Distinguishing between congenital and infantile hemangiomas is challenging, but essential for appropriate treatment. The immunohistochemical marker glucose transporter type 1 is helpful, but biopsies are uncommon in this setting. The aim of this retrospective study was to describe and compare epidemiological, clinical, and treatment characteristics of congenital and infantile hemangiomas diagnosed at a tertiary care hospital over 3 years. We studied 107 hemangiomas: 34 congenital hemangiomas (rapidly involuting, partially involuting, and noninvoluting), 70 infantile hemangiomas, and 3 hemangiomas pending classification. Superficial infantile hemangiomas of the head and neck were the most prevalent tumors. Congenital hemangiomas were most often located on the trunk. Studied risk factors were more common in patients with infantile hemangiomas. In this group of patients, treatment response was independent of sex, in vitro fertilization, lesion depth and location, and type of treatment.
El diagnóstico diferencial clínico entre los hemangiomas congénitos (HC) y los infantiles (HI) es complicado pero esencial para el tratamiento. El marcador inmunohistoquímico GLUT-1 ayuda a distinguirlos, sin embargo, la biopsia no es habitual. Se diseñó un estudio retrospectivo incluyendo los HI y a los HC diagnosticados en un hospital terciario en un periodo de tres años, con el objetivo de describir y comparar los principales aspectos clínicos, epidemiológicos y terapéuticos. Se incluyeron un total de 107 hemangiomas, 34 HC (NICH/PICH/RICH), 70 HI y tres pendientes de clasificar. El HI superficial de cabeza y cuello fue el tumor más frecuente. El tronco fue la localización más frecuente de los HC. Los factores de riesgo estudiados fueron más frecuentes en el grupo de los HI. Para los HI, el tipo de respuesta obtenida fue independiente de las variables (sexo, fecundación in vitro, profundidad, localización y tipo de tratamiento).
Infantile hemangioma is the most common benign vascular tumor1 of childhood.2,3 It typically follows 3 sequential growth phases (rapid/proliferative, stable, and slow involution) and may sometimes cause cosmetic or functional impairment.2 If present, precursor lesions in infantile hemangioma can make it more difficult to distinguish these lesions from biologically distinct vascular tumors such as congenital hemangioma. The distinction, however, is crucial for proper treatment.1,2 The immunohistochemical marker GLUT-1, which is positive in all the development phases of infantile hemangioma and negative in congenital hemangioma, can help, but biopsy is usually reserved for selected cases in this setting.2,3 Diagnosis is based on proper clinical evaluation and imaging and is often delayed.
The main aim of this study was to describe and compare the main clinical, epidemiological, and treatment characteristics of infantile and congenital hemangiomas diagnosed in a tertiary care hospital.
Material and MethodsWe conducted a retrospective, observational study of patients diagnosed with infantile or congenital hemangioma in the Pediatric Dermatology Department of Hospital Universitario Dr. Peset in Valencia, Spain, between December 2019 and December 2022. In the absence of histopathology findings, hemangiomas were classified as congenital or infantile depending on a range of criteria, namely detection of the tumor during pregnancy, presence of a fully formed lesion at birth, and absence of a well-defined growth phase for congenital hemangiomas and absence of a lesion at birth, presence of a precursor lesion, and identification of clearly differentiated development phases for infantile hemangiomas. We collected information on hemangioma subtypes2,4,5 and characteristics; syndromic associations (PHACE [posterior fossa malformations, hemangioma, arterial anomalies, cardiac defects, eye anomalies] syndrome or LUMBAR [lower-body hemangioma, urogenital abnormalities, ulceration, myelopathy, bony deformities, and anorectal malformation] syndrome); sociodemographic data (including maternal risk factors and comorbidities); treatment strategies for infantile hemangiomas6 (no treatment, 0.5% timolol maleate ophthalmic solution,7 or propranolol 3.75mg/mL oral solution8,9); and treatment-related adverse effects. Oral therapy was used to treat large lesions, lesions on the face or in areas considered at risk for disfigurement, multiple lesions, and/or lesions with a risk of ulceration. Treatment response was categorized as no response (<30% clearance), partial response (30%–80% clearance), or complete response (80%–100% clearance). The characteristics analyzed were compared between the groups (infantile vs. congenital hemangioma) and with recent findings in the literature. Quantitative variables are expressed as mean (SD) and qualitative variables as percentages. A regression model was built for infantile hemangiomas to analyze the influence on treatment response of sex (male, female), tumor location (head and neck, trunk, upper extremities, lower extremities/perineum), tumor type (superficial, deep, mixed), conception through in vitro fertilization (IVF) or not, and type of treatment (propranolol, timolol). A P value of less than .05 was considered significant. The analyses were performed in IBM SPSS (version 21.0).
