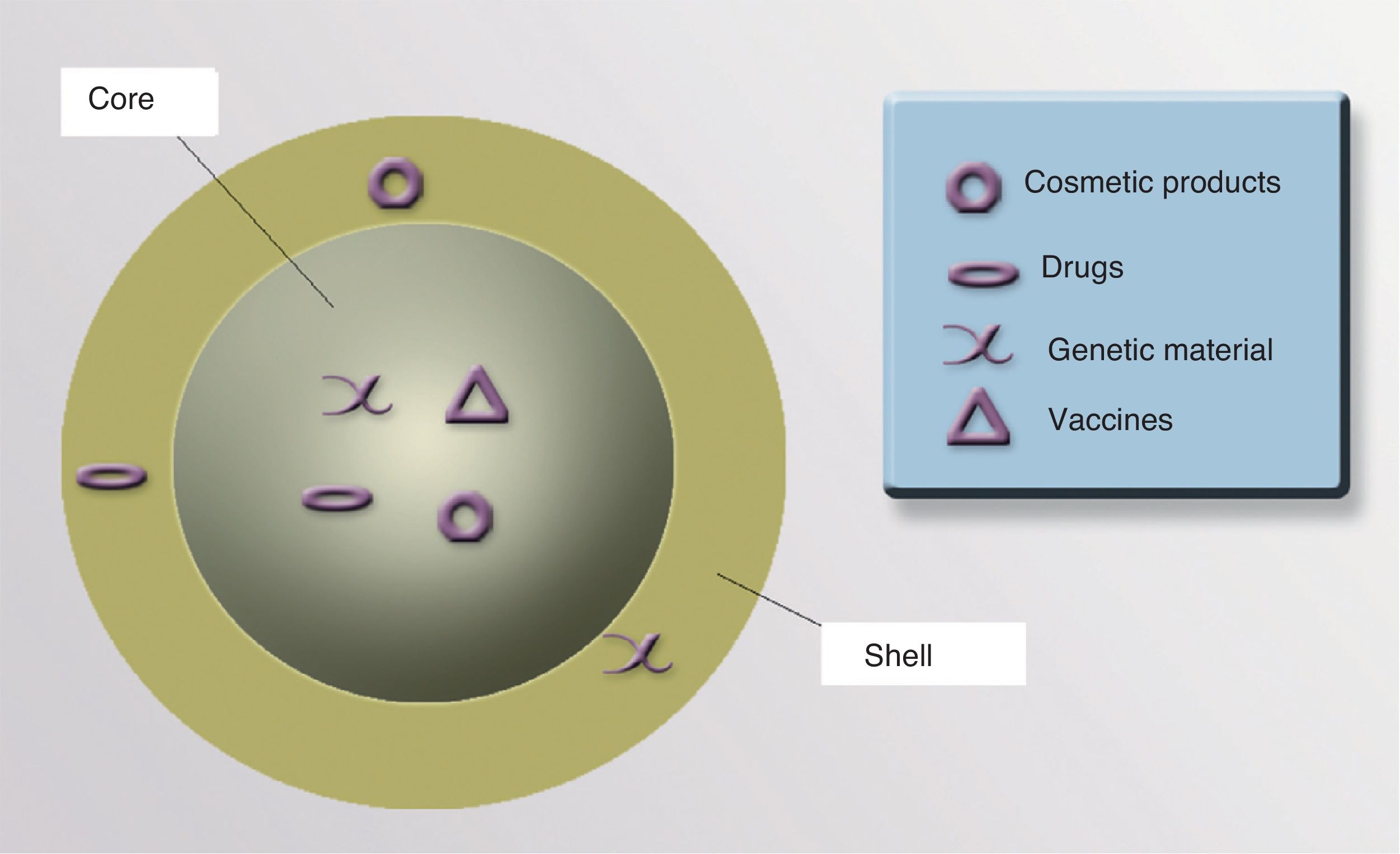
CoreThe core of a NP can be used to transport drugs, modified viruses, proteins, genetic material, vaccines, or cosmetics to precise target sites (Fig. 4).
Nanometric Devices for Diagnostic UseQuantum dots are crystals several nanometers in diameter made from semiconductor materials such as cadmium selenide (CdSe), cadmium telluride (CdTe), indium phosphide (InP), and indium arsenide (InAs). The special optical, electrical, and thermal properties of quantum dots are determined by quantum confinement—that is, the spatial confinement of the charged particles (electrons) of the nanocrystal.
The main application of quantum dots in the biomedical sciences is their use as fluorescent markers of molecules in cells and tissues. Illuminated by a laser, they produce intense fluorescence that is useful for locating tumors. Quantum dots have been injected into tumors in mice and used to identify the sentinel lymph node, achieving more precise sentinel lymph node mapping.92–98 This application could be useful for the selective marking of tumor cells with tumor-specific antibodies—which would facilitate Mohs surgery—as well as for the early diagnosis of tumors and PDT, given that quantum dots can generate O2 radicals without the need for photosensitizer molecules. Quantum dots are therefore capable of acting as new active agents in PDT. However, quantum dots—which, remember, are made of heavy metals—have an important limitation: their toxicity is unknown.99
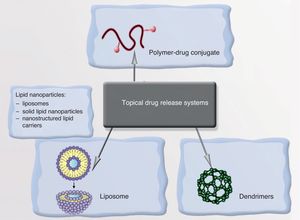
Drug Transport and Release SystemsAnother solution offered by nanomedicine is the development of systems for the transport and controlled release of drugs. The idea consists in using nanostructures to transport the drug and release it in response to a particular stimulus only after recognizing the target. Systems of this sort could reduce toxicity in healthy tissue and increase therapeutic effectiveness.
