Trichoscopy is an essential tool in the evaluation of alopecia. The current compilation of trichoscopic signs in this setting helps distinguish between different forms of hair loss and has improved our understanding of the pathogenic mechanisms involved. The trichoscopic signs are always linked to the pathogenic mechanisms of the alopecia being examined. We examine correlations between the main trichoscopic and histopathologic findings in nonscarring alopecias.
La tricoscopia es una herramienta esencial en el examen de las alopecias. La semiología elaborada durante las últimas décadas ha contribuido a la comprensión patogénica y al diagnóstico diferencial entre las distintas formas de alopecia. Todos los signos tricoscópicos tienen su base en la patogenia de la alopecia examinada. En el presente artículo, examinamos la correlación entre los principales hallazgos tricoscópicos de las alopecias no cicatriciales y la base histopatológica que sustenta esos signos tricoscópicos.
Trichoscopy is a very useful tool for evaluating the different types of alopecia. The signs revealed by trichoscopy reflect microscopic changes in the hair follicle with respect to alterations in the perifollicular epidermis and dermis. Therefore, a knowledge of these changes would enhance our understanding of the signs observed, pathogenesis, and the differential diagnosis with other forms of alopecia.
In this article, we address the correlation between histopathology findings for the main types of nonscarring alopecia and key trichoscopy findings in the literature (Table 1). All the images (clinical and histopathological) are taken from cases seen in our practice. The relevant consent for use of these images was obtained.
Correlation Between the Main Trichoscopy Signs of Nonscarring Alopecia and Corresponding Histopathology Findings.
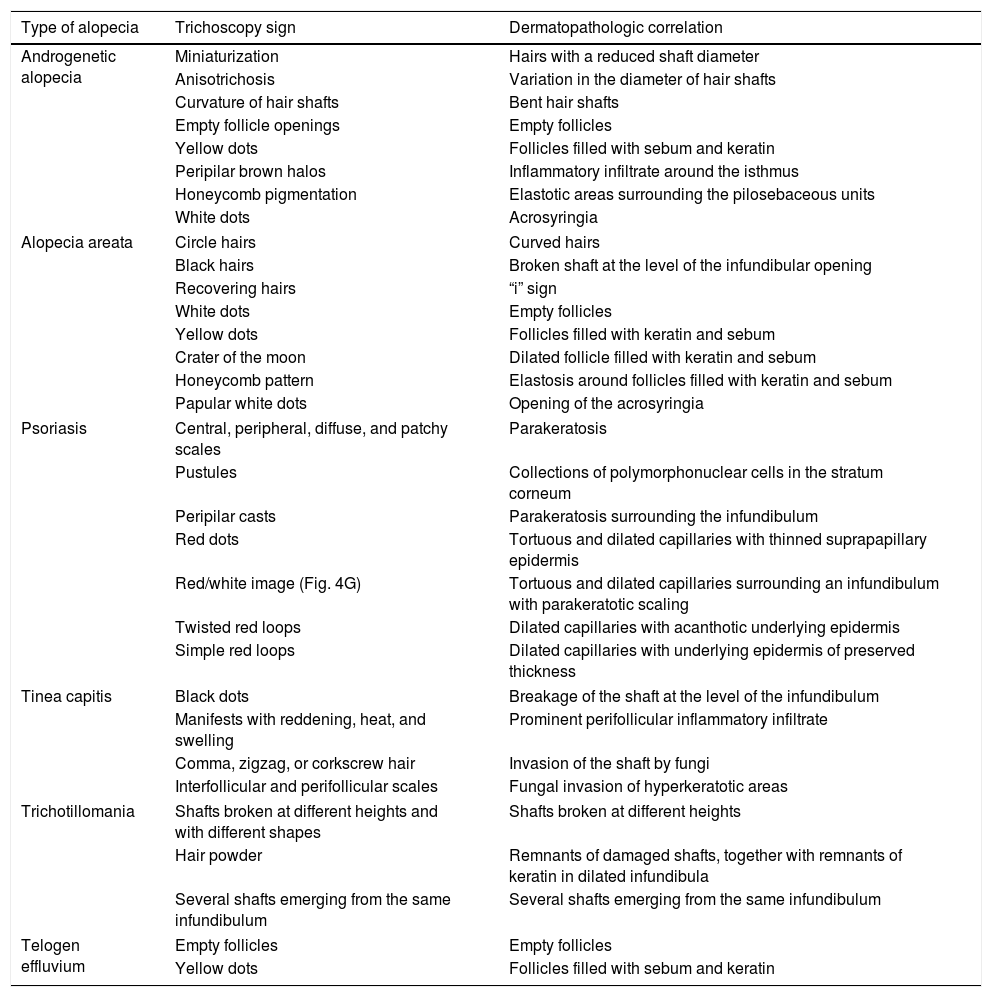
| Type of alopecia | Trichoscopy sign | Dermatopathologic correlation |
|---|---|---|
| Androgenetic alopecia | Miniaturization | Hairs with a reduced shaft diameter |
| Anisotrichosis | Variation in the diameter of hair shafts | |
| Curvature of hair shafts | Bent hair shafts | |
| Empty follicle openings | Empty follicles | |
| Yellow dots | Follicles filled with sebum and keratin | |
| Peripilar brown halos | Inflammatory infiltrate around the isthmus | |
| Honeycomb pigmentation | Elastotic areas surrounding the pilosebaceous units | |