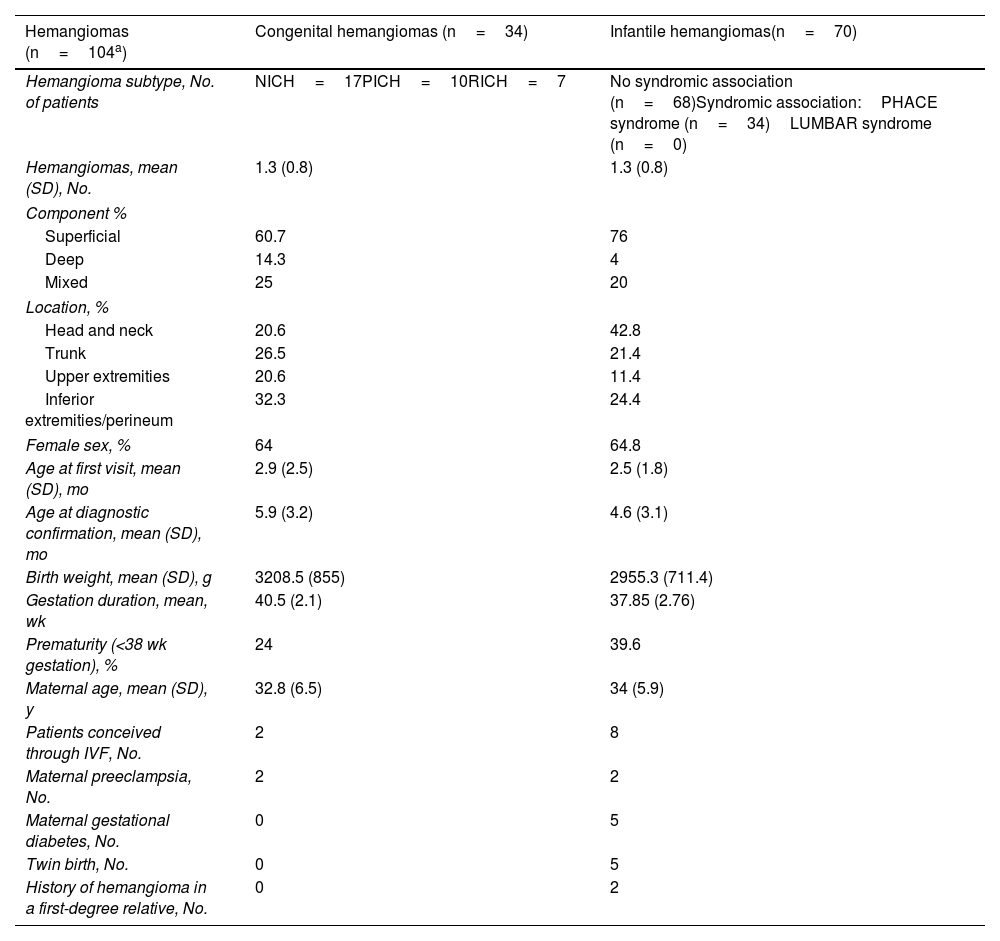
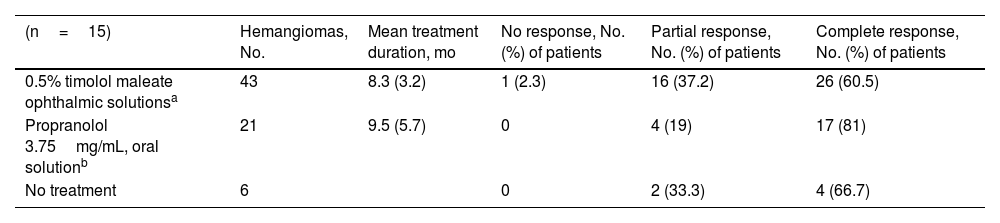
ResultsEighty-one patients (53 girls and 28 boys) with 107 hemangiomas (34 congenital and 70 infantile) were included. The mean (SD) number of hemangiomas per person was 1.3 (0.8). The diagnosis (congenital vs. infantile) could not be confirmed in 3 lesions because of loss to follow-up. The most common locations were the head and neck (42.8%) for infantile hemangiomas and the lower limbs/perineum for congenital hemangiomas (32.3%). Both tumors were more common in girls (64% for congenital hemangiomas and 64.8% for infantile hemangiomas). The main tumor and sociodemographic characteristics of each group are compared in Table 1. Apart from clinical examination, 55 patients (67.5%) underwent B-mode and Doppler ultrasound, and 10 of these (12.4% of the overall group) also underwent magnetic resonance imaging to examine centrofacial or anterior cervical lesions and/or to rule out dysraphism or associated brain alterations.2 Abdominal ultrasound was performed in 5 patients (6.2%) with multiple lesions; no liver lesions were detected.2 The correlation between first diagnostic impressions and diagnosis was 92.1%, with a mean time to confirmation of 2.7 (1.2) months. Gestational diabetes (6.2%) and maternal preeclampsia (4.9%) were among the most common maternal risk factors. Mean maternal age was 32.8 (6.5) years in the congenital hemangioma group and 34 (5.6) years in the infantile hemangioma group (Table 1). Eight patients with congenital hemangioma (23.5%) were treated with timolol after discussion with their parents, but none of them responded. None of the congenital hemangiomas were surgically treated. Six patients did not receive any treatment because of the characteristics of their lesions (size, growth, and/or location) or because their parents decided against this option. Sixty-four infantile hemangiomas were treated: 21 (32.8%) with propranolol and 43 (67.2%) with timolol. Mean treatment duration was 9.5 (5.7) and 8.3 (3.2) months, respectively. The treatment responses are summarized in Table 2.