NanoemulsionsNanoemulsions are a very promising system for administering drugs in dermatology.100 Applications include the administration of antioxidants, nonsteroidal anti-inflammatory drugs, photosensitizing agents for PDT, antimicrobial agents, and sunscreens in the form of nanoemulsions. Physical sunscreens containing titanium dioxide and iron oxide are already commercially available as topical sun protection. Because they are transparent, these sunscreens do not disperse incident light, leading to better patient acceptance and adherence.
NanoparticlesNPs can transport a drug and, on recognizing the target, release it in response to a particular stimulus, thereby providing enormous selectivity and therapeutic precision. The vehicles of these drugs can be liposomes, polymeric NPs, or dendrimers (Fig. 5). An example is the encapsulation of minoxidil in liposomes to increase absorption in pilosebaceous follicles, thereby increasing the bioavailability of the drug in the root of the follicle. Solutions involving antioxidant vitamins encapsulated in liposomes—which increases the dermal bioavailability of the vitamins and prevents them from oxidizing on contact with air—are now commercially available. Antiandrogenic substances, finasteride, and retinoids can also be encapsulated, making it possible to reduce the side effects associated with systemic administration of these drugs.101–107
Photothermal Ablation With Metallic NanoparticlesThanks to their photothermal properties, metallic nanostructures are candidates for use in ablative laser treatment. A laser of appropriate wavelength could be used to excite the plasmons on the metallic surface of a nanostructure consisting of a metallic shell over a glass surface, producing heat. This technology could revolutionize light-source-based therapies, mainly those which involve red or near-infrared light, which is capable of penetrating tissues to a depth of several centimeters.
By using appropriate surface molecules (antibodies), it could be possible to select specific molecular targets that would allow the selective thermal destruction of malignant cells and/or the blood vessels supporting the skin tumor. In therapeutics, it could be possible to use lasers or light sources on any area of the skin surface and target cell populations that do not contain any of the classic chromophores. In mice, gold NPs conjugated with anti-EGFR antibodies have been used in the treatment of squamous cell carcinoma to destroy malignant cells with half the energy required for benign cells. Selective photothermal ablation of melanomas has also been achieved in mice using gold NPs attached to a peptide that is an agonist for the melanocortin 1 receptor overexpressed in melanoma.108–113
Carbon nanotubes—molecules in the fullerene family consisting of sheets of graphite arranged in a tubular structure—also absorb light in the near-infrared spectral range and are capable of generating heat. As in the case of NPs, it could be possible to take advantage of the fact that, due to their greater permeability, carbon nanotubes accumulate in the tumor stroma through the capillary vessels that form in order to nourish the tumor. Following the application of a laser or other light source, the tumor could be destroyed by photoablation.114–116
Cutaneous vascular lesions such as telangiectasias and capillary malformations are another interesting area for exploration. If metallic nanostructures are designed that are capable of accumulating in the periphery or endothelial coating of these lesions, photothermal ablation could be used thanks to the surface plasmonic properties of the nanostructures following irradiation with a light energy source.
NanobiosensorsNPs can act as biosensors that allow the adjustment of dosage and treatment regimen, thereby reducing systemic toxicity without relinquishing the maximum therapeutic effect.
As the reader may have deduced, research on the biomedical applications of nanotechnology is filled with possibilities for improving techniques for diagnosis, monitoring and treatment in dermatology. This field is poised to revolutionize our specialty in the coming decades.
New ToxicitiesThe development of new technologies is inseparable from the appearance of new toxicities that affect both patients and health care workers. Gas chromatography–mass spectrometry studies of smoke plumes from laser hair removal have demonstrated the presence of more than 14 potentially toxic substances including 2-methylpyridine, diethyl phthalate, and trimethyl disulfide.117
As new technologies with therapeutic and, especially, cosmetic applications have spread and demand for them has grown, these techniques have been widely and almost indiscriminately applied to all sorts of skin lesions before their real possibilities and side effects are fully understood.
The high reactivity of NPs could also alter other biological mechanisms besides those identified during the design of the particles; this phenomenon has become known as nanotoxicity. NPs could also become new irritants and allergens. Quantum dots contain heavy metals and could therefore have cytotoxic effects, especially if they are not designed with a hydrophilic coating. Moreover, they remain inside the cells for weeks or months, and we know almost nothing about their excretion pathways or how they are metabolized. In fact, some nonbiodegradable particles could remain in the body indefinitely and cause permanent activation of the immune system as the body attempts to eliminate them, possibly triggering autoimmune diseases. The following factors are essential to determining the safety profile of NPs118:
- 1)
How the NP enters the body.
- 2)
Size (the smaller the NP, the more toxic it is).
- 3)
Biocompatibility.
- 4)
Biodegradability.
Therefore, in our opinion and that of experts, more exhaustive studies are needed to ensure the safety of these techniques.
Scientific and technical advances are gradually making their way into the routine practice of dermatologists. Concepts and terms that are strange to us today may become the standard diagnostic and therapeutic methods of tomorrow. We have reviewed some recent advances that are currently experimental or have limited applications, but which in the future could complement or even replace present techniques.
Conflicts of InterestThe authors declare that they have no conflict of interest.
We thank Dr Izaskun Ocerin Guerta for allowing us to use her images.
Please cite this article as: Boixeda P, Feltes F, Santiago JL, Paoli J. Perspectivas de futuro en láseres, nuevas tecnologías y nanotecnología en dermatología. Actas Dermosifiliogr. 2015;106:168–179.