Vascular occlusion has multiple, diverse clinical manifestations, some of which can have grave consequences for patients. The causes of vascular occlusion are also highly variable, ranging from thrombi triggered by the uncontrolled activation of coagulation mechanisms, on the one hand, to endothelial dysfunction or occlusion by material extrinsic to the coagulation system on the other. In a 2-part review, we look at the main causes of vascular occlusion and the key clinical and histopathologic findings. In this first part, we focus on vascular occlusion involving thrombi.
La patología vascular oclusiva es causante de diversas y variadas manifestaciones clínicas, algunas de las cuales son de catastróficas consecuencias para el paciente. Sin embargo, las causas de tal oclusión son muy variadas, extendiéndose desde trombos por acción descontrolada de los mecanismos de coagulación, hasta anomalías de los endotelios de los vasos u oclusión por materiales extrínsecos. En una serie de dos artículos hacemos una revisión de las principales causas de oclusión vascular, resumiendo sus manifestaciones clínicas principales y los hallazgos histopatológicos fundamentales. Esta primera parte corresponde a las oclusiones vasculares que cursan con trombos.
Blood vessels become occluded for diverse reasons, some intrinsic to the patient, others not.
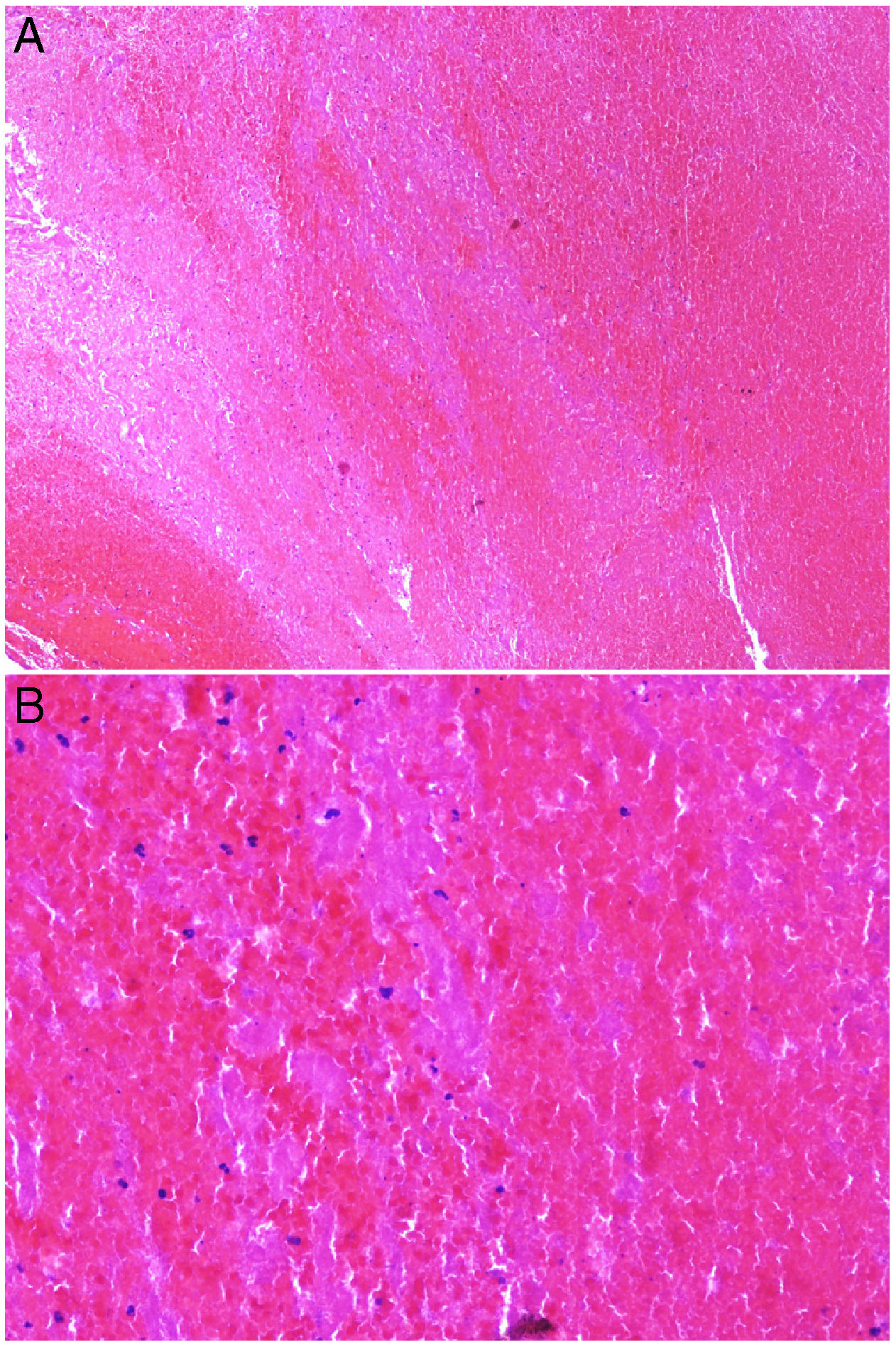
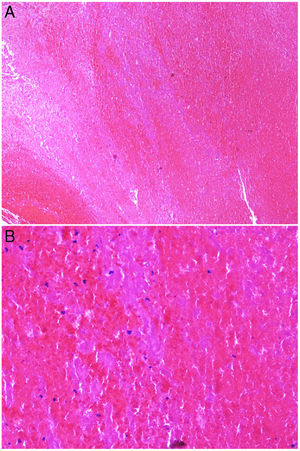
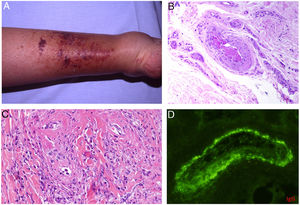
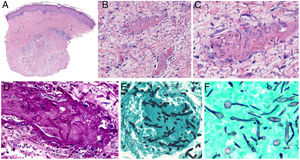
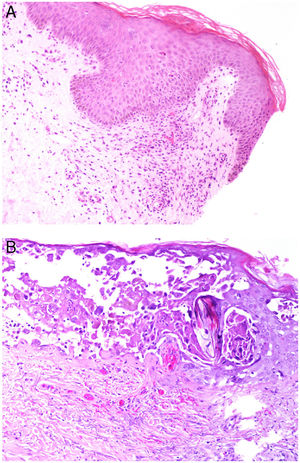