Comparison of Clinical and Sociodemographic Characteristics and Associated Risk Factors Between Infantile and Congenital Hemangiomas.
| Hemangiomas (n=104a) | Congenital hemangiomas (n=34) | Infantile hemangiomas(n=70) |
|---|---|---|
| Hemangioma subtype, No. of patients | NICH=17PICH=10RICH=7 | No syndromic association (n=68)Syndromic association:PHACE syndrome (n=34)LUMBAR syndrome (n=0) |
| Hemangiomas, mean (SD), No. | 1.3 (0.8) | 1.3 (0.8) |
| Component % | ||
| Superficial | 60.7 | 76 |
| Deep | 14.3 | 4 |
| Mixed | 25 | 20 |
| Location, % | ||
| Head and neck | 20.6 | 42.8 |
| Trunk | 26.5 | 21.4 |
| Upper extremities | 20.6 | 11.4 |
| Inferior extremities/perineum | 32.3 | 24.4 |
| Female sex, % | 64 | 64.8 |
| Age at first visit, mean (SD), mo | 2.9 (2.5) | 2.5 (1.8) |
| Age at diagnostic confirmation, mean (SD), mo | 5.9 (3.2) | 4.6 (3.1) |
| Birth weight, mean (SD), g | 3208.5 (855) | 2955.3 (711.4) |
| Gestation duration, mean, wk | 40.5 (2.1) | 37.85 (2.76) |
| Prematurity (<38 wk gestation), % | 24 | 39.6 |
| Maternal age, mean (SD), y | 32.8 (6.5) | 34 (5.9) |
| Patients conceived through IVF, No. | 2 | 8 |
| Maternal preeclampsia, No. | 2 | 2 |
| Maternal gestational diabetes, No. | 0 | 5 |
| Twin birth, No. | 0 | 5 |
| History of hemangioma in a first-degree relative, No. | 0 | 2 |
IVF: in vitro fertilization; LUMBAR: lower-body hemangioma, urogenital abnormalities, ulceration, myelopathy, bony deformities, and anorectal malformation; NICH: noninvoluting congenital hemangiomas; PHACE: posterior fossa malformations, hemangioma, arterial anomalies, cardiac defects, eye anomalies; PICH: partially involuting congenital hemangiomas; RICH: rapidly involuting congenital hemangiomas.
Treatment Responses by Strategy in Patients with Infantile Hemangiomas.
| (n=15) | Hemangiomas, No. | Mean treatment duration, mo | No response, No. (%) of patients | Partial response, No. (%) of patients | Complete response, No. (%) of patients |
|---|---|---|---|---|---|
| 0.5% timolol maleate ophthalmic solutionsa | 43 | 8.3 (3.2) | 1 (2.3) | 16 (37.2) | 26 (60.5) |
| Propranolol 3.75mg/mL, oral solutionb | 21 | 9.5 (5.7) | 0 | 4 (19) | 17 (81) |
| No treatment | 6 | 0 | 2 (33.3) | 4 (66.7) |
No serious adverse effects were reported during the period evaluated. One girl with an ulcerated gluteal hemangioma10 required a combination of low-dose propranolol (1.5mg/kg/d) and vascular laser treatment and achieved a satisfactory cosmetic outcome. Following a complete cardiology examination, the 2 patients with PHACE syndrome were treated with propranolol 1mg/kg/d; the dosages were increased more slowly than usual11 up to a maximum of 2mg/kg/d. No significant differences were observed for the influence of sex, tumor depth, anatomic location, conception through IVF or not, or treatment on treatment response (supplementary material).
DiscussionThe clinical and sociodemographic characteristics of patients with congenital and infantile hemangiomas in our series are very similar to those reported in the literature, but with a notably high rate of congenital hemangiomas (31.8%). Although a white perilesional halo and thick telangiectasias have been traditionally described as an almost distinctive clinical mark of congenital hemangioma, in our series, these features were also present in infantile hemangiomas.2 Congenital and infantile hemangiomas were both more common in girls. This female predominance has been described for infantile hemangiomas and noninvoluting congenital hemangiomas, but not for rapidly or partially involuting hemangiomas, where sex-related differences have typically not been observed.4,5,12 An exclusively superficial component was most common in both congenital and infantile hemangiomas, while an exclusively deep component was more common in congenital hemangiomas (14% vs. 4%). The predominant locations were the head and neck for infantile hemangiomas (42.8%) and the lower extremities/perineum for congenital hemangiomas (32.3%). This second finding contrasts with previous reports showing that most hemangiomas, both congenital and infantile, are located on the head and neck.2,5 Mean age at the first visit was similar in both groups, but time to diagnostic confirmation was 1.3 (0.1) months longer in patients with congenital hemangiomas. In line with recent reports, low birth weight, premature birth, conception through IVF, advanced gestational age, and some of the maternal risk factors analyzed (preeclampsia, gestational diabetes) were more common in the infantile hemangioma group.2,12
Complete response rates for timolol and propranolol were slightly higher than those reported in clinical trials,6–8,13 possibly because we used a clearance rate of 80% to 100% to define complete response, and because patients in routine clinical settings are prescribed what is considered to be the most suitable treatment rather than being randomly allocated to a given treatment. Treatment response was independent of sex, location, conception through IVF or not, treatment type, and tumor depth. This contrasts with previous findings showing superior responses for superficial hemangiomas.6 One group of authors recently suggested that timolol combined with laser treatment might be more effective than timolol alone and might provide a similar level of effectiveness to that of propranolol.14 Atenolol15 and nadolol16 may be an alternative for patients intolerant of propranolol, with clinical trial data indicating that oral nadolol is noninferior to propranolol and may have a better safety profile. At our hospital, a patient with bronchial hyperreactivity who was not included in this series as his case occurred outside the follow-up time had a complete response to atenolol and optimal tolerance. Sirolimus has also been proposed as a potentially useful treatment for refractory infantile hemangioma.17 Timolol is ineffective in congenital hemangiomas and might even increase the likelihood of adverse effects. Finally, it has been suggested that GNAQ/GNAS11 mutations and alterations affecting related molecular pathways could be a target for future treatment.18
The main limitations of this study are its retrospective design and small sample.19 We have described the differences between the main characteristics of congenital and infantile hemangiomas at a tertiary care hospital and reviewed the latest evidence with the aim of aiding the distinction between these 2 tumors.
FundingNone declared.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We are grateful to Dr. Cecilia Alonso Díez for her help with the database design.