| White dots | Acrosyringia | |
| Alopecia areata | Circle hairs | Curved hairs |
| Black hairs | Broken shaft at the level of the infundibular opening | |
| Recovering hairs | “i” sign | |
| White dots | Empty follicles | |
| Yellow dots | Follicles filled with keratin and sebum | |
| Crater of the moon | Dilated follicle filled with keratin and sebum | |
| Honeycomb pattern | Elastosis around follicles filled with keratin and sebum | |
| Papular white dots | Opening of the acrosyringia | |
| Psoriasis | Central, peripheral, diffuse, and patchy scales | Parakeratosis |
| Pustules | Collections of polymorphonuclear cells in the stratum corneum | |
| Peripilar casts | Parakeratosis surrounding the infundibulum | |
| Red dots | Tortuous and dilated capillaries with thinned suprapapillary epidermis | |
| Red/white image (Fig. 4G) | Tortuous and dilated capillaries surrounding an infundibulum with parakeratotic scaling | |
| Twisted red loops | Dilated capillaries with acanthotic underlying epidermis | |
| Simple red loops | Dilated capillaries with underlying epidermis of preserved thickness | |
| Tinea capitis | Black dots | Breakage of the shaft at the level of the infundibulum |
| Manifests with reddening, heat, and swelling | Prominent perifollicular inflammatory infiltrate | |
| Comma, zigzag, or corkscrew hair | Invasion of the shaft by fungi | |
| Interfollicular and perifollicular scales | Fungal invasion of hyperkeratotic areas | |
| Trichotillomania | Shafts broken at different heights and with different shapes | Shafts broken at different heights |
| Hair powder | Remnants of damaged shafts, together with remnants of keratin in dilated infundibula | |
| Several shafts emerging from the same infundibulum | Several shafts emerging from the same infundibulum | |
| Telogen effluvium | Empty follicles | Empty follicles |
| Yellow dots | Follicles filled with sebum and keratin | |
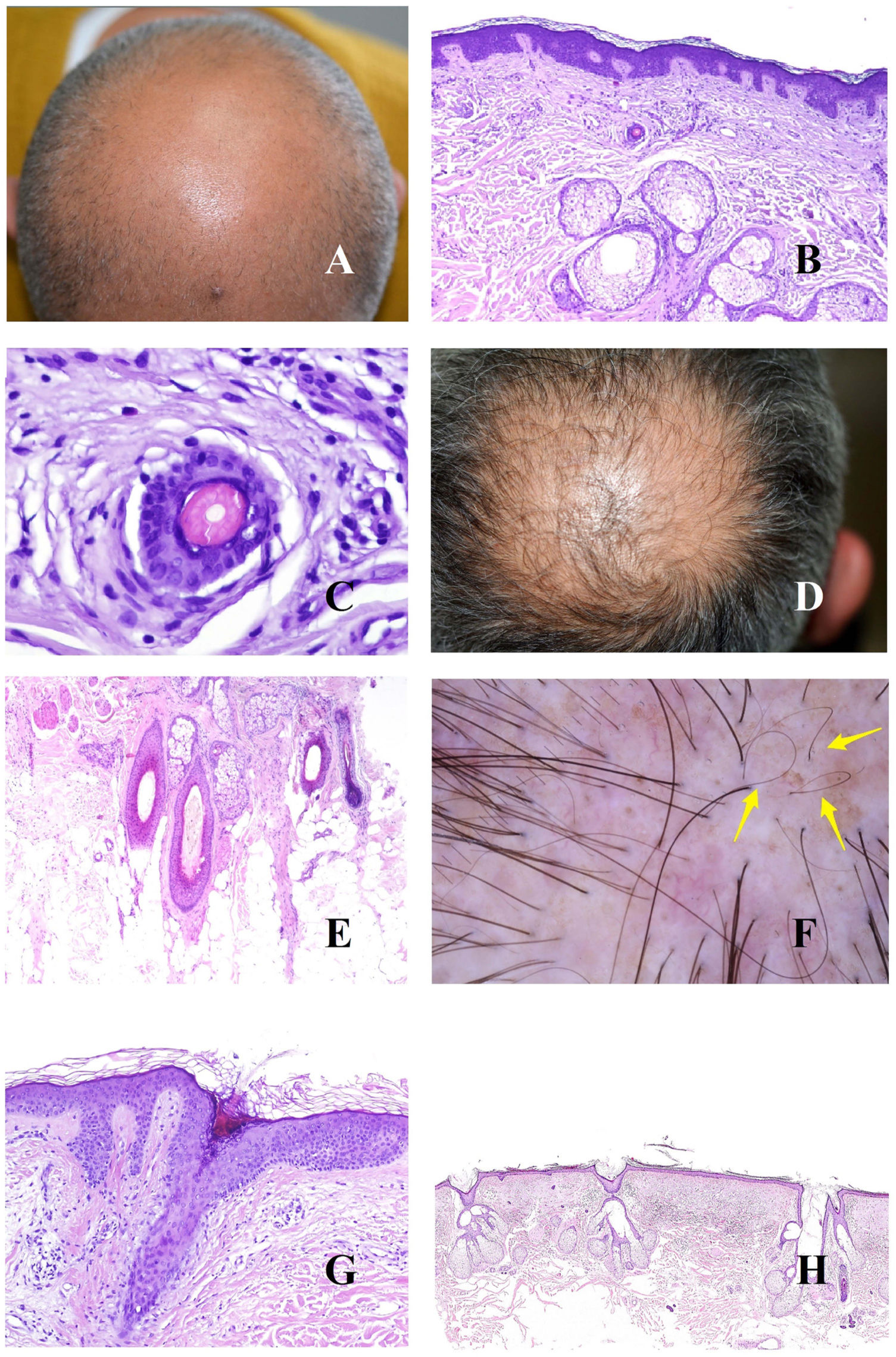
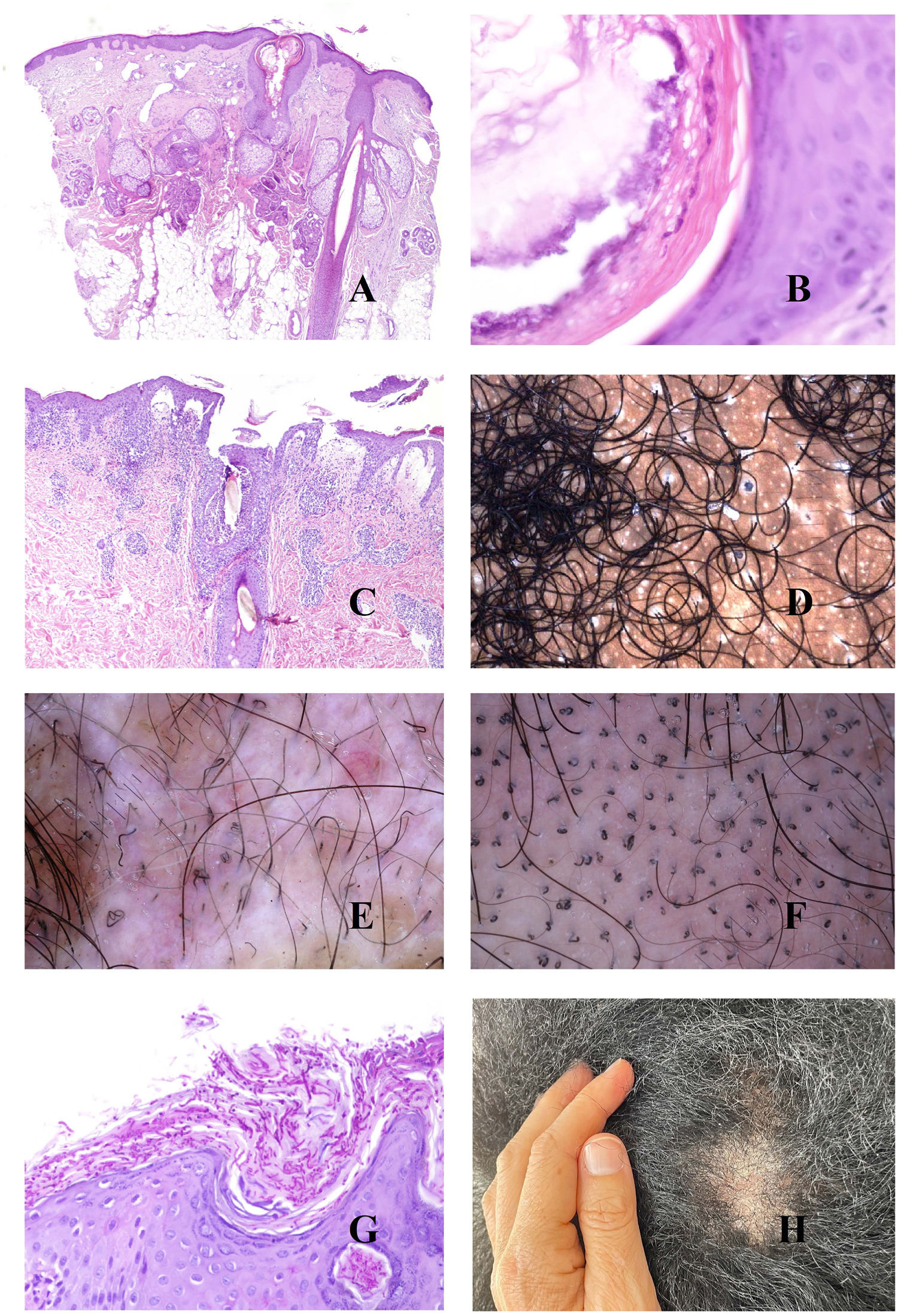
The underlying cause of androgenetic alopecia is a shortening of the hair follicle growth cycle,1 with an increase in vellus hair follicles and an alteration of the terminal-to-vellus ratio, which, in many cases falls below 4:1. A ratio lower than 3:1 is considered diagnostic.2 Trichoscopy reveals miniaturization (Fig. 1A), as does biopsy, in which the thickness of the shaft is smaller than that of the inner root sheath (Fig. 1B and C).
A, Androgenetic alopecia. Numerous miniaturized hair shafts. B, Androgenetic alopecia showing a miniaturized hair follicle in the upper part of the dermis. Compare with the sebaceous glands, which appear large in size, despite their normal morphology (hematoxylin-eosin [H-E], ×40). C, Detail of a miniaturized hair follicle showing a shaft that is thinner than the internal root sheath (H-E, ×400). D, Androgenetic alopecia. Anisotrichosis, with marked variation between shaft diameters. E, Androgenetic alopecia. While anisotrichosis is not easily evaluated in biopsy specimens, variations in shaft diameter can even be seen in small specimens (H-E, ×40). F, Androgenetic alopecia. Occasional circle hairs. G, Androgenetic alopecia. Circle hairs are above the epidermis in most cases (H-E, ×40). H, Androgenetic alopecia. Abundant empty hair follicles, with no shaft (H-E, ×20).
The abundance of vellus hair follicles also underlies one of the early signs of androgenetic alopecia, namely, anisotrichosis,3 which is defined as a variation in hair shaft diameter greater than 20% in men and 10% in women (Fig. 1D). Clearly, anisotrichosis cannot be evaluated using biopsy, although we do observe co-occurrence of shafts with different diameters (Fig. 1E).
The shafts of the vellus hair follicles bend easily, as they are weak and thin (Fig. 1F). They are usually found above the epidermis (Fig. 1G), although they may occasionally be trapped below it.4
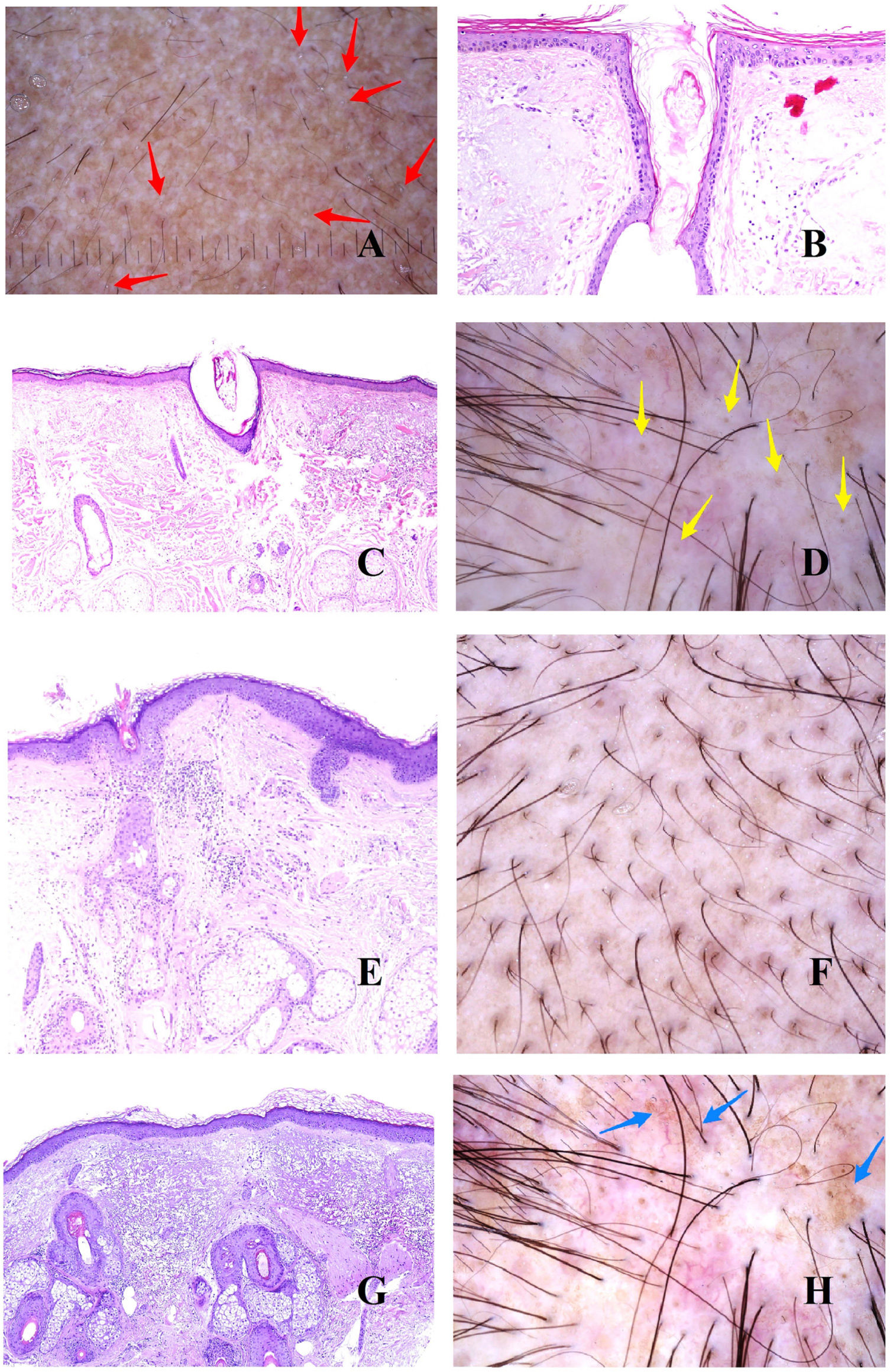
In addition to vellus hair follicles, we can see empty follicular openings, i.e., without a hair shaft (Fig. 1H). These are easily visible in trichoscopy5 (Fig. 2A). However, follicular openings may be filled with other materials, such as sebum and keratin (Fig. 2B and C), which are seen as yellow dots in trichoscopy (Fig. 2D).3
A, Androgenetic alopecia. The red arrows indicate the white dots of the acrosyringia. The widest white dots correspond to empty hair follicles. B and C, Androgenetic alopecia. Follicular infundibula without a shaft may be filled with sebaceous material and keratin (B, hematoxylin-eosin [H-E], ×100; C, H-E, ×20). D, Androgenetic alopecia. Yellow dots corresponding to infundibula without hair shafts filled with sebum and keratin. E, Androgenetic alopecia. Discrete lymphohistiocytic inflammatory infiltrate surrounding the isthmus of the hair follicle (H-E, ×40). F, Androgenetic alopecia. Brownish halo surrounding the follicular opening. G, Androgenetic alopecia. Intense solar elastosis surrounding miniaturized hair follicles (H-E, ×20). H, Androgenetic alopecia. Pigmented area with honeycomb pattern.