Intrinsic causes include coagulation disorders. Coagulation is a natural, physiological process whose purpose is to form an occlusive clot at even small points of rupture in blood vessels to prevent continued bleeding and exsanguination. Hemostasis is achieved through normal clotting to patch a vessel wall while a defect is repaired. However, various abnormalities can lead to an exaggerated hemostatic response that causes the aggregation of platelets, fibrin, cells and/or coagulation factors that may partially or completely occlude the lumen, the phenomenon we call a thrombus. Thrombi that are rich in fibrin and platelets are lighter colored than those with cellular components. However, thrombi generally tend to have alternating lighter/whitish layers and darker reddish ones, forming lines of Zahn (Fig. 1, A-B). Platelet presence in a thrombus can be studied by immunohistochemical staining with antibodies against the platelet marker glycoprotein CD61.
A, Alternating layers within a thrombus. The darker red layers rich in red blood cells (RBCs) alternate with lighter fibrin-rich areas. Hematoxylin-eosin, magnification ×40. B, The red areas replete with RBCs and lighter-colored fibrin-rich areas at higher magnification. Hematoxylin-eosin, magnification ×200.
Thrombosis can be caused by a number of conditions, among them a hypercoagulable state, endothelial damage in vessel walls, and immunoglobulin or antigen-antibody complex deposition.
Also relevant is the possibility of occlusion by foreign bodies or material unrelated to coagulation. They may be organic or inorganic. Even parasites living in the circulatory system may be responsible.
This article on thrombi is the first in a 2-part series that summarizes the main causes of vascular occlusion and reviews the clinical and histopathologic signs of thrombosis.
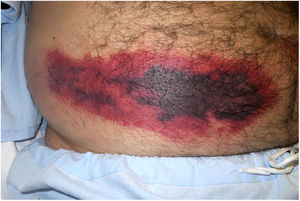
Platelet-Rich RhrombiHeparin-induced necrosisDefinition: Heparin-induced necrosis is a rare condition secondary to subcutaneous or intravenous administration of heparin, especially in unfractionated forms. An immune complex of platelet factor 4 and heparin forms at the site of injection, leading to platelet activation, vascular occlusion and thrombocytopenia secondary to platelet consumption.1
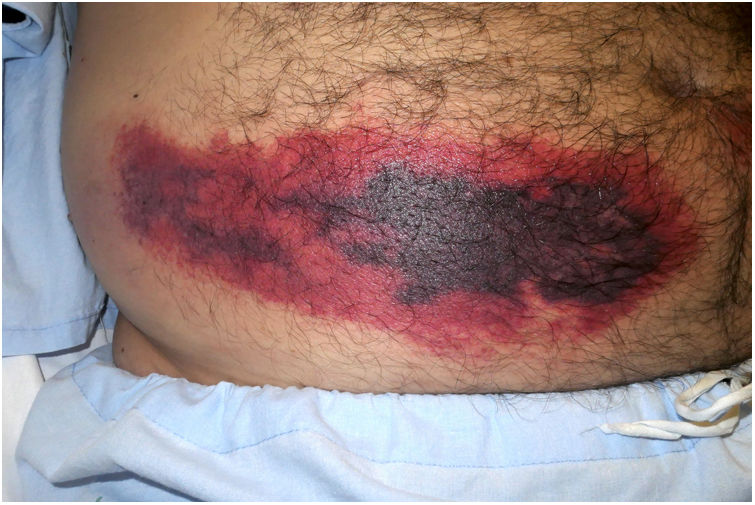
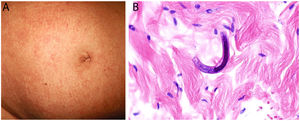
Clinical presentation: This condition manifests as painful patches of retiform purpura with necrotic centers at the point of heparin injection (Fig. 2) and a 50% decrease in the platelet count.1 Treatment consists of discontinuing heparin therapy and using an alternative anticoagulant. Vitamin K antagonists are contraindicated.
Histopathology: Findings include non-inflammatory occlusive vasculopathy with fibrin thrombi inside dermal and subcutaneous arterioles and venules along with variable degrees of bleeding in tissues and signs of secondary ischemic skin necrosis.
Thrombocytosis secondary to myeloproliferative disordersDefinition: Thrombosis secondary to myeloproliferative disorders is defined by the presence in blood of a large number of platelets, often dysfunctional ones. The myeloproliferative disorder responsible is usually essential thrombocythemia or polycythemia vera.2,3
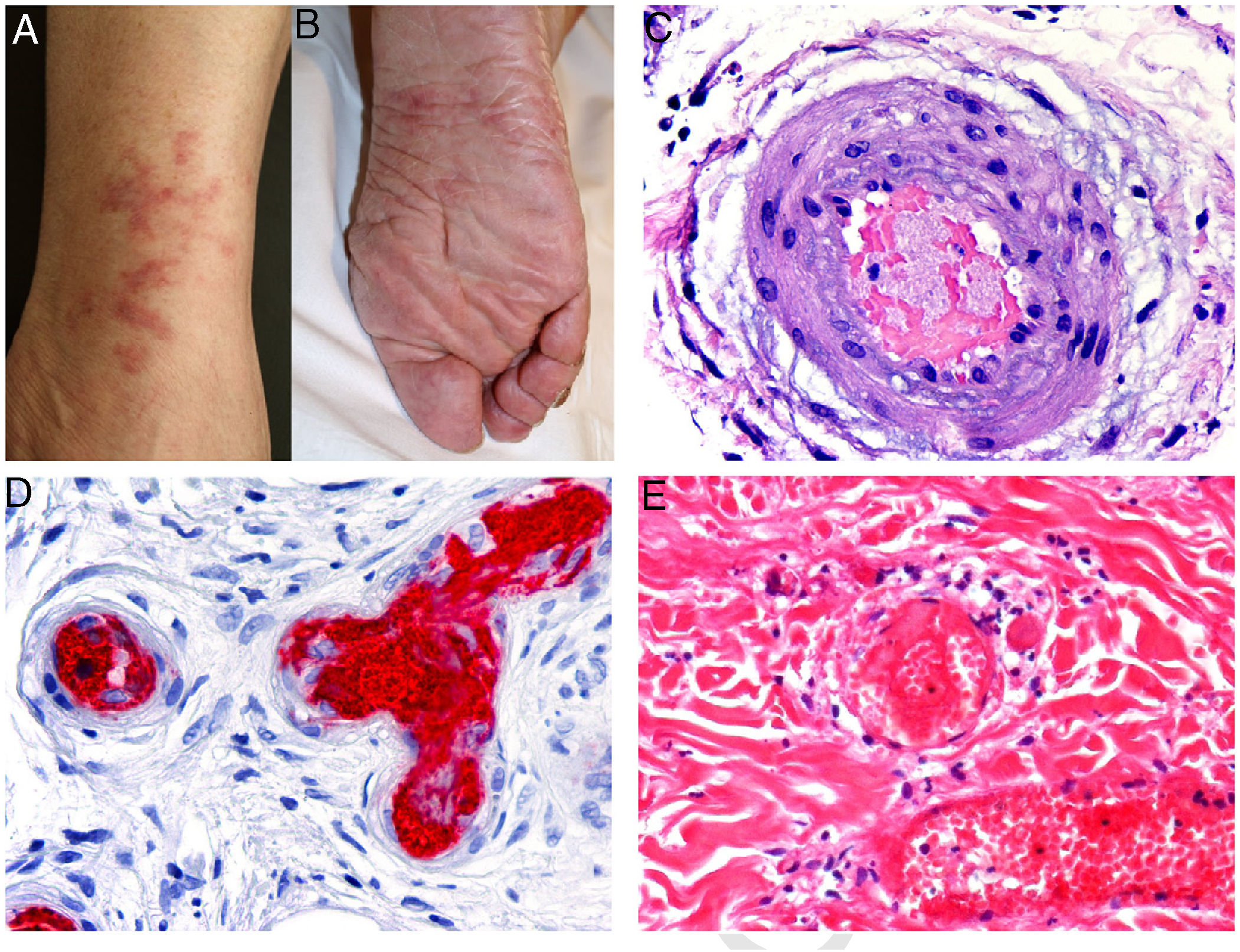
Clinical presentation: Lesions may be hemorrhagic (petechiae, purpura, hematomas) or occlusive (livedo racemosa, livedo reticularis, erythromelalgia, erythematous plaques, Raynaud phenomenon, ulceration, or gangrene). Cutaneous manifestations occur in 10% of cases and may be the first clinical signs of essential thrombocythemia vera4 (Fig. 3, A-B).
A and B, Livedo reticularis lesions on the lower leg and sole, a presentation of essential thrombocytopenia in a 77-year-old woman with no noteworthy medical history. The blood work-up showed thrombocytosis (919 000 platelets/mm3). We thank Drs Rosario Haro and Luis Requena (Dermatology Department, Fundación Jiménez Díaz, Madrid) for the photographs. C, In essential thrombocytopenia, a thrombocytosis secondary to myeloproliferative disorders, arteriolar occlusion is due to the presence of granular material and red blood cells. Hematoxylin-eosin (H&E), magnification ×40. D, Also in essential thrombocytopenia, immunostaining for CD61 confirms vascular occlusion by thrombocytes. Magnification ×40. E, In paroxysmal nocturnal hemoglobinuria, platelet and fibrin thrombi can be seen in dermal vessels; scant perivascular lymphocytic infiltrate. H&E, magnification ×400.
Histopathology: Extravasation of red blood cells (RBCs) can be seen in hemorrhagic lesions. In vascular occlusion platelets are abundant, accounting for most of the material occluding the lumen5 (Fig. 3, C-D).
Paroxysmal nocturnal hemoglobinuriaDefinition: Paroxysmal nocturnal hemoglobinuria (PHN) is a nonmalignant acquired X-linked disease involving expansion of a hematopoietic stem cell clone on the phosphatidylinositol glycan class A gene (PIG-A), which induces a rare sensitivity of these cells to lysis by complement activity.6
Clinical presentation: PHN is a chronic condition with hemolytic crises that can be triggered by such events as a common infection, vaccination, surgery, or certain antibiotics. The incidence is 15.9 cases/100 000 population. No sex-related differences have been found. Patients with PNH are usually young adults around 35 years of age, although onset may occur at any time. The usual clinical signs are pallor; fatigue; dyspnea on exertion; intravascular hemolytic anemia (hemoglobinuria at onset in 26% and in 62% at some point in disease progression). Thrombosis is common (in 30% to 40%, with occasional deep vein thrombosis, especially of suprahepatic veins (Budd-Chiari syndrome). Mesenteric, central nervous system, and cutaneous vasculature can be affected. Arteries are occasionally involved (in 15%). Bone marrow function may also become impaired, leading to severe pancytopenia (in 32%). Mortality is around 24% at 10 years. Death is usually due to thrombotic complications (in 40% to 60%) and hemorrhagic ones. There have been cases of spontaneous recovery, but survival averages around 22 years.
Skin lesions associated with PHN are rare. When present, they are generally found in acral locations: soles of the feet, fingers and toes, the nose, and the external ear. They may consist of petechiae, hemorrhagic blisters, leg ulcers, pyoderma gangrenosum, or noninflammatory purpura retiform that may progress to necrosis.7–9 PHN-associated cutaneous necrosis occurs in 0.6% of cases, possibly explained by cases of parvovirus B19 infection, which have been reported during phases of hemolytic anemia in these patients.10
Histopathology: Findings in skin biopsies from patients with PNH reveal it to be an occlusive vascular disease without vasculitis.11 Intraluminal thrombi, whose origin is uncertain, are probably related to increased platelet aggregation. Depending on the degree of intraluminal compromise of cutaneous vasculature, there are usually morphologic epiphenomena mainly evident in skin necrosis (Fig. 3E).
Thrombotic thrombocytopenic purpura and hemolytic uremic syndromeDefinition: Both thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS) predispose to thrombosis. Although TTP and HUS are believed to be related, their mechanisms of thrombus formation are different.
Clinical presentation: Capillary and arteriolar thrombosis in different organs accounts for the symptoms that accompany hemolytic anemia and thrombocytopenia. Kidney involvement predominates in HUS whereas the central nervous system is usually involved in TTP. Petechiae may form in the skin.
Histopathology: Intraluminal thrombi are found in both TTP and HUS. Overlying ulcers may appear if occlusion is severe enough, but as the thrombogenic mechanism is different in each disorder, subtle differences in morphology can be observed.
Thrombosis in TTP is due to the presence of ultralarge von Willebrand multimers in plasma with insufficient protease to break them down, resulting in platelet consumption and thrombopenia. The microthrombi are therefore platelet rich and fibrin poor (whitish), and perivascular inflammation is minimal or absent.12
In contrast, HUS causes primary damage to the endothelium, and thrombosis is secondary. Affected vessels show focal endothelial detachment. The thrombi are fibrin rich (red) and may be accompanied by a perivascular inflammatory infiltrate.12,13
Thrombi Caused by Cold-Induced AgglutinationCryoglobulins are proteins that precipitate at low temperatures and dissolve when warmed. They usually appear in acral regions. Most cryoglobulins are immunoglobulins.
Cold agglutinins are autoantibodies that function optimally at low temperatures, generally below 37°C. Most are immunoglobulin (Ig) type M molecules, which anomalously recognize antigens on RBCs.
CryoglobulinemiaDefinition: Cryoglobulinemia is a rare thrombosing vasculitis and/or a vasculitis secondary to circulating cryoglobulins, the immunoglobulins that precipitate at cold temperatures. The cause is usually hepatitis C virus (HCV) infection, an autoimmune disorder, or a B-cell type lymphoid neoplasm.
Clinical presentation: Three types of cryoglobulinemia have been distinguished, corresponding to the mono- and/or polyclonal nature of the immunoglobulins deposited.14
Monoclonal type I cryoglobulinemia is caused by κ or λ light-chain cryoglobulins produced mainly by well-differentiated B-cell lymphomas or myelomas.
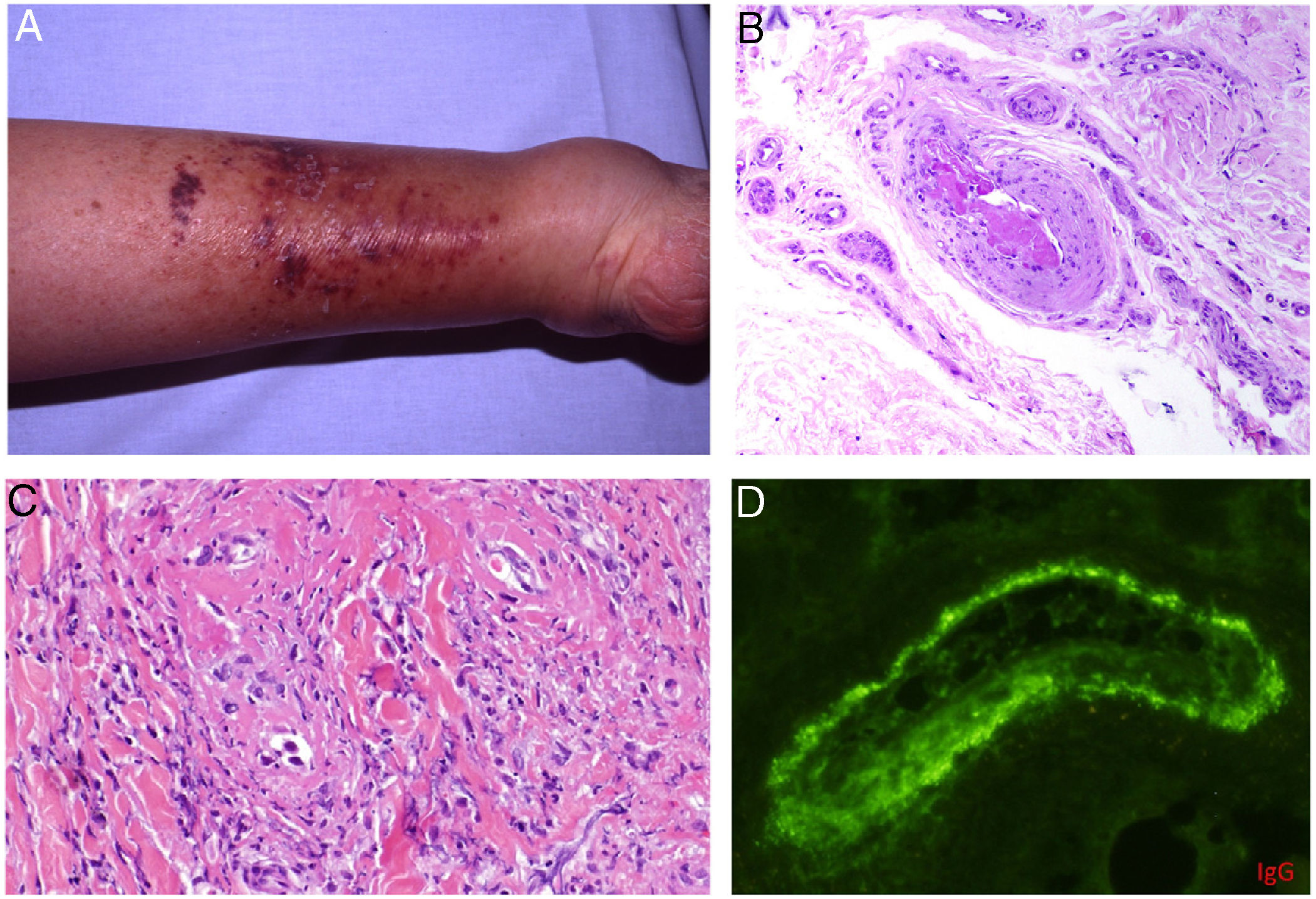
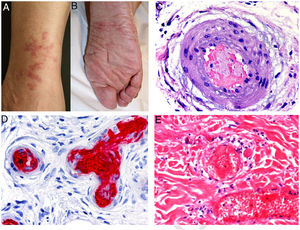
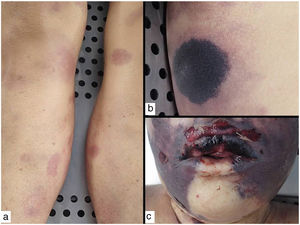
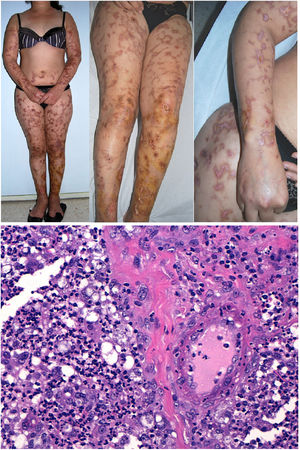
Types II and III (also called mixed cryoglobulinemias) are due to monoclonal IgM antibodies or polyclonal IgG antibodies, respectively, which form circulating immune complexes.15 The usual causes are HCV infection, autoimmune diseases, or occasionally B-cell lymphoid neoplasms.16 Only 8% of these cryoglobulinemias produce symptoms, which consist of purpura, muscle weakness, and joint pain. The kidney may be involved, causing proteinuria, hematuria, and edema, as well as neuropathy due to the involvement of the vasa nervorum. Skin lesions develop typically on the lower extremities in the form of purpura tending to ulcers and discoloration (Fig. 4A).