In biopsy specimens from patients with androgenetic alopecia, it is not uncommon to find a slight lymphocytic infiltrate around the isthmus (Fig. 2E), which trichoscopy shows as the peripilar sign (Fig. 2F).
As hair loss progresses and bald areas spread, the exposed scalp begins to be affected by sunlight, thus increasing dermal elastosis. The elastotic areas are seen as brownish in trichoscopy.3,5 No matter how the elastotic areas surround the remaining vellus follicles with conserved sebaceous glands (which are yellowish in color) (Fig. 2G), the result is a pigmented “honeycomb” pattern around the yellow papules (Fig. 2H).6
Given this background of elastosis and very low follicular density, the eccrine glands are easily visible, especially the openings of the acrosyringia, which are seen in trichoscopy as white dots3 (Fig. 2A).
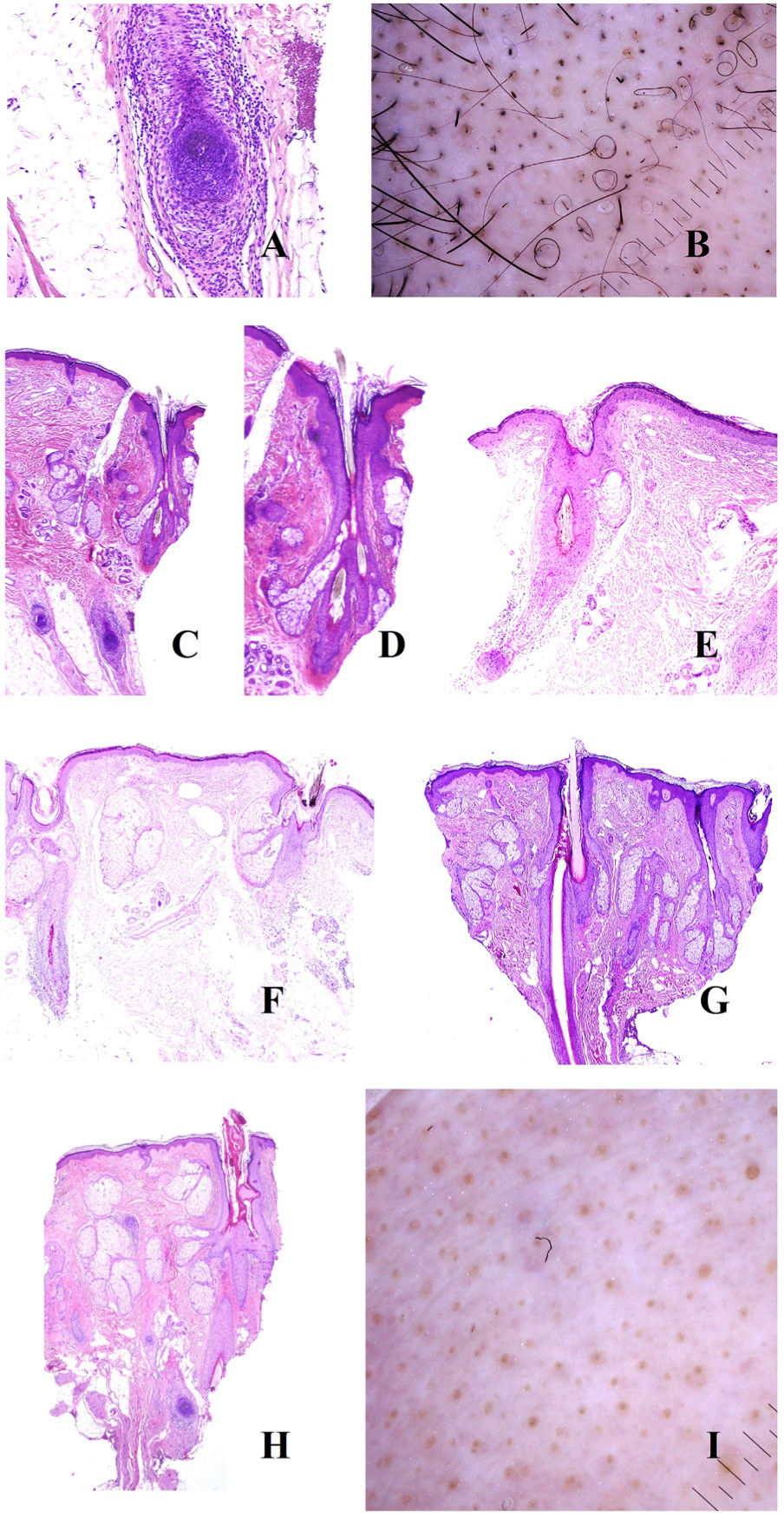
Alopecia AreataThe pathogenesis of alopecia areata is characterized by the key role of an autoimmune lymphocytic infiltrate targeting the hair bulb (Fig. 3A), which is associated with the loss of immune status by the bulb.7 This affects the hair growth cycle in 3 ways.
A, Alopecia areata. Intense peribulbar inflammatory infiltrate (hematoxylin-eosin [H-E], ×100). B, Alopecia areata. Numerous circle hairs. Note also broken hairs and black dots. C, Alopecia areata. Peribulbar inflammatory infiltrate. Two hair shafts emerging from the same infundibulum (H-E, ×20). D, Alopecia areata. Detail of the point where 2 shafts emerge from the same infundibulum (H-E, ×40). E, Alopecia areata. Evidence of a peribulbar inflammatory infiltrate. The hair shaft is seen to be broken (H-E, ×20). F, Alopecia areata. Broken hair shaft at the point where it emerges from the infundibulum (H-E, ×20). G, Alopecia areata. New hair follicle pushing the old one (H-E, ×20). H, Alopecia areata. Infundibulum filled with sebaceous secretion and keratin (H-E, ×20). I, Alopecia areata. Numerous yellow dots, together with an occasional dystrophic hair.
First, the cycle is shortened. This is accompanied by an increase in the number of vellus hair follicles to the detriment of the terminal follicles. However, the terminal-to-vellus ratio does not reach the values observed in androgenetic alopecia. Biopsy reveals follicles with hairs that tend to bend, which are seen as circle hairs in trichoscopy8,9 (Fig. 3B). In fact, the presence of a large number of circle hairs almost confirms a diagnosis of alopecia areata.9,10
The second consequence is a dystrophic anagen phase, which manifests in 2 ways: as the emergence of 2 or more hair shafts from each infundibulum, on the one hand (Fig. 3C and D), and as breakage of the shafts, on the other8 (Fig. 3E and B). When the latter occurs right at the infundibular opening (Fig. 3F), it is seen clinically as a black dot8,9,11,12 (Fig. 3B), which is one of the most common findings in alopecia areata.13 The variability between the broken shafts is never as marked as in trichotillomania.13 It is not clear how these breaks arise, although one hypothesis is that inflammation can lead to progressive thinning until the hair breaks above the epidermis.13 Therefore, in alopecia areata, the hair shafts may take on a monilethrix-like pattern.13
When alopecia areata begins to resolve, we can observe the new follicle pushing out from the old one (Fig. 3G). On emerging, the new shafts are seen in trichoscopy as having a pointed tip.13 As the new shaft grows, the old shaft is displaced upwards until it only accounts for a small segment in the upper part of the new shaft (the dot on the “i”). This is known as the “i sign” and is a good indicator that alopecia is healing. As mentioned above, recovering hair shafts can bend back on themselves (circle or “pigtail” hairs).13
The third consequence of inflammation of the bulb is prolongation of the telogen phase, which in turn leads to an abundance of empty follicles (kenogen), seen as white dots in trichoscopy.9 As in androgenetic alopecia, the infundibula may also be filled with keratin and sebum (Fig. 3H), seen as yellow dots in trichoscopy8,9,11,12 (Fig. 3I). As they contain sebum, these yellow dots are not seen in preadolescent patients.9 One characteristic of yellow dots in alopecia areata is their regular distribution, clustered in groups of 2 or 3, reflecting the number of hair shafts per follicular unit.13 Widening of the infundibulum is seen as a crater in trichoscopy, and the “craters of the moon” sign has been reported in severe alopecia areata.14 Furthermore, if the yellow dots appear on sun-exposed skin in patients with a high skin phototype, then they may be accompanied by honeycomb-pattern pigmentation13 owing to the elastosis surrounding the follicles, as in androgenetic alopecia (see above).
This type of alopecia is also characterized by eccrine openings that are easily identifiable as papular white dots in trichoscopy.
PsoriasisPsoriatic alopecia is a controversial disease, since its symptoms overlap with those of seborrheic dermatitis.
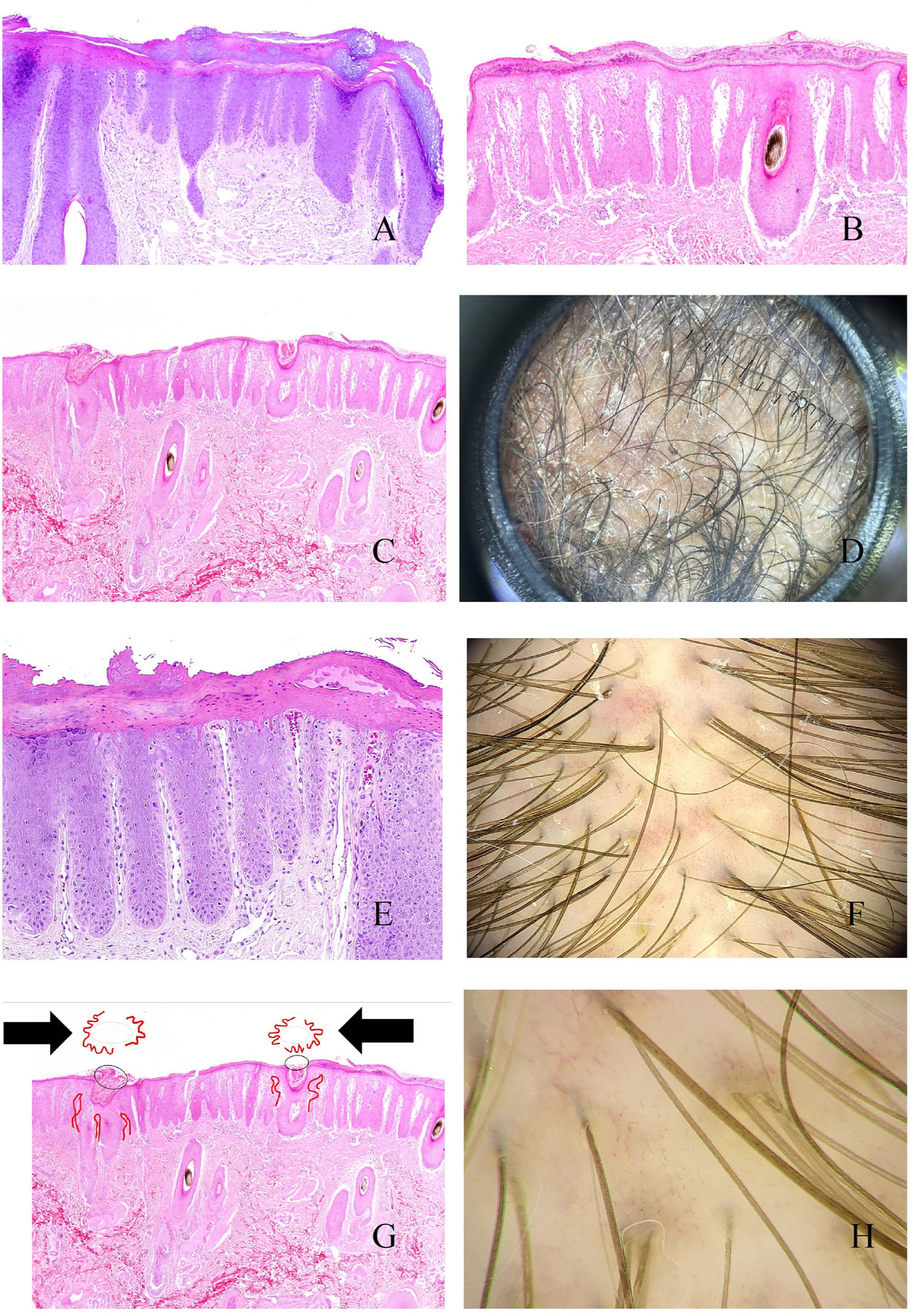
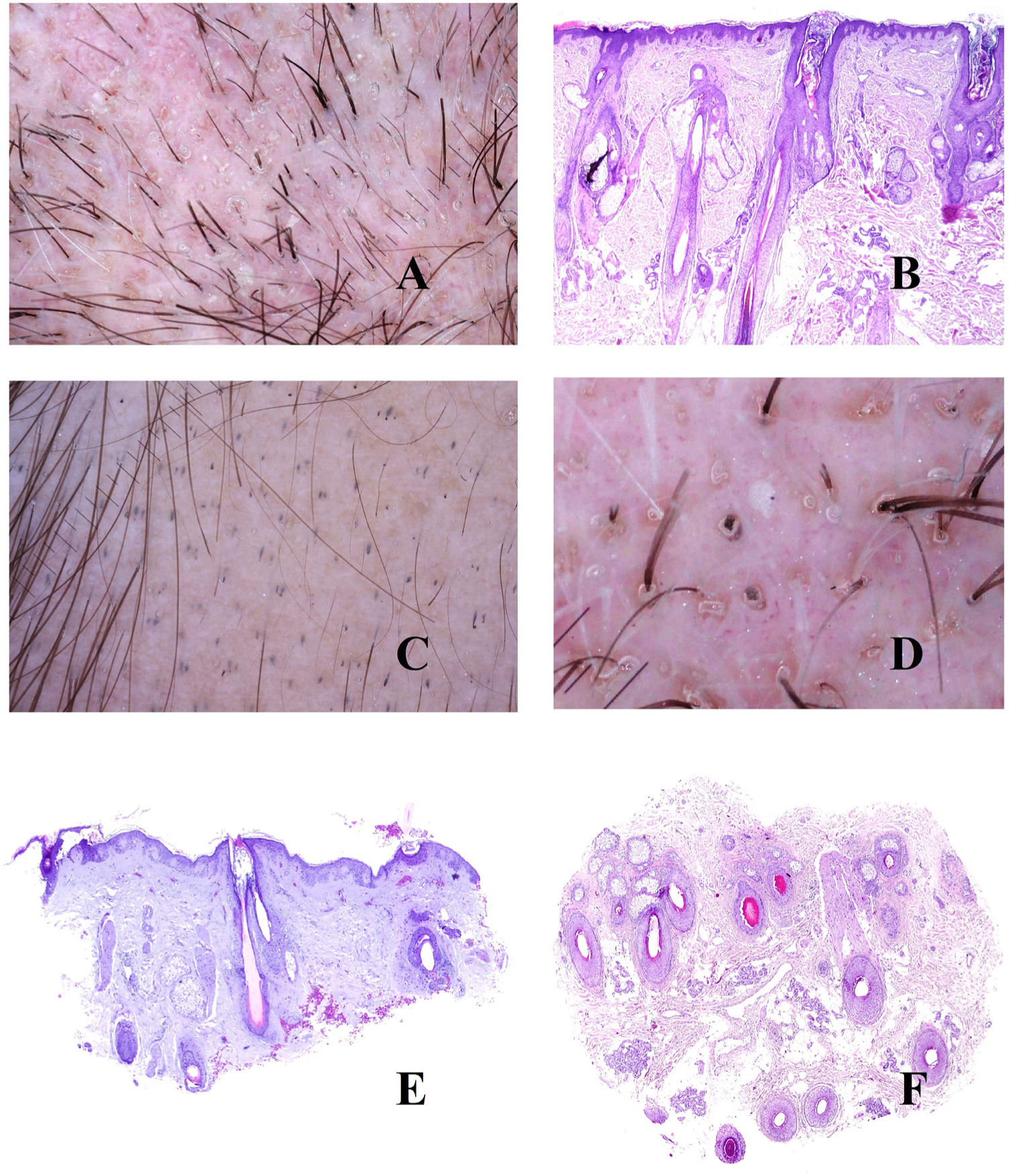
As with other parts of the skin, lengthening of the interpapillary ridges can lead to thickening of the scalp (Fig. 4A). Above this level, and in the stratum corneum, parakeratotic layers of varying sizes are easily seen (Fig. 4B), correlating with various trichoscopic patterns, namely, central, peripheral, diffuse, and patchy scaling.15 When these scales show abscesses composed of polymorphonuclear cells, the disease may manifest as pustules.16
A, Psoriatic alopecia. Epidermal thickening involving the interpapillary ridges (hematoxylin-eosin [H-E], ×20). B, Psoriatic alopecia. Parakeratotic scaling (H-E, ×20). C, Psoriatic alopecia. In this biopsy specimen, the parakeratotic scales are located in the infundibulum (H-E, ×20). D, Psoriatic alopecia. Peripilar casts corresponding to infundibular parakeratotic scales eliminated by growth of the shaft (image courtesy of Dr. Nerea Landa). E, Psoriatic alopecia. The vessels of the papillary dermis appear tortuous and dilated. Those on the right lie in areas of marked suprapapillary epidermal thickening and are seen as twisted red loops, whereas those on the left lie under an epidermis with a slightly more preserved thickness and are seen in trichoscopy as simple red loops (H-E, ×40). F, Red dots (image courtesy of Dr. David Saceda). G, When the tortuous papillary capillary loops appear surrounded by parakeratotic scale, trichoscopy shows a very characteristic image (H-E, ×20). H, Tortuous vessels (image courtesy of Dr. David Saceda).
Parakeratotic scales occasionally appear on the infundibulum (Fig. 4C), surrounding the point where the hair shaft emerges, and are eliminated with the shaft in the form of peripilar casts (Fig. 4D).
In the papillary dermis, the capillaries are seen as tortuous and dilated (Fig. 4E). Owing to suprapapillary epidermal thinning, these capillaries are seen as red dots in trichoscopy15 (Fig. 4F). Tortuous capillaries surrounding an infundibulum containing a parakeratotic scale produce a very characteristic trichoscopy image (Fig. 4G).15 Dilated capillaries are sometimes seen in areas of epidermal acanthosis, without as much suprapapillary thinning, in the form of twisted red loops15,16 (Fig. 4H). In contrast, capillaries with a more preserved thickness under the epidermis are seen as simple red loops.15,16
Tinea CapitisFungal microorganisms usually invade the epidermal or infundibular stratum corneum (Fig. 5A and B), although they can also affect the hair shaft itself, leading to breakage at different levels (Fig. 5C). When the shaft breaks in the opening of the infundibulum, trichoscopy reveals black dots17,18 (Fig. 5D).
A and B, Tinea capitis. Fungal invasion of infundibular keratin (A, hematoxylin-eosin [H-E], ×20; B, H-E, ×400). C, Breakage of the shaft in a case of tinea capitis (H-E, ×20). D, Tinea capitis. Black dots. E, Tinea capitis. Numerous corkscrew hairs. F, Tinea capitis. Numerous comma hairs. G, Tinea capitis. Keratotic scale with very abundant fungal microorganisms (H-E, ×200). H, Tinea capitis. Peri- and interfollicular scales (image courtesy of Dr. Nerea Landa).
The condition may present as inflammation, with reddening, heat, and swelling. In histopathology, this is seen as a prominent perifollicular inflammatory infiltrate that is very often mixed (acute and chronic).
Direct invasion of the shaft by fungi can alter its shape and architecture, leading to comma hairs, zigzag hairs, and corkscrew hairs17,18 (Fig. 5E and F). In contrast, fungal invasion of hyperkeratotic areas leads to interfollicular and perifollicular scaling17,18 (Fig. 5G and H).
TrichotillomaniaOwing to a mechanical effect, hair shafts break at different heights in trichotillomania. This is visible both in trichoscopy and in biopsy (Fig. 6A)9,19 and takes the form of question mark hair, exclamation mark hair, tulip hair, or flame hair.9,19 The presence of broken shafts is not pathognomonic of trichotillomania, since this is observed in other conditions such as alopecia areata, tinea, and trichorrhexis nodosa. However, none of these entities is characterized by such high variability in the shape and length of the hairs as that seen in trichotillomania.19 Breaks at different levels can also be seen in trichoscopy, with evidence of broken shafts below the skin surface.19 Similarly, the end portions of the broken shafts vary considerably in shape, with frequent observation of trichoptilosis (end of the hair split or frayed).19 This sign, however, is characteristic of shorter shafts.19
A, Trichotillomania. Shafts broken at different levels. B, Trichotillomania. Melanin pigment deposit on the follicular shaft on the left. The 2 infundibula on the right appear dilated and with marked keratin content. C, Trichotillomania. Presence of hair powder in many infundibula. D, Trichotillomania. Trichoscopy shows some V hairs. E and F, Telogen effluvium. Hair follicles in telogen, together with empty hair follicles, visible both in vertical and in horizontal slices (E, hematoxylin-eosin [H-E], ×20; F, H-E, ×20).
Bleeding and pigment deposits are both useful signs when attempting to identify breakages in the hair shaft. Bleeding is seen in histology samples as blood separating 2 hair fragments. When this break is horizontal, the image resembles a hamburger, whereas when it is longitudinal, it resembles a hot dog. Furthermore, deposition of melanin on the hair shaft (Fig. 6B) is suggestive, although not pathognomonic, since it can be observed in alopecia involving endogenous lesions of the shaft, for example, alopecia areata.
Remnants of damaged hair shafts accumulating alongside remnants of keratin in dilated infundibula (Fig. 6B) are seen as hair powder in trichoscopy9,19,20 (Fig. 6C). Hair powder must be distinguished from dirty dots, which can be seen in healthy children.19 Hair powder can be differentiated based on its uniform coloring, fine grain, and proximity to shafts showing other signs of mechanical damage.19
It is not uncommon for 1 or more broken shafts to emerge from the same follicular opening20 (Fig. 6D).
Telogen EffluviumIn telogen effluvium, various stimuli (e.g., surgery, severe disease, labor, extreme diet, various medications) lead to massive migration of follicles from the anagen to the telogen phase.2 The trichogram shows abundant hair shafts with telogen morphology.
While biopsy findings for acute telogen effluvium are relatively banal, those for chronic telogen effluvium, both in horizontal and in vertical sections, may comprise follicles in telogen, together with empty follicles (Fig. 6D and E). Under normal conditions, follicles in telogen account for no more than 10%. In biopsy of telogen effluvium, on the other hand, they can account for up to 20–30%.2
The fall of a hair shaft is almost immediately followed by the growth of a new shaft. The cases where growth of the new hair is not immediate are characterized by empty or sebum-filled follicles (yellow dots), which are indistinguishable from those seen in androgenetic alopecia.21 When there is doubt, the trichogram can prove very useful.
Recovering new hair shafts are seen as fine and pointed in trichoscopy.9,21 When these are present in large numbers, they are characteristic of acute telogen effluvium.21
While telogen effluvium may be accompanied by a certain percentage of vellus hairs, a terminal-to-vellus ratio below 4:1 is more indicative of androgenetic alopecia than of effluvium.
Perifollicular lymphocytic infiltrates are not unusual in telogen effluvium, correlating with the findings of brown peripilar pigmentation (also seen in androgenetic alopecia).21
Most authors agree that trichoscopy is inconclusive in telogen effluvium21 and that the disease should be considered a diagnosis of exclusion.22
ConclusionsKnowledge of the histopathologic findings observed in each of these entities enhances our understanding of trichoscopy findings, as well as the differential diagnosis with conditions in which clinical findings may overlap considerably.
Conflicts of InterestThe authors declare that they have no conflicts of interest.


![A, Androgenetic alopecia. Numerous miniaturized hair shafts. B, Androgenetic alopecia showing a miniaturized hair follicle in the upper part of the dermis. Compare with the sebaceous glands, which appear large in size, despite their normal morphology (hematoxylin-eosin [H-E], ×40). C, Detail of a miniaturized hair follicle showing a shaft that is thinner than the internal root sheath (H-E, ×400). D, Androgenetic alopecia. Anisotrichosis, with marked variation between shaft diameters. E, Androgenetic alopecia. While anisotrichosis is not easily evaluated in biopsy specimens, variations in shaft diameter can even be seen in small specimens (H-E, ×40). F, Androgenetic alopecia. Occasional circle hairs. G, Androgenetic alopecia. Circle hairs are above the epidermis in most cases (H-E, ×40). H, Androgenetic alopecia. Abundant empty hair follicles, with no shaft (H-E, ×20). A, Androgenetic alopecia. Numerous miniaturized hair shafts. B, Androgenetic alopecia showing a miniaturized hair follicle in the upper part of the dermis. Compare with the sebaceous glands, which appear large in size, despite their normal morphology (hematoxylin-eosin [H-E], ×40). C, Detail of a miniaturized hair follicle showing a shaft that is thinner than the internal root sheath (H-E, ×400). D, Androgenetic alopecia. Anisotrichosis, with marked variation between shaft diameters. E, Androgenetic alopecia. While anisotrichosis is not easily evaluated in biopsy specimens, variations in shaft diameter can even be seen in small specimens (H-E, ×40). F, Androgenetic alopecia. Occasional circle hairs. G, Androgenetic alopecia. Circle hairs are above the epidermis in most cases (H-E, ×40). H, Androgenetic alopecia. Abundant empty hair follicles, with no shaft (H-E, ×20).](https://static.elsevier.es/multimedia/00017310/0000011400000006/v1_202306061231/S0001731023003538/v1_202306061231/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![A, Androgenetic alopecia. The red arrows indicate the white dots of the acrosyringia. The widest white dots correspond to empty hair follicles. B and C, Androgenetic alopecia. Follicular infundibula without a shaft may be filled with sebaceous material and keratin (B, hematoxylin-eosin [H-E], ×100; C, H-E, ×20). D, Androgenetic alopecia. Yellow dots corresponding to infundibula without hair shafts filled with sebum and keratin. E, Androgenetic alopecia. Discrete lymphohistiocytic inflammatory infiltrate surrounding the isthmus of the hair follicle (H-E, ×40). F, Androgenetic alopecia. Brownish halo surrounding the follicular opening. G, Androgenetic alopecia. Intense solar elastosis surrounding miniaturized hair follicles (H-E, ×20). H, Androgenetic alopecia. Pigmented area with honeycomb pattern. A, Androgenetic alopecia. The red arrows indicate the white dots of the acrosyringia. The widest white dots correspond to empty hair follicles. B and C, Androgenetic alopecia. Follicular infundibula without a shaft may be filled with sebaceous material and keratin (B, hematoxylin-eosin [H-E], ×100; C, H-E, ×20). D, Androgenetic alopecia. Yellow dots corresponding to infundibula without hair shafts filled with sebum and keratin. E, Androgenetic alopecia. Discrete lymphohistiocytic inflammatory infiltrate surrounding the isthmus of the hair follicle (H-E, ×40). F, Androgenetic alopecia. Brownish halo surrounding the follicular opening. G, Androgenetic alopecia. Intense solar elastosis surrounding miniaturized hair follicles (H-E, ×20). H, Androgenetic alopecia. Pigmented area with honeycomb pattern.](https://static.elsevier.es/multimedia/00017310/0000011400000006/v1_202306061231/S0001731023003538/v1_202306061231/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![A, Alopecia areata. Intense peribulbar inflammatory infiltrate (hematoxylin-eosin [H-E], ×100). B, Alopecia areata. Numerous circle hairs. Note also broken hairs and black dots. C, Alopecia areata. Peribulbar inflammatory infiltrate. Two hair shafts emerging from the same infundibulum (H-E, ×20). D, Alopecia areata. Detail of the point where 2 shafts emerge from the same infundibulum (H-E, ×40). E, Alopecia areata. Evidence of a peribulbar inflammatory infiltrate. The hair shaft is seen to be broken (H-E, ×20). F, Alopecia areata. Broken hair shaft at the point where it emerges from the infundibulum (H-E, ×20). G, Alopecia areata. New hair follicle pushing the old one (H-E, ×20). H, Alopecia areata. Infundibulum filled with sebaceous secretion and keratin (H-E, ×20). I, Alopecia areata. Numerous yellow dots, together with an occasional dystrophic hair. A, Alopecia areata. Intense peribulbar inflammatory infiltrate (hematoxylin-eosin [H-E], ×100). B, Alopecia areata. Numerous circle hairs. Note also broken hairs and black dots. C, Alopecia areata. Peribulbar inflammatory infiltrate. Two hair shafts emerging from the same infundibulum (H-E, ×20). D, Alopecia areata. Detail of the point where 2 shafts emerge from the same infundibulum (H-E, ×40). E, Alopecia areata. Evidence of a peribulbar inflammatory infiltrate. The hair shaft is seen to be broken (H-E, ×20). F, Alopecia areata. Broken hair shaft at the point where it emerges from the infundibulum (H-E, ×20). G, Alopecia areata. New hair follicle pushing the old one (H-E, ×20). H, Alopecia areata. Infundibulum filled with sebaceous secretion and keratin (H-E, ×20). I, Alopecia areata. Numerous yellow dots, together with an occasional dystrophic hair.](https://static.elsevier.es/multimedia/00017310/0000011400000006/v1_202306061231/S0001731023003538/v1_202306061231/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![A, Psoriatic alopecia. Epidermal thickening involving the interpapillary ridges (hematoxylin-eosin [H-E], ×20). B, Psoriatic alopecia. Parakeratotic scaling (H-E, ×20). C, Psoriatic alopecia. In this biopsy specimen, the parakeratotic scales are located in the infundibulum (H-E, ×20). D, Psoriatic alopecia. Peripilar casts corresponding to infundibular parakeratotic scales eliminated by growth of the shaft (image courtesy of Dr. Nerea Landa). E, Psoriatic alopecia. The vessels of the papillary dermis appear tortuous and dilated. Those on the right lie in areas of marked suprapapillary epidermal thickening and are seen as twisted red loops, whereas those on the left lie under an epidermis with a slightly more preserved thickness and are seen in trichoscopy as simple red loops (H-E, ×40). F, Red dots (image courtesy of Dr. David Saceda). G, When the tortuous papillary capillary loops appear surrounded by parakeratotic scale, trichoscopy shows a very characteristic image (H-E, ×20). H, Tortuous vessels (image courtesy of Dr. David Saceda). A, Psoriatic alopecia. Epidermal thickening involving the interpapillary ridges (hematoxylin-eosin [H-E], ×20). B, Psoriatic alopecia. Parakeratotic scaling (H-E, ×20). C, Psoriatic alopecia. In this biopsy specimen, the parakeratotic scales are located in the infundibulum (H-E, ×20). D, Psoriatic alopecia. Peripilar casts corresponding to infundibular parakeratotic scales eliminated by growth of the shaft (image courtesy of Dr. Nerea Landa). E, Psoriatic alopecia. The vessels of the papillary dermis appear tortuous and dilated. Those on the right lie in areas of marked suprapapillary epidermal thickening and are seen as twisted red loops, whereas those on the left lie under an epidermis with a slightly more preserved thickness and are seen in trichoscopy as simple red loops (H-E, ×40). F, Red dots (image courtesy of Dr. David Saceda). G, When the tortuous papillary capillary loops appear surrounded by parakeratotic scale, trichoscopy shows a very characteristic image (H-E, ×20). H, Tortuous vessels (image courtesy of Dr. David Saceda).](https://static.elsevier.es/multimedia/00017310/0000011400000006/v1_202306061231/S0001731023003538/v1_202306061231/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![A and B, Tinea capitis. Fungal invasion of infundibular keratin (A, hematoxylin-eosin [H-E], ×20; B, H-E, ×400). C, Breakage of the shaft in a case of tinea capitis (H-E, ×20). D, Tinea capitis. Black dots. E, Tinea capitis. Numerous corkscrew hairs. F, Tinea capitis. Numerous comma hairs. G, Tinea capitis. Keratotic scale with very abundant fungal microorganisms (H-E, ×200). H, Tinea capitis. Peri- and interfollicular scales (image courtesy of Dr. Nerea Landa). A and B, Tinea capitis. Fungal invasion of infundibular keratin (A, hematoxylin-eosin [H-E], ×20; B, H-E, ×400). C, Breakage of the shaft in a case of tinea capitis (H-E, ×20). D, Tinea capitis. Black dots. E, Tinea capitis. Numerous corkscrew hairs. F, Tinea capitis. Numerous comma hairs. G, Tinea capitis. Keratotic scale with very abundant fungal microorganisms (H-E, ×200). H, Tinea capitis. Peri- and interfollicular scales (image courtesy of Dr. Nerea Landa).](https://static.elsevier.es/multimedia/00017310/0000011400000006/v1_202306061231/S0001731023003538/v1_202306061231/en/main.assets/thumbnail/gr5.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![A, Trichotillomania. Shafts broken at different levels. B, Trichotillomania. Melanin pigment deposit on the follicular shaft on the left. The 2 infundibula on the right appear dilated and with marked keratin content. C, Trichotillomania. Presence of hair powder in many infundibula. D, Trichotillomania. Trichoscopy shows some V hairs. E and F, Telogen effluvium. Hair follicles in telogen, together with empty hair follicles, visible both in vertical and in horizontal slices (E, hematoxylin-eosin [H-E], ×20; F, H-E, ×20). A, Trichotillomania. Shafts broken at different levels. B, Trichotillomania. Melanin pigment deposit on the follicular shaft on the left. The 2 infundibula on the right appear dilated and with marked keratin content. C, Trichotillomania. Presence of hair powder in many infundibula. D, Trichotillomania. Trichoscopy shows some V hairs. E and F, Telogen effluvium. Hair follicles in telogen, together with empty hair follicles, visible both in vertical and in horizontal slices (E, hematoxylin-eosin [H-E], ×20; F, H-E, ×20).](https://static.elsevier.es/multimedia/00017310/0000011400000006/v1_202306061231/S0001731023003538/v1_202306061231/en/main.assets/thumbnail/gr6.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)