A, Cryoglobulinemia with multiple purpuric papules near the ankle, with hemorrhagic skin discoloration. B, Type I cryoglobulinemia, showing an intravascular hyaline mold partially occluding the vessel. Hematoxylin-eosin, magnification ×100. C, In mixed cryoglobulinemia, a skin sample shows vessels with fibrinoid necrosis in the reticular dermis, vascular subocclusion, leukocytoclasia, and endothelial necrosis. Hematoxylin-eosin, magnification ×200. D, In cryoglobulinemia, immunoglobulin G deposits are detected in the inflamed vessel wall. Direct immunofluorescence, magnification ×200.
Histopathology: No pathognomonic histopathologic findings have been identified for cryoglobulinemia.
In type I disease, hyaline thrombi, molded to resemble the affected vessel, can be observed in the lumina of small dermal blood vessels (Fig. 4B). Periodic acid-Schiff (PAS) can be used to stain the glucosic component of the precipitated immunoglobulins in deposits.
In types II and III cryoglobulinemias, necrotizing vasculitis accompanied by bleeding and thrombosis can be observed. Leukocytoclasia is sometimes present (Fig. 4C). Immunofluorescence assays can reveal immunoglobulin and complement deposition (Fig. 4D).
Correlation between clinical signs and pathology and the identification of serum cryoglobulins will be essential for the differential diagnosis of cryoglobulinemia-associated vasculopathy. PAS-positive findings in deposits are useful for distinguishing type I cryoglobulinemia from other thrombus-forming vascular diseases. Unlike leukocytoclastic vasculitis, cryoglobulinemias do not produce glomerular aggregation. In mixed-type cryoglobulinemias it is very important to correlate clinical signs and pathology to distinguish between vasculitis associated with infections, medications, or autoimmune diseases not involving cryoglobulins.
CryofibrinogenemiaDefinition: This fibrin-fibrinogen complex precipitation disorder occurs at low temperatures. In contrast to cryoglobulins, the complexes precipitate in plasma rather than in serum. Therefore, detection requires extracting blood into tubes containing oxalic, ethylenediaminetetraacetic, or citric acid. The extracted blood must be centrifuged immediately at a temperature of 37°C and then refrigerated at 4°C for 72hours. Cryofibrinogenemia may be a primary condition or secondary to infection, connective tissue disease, or above all, to hematologic diseases.17
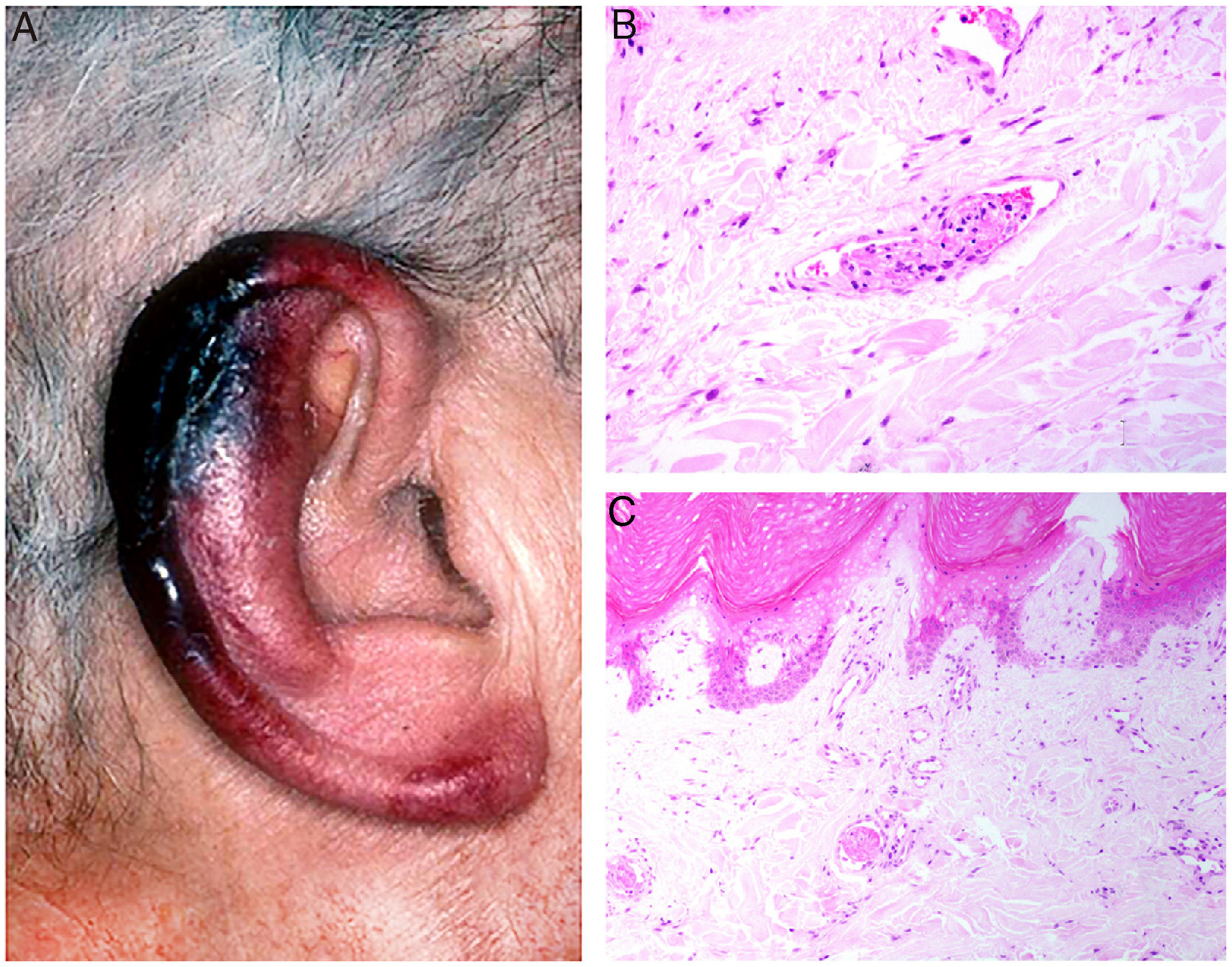
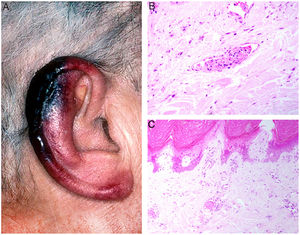
Clinical presentation: Presentations include ulcers, Raynaud phenomenon, or purpuric retiform lesions. These signs may be accompanied by general symptoms, even fever. Cold exposure triggers the condition. The lower extremities and acral areas, such as the ears (Fig. 5A), are preferentially affected.18 The correlation between the severity of skin lesions and the concentration of the cryoprecipitate is especially clear when fibrinogen is a component.18
A, In cryofibrinogenemia, erythematous-purpuric lesions have formed on the outer ear, especially the lobe and helix, where necrosis has developed. B, In cold agglutinin disease, intraluminal thrombi are aggregates of red blood cells, fibrin, and polymorphonuclear neutrophils. Hematoxylin-eosin (H&E), magnification ×200. C, Necrosis of the overlying epidermis and occlusion of vessels in the superficial dermis in cold agglutinin disease. H&E, magnification ×100.
Histopathology: Findings in cryofibrinogenemia and cryoglobulinemia overlap: both show homogeneous, eosinophilic, PAS-positive intraluminal material. Dermal necrosis may be present. When frank leukocytoclastic vasculitis is also found, both entities may coexist.18 The ischemia that results may induce reactive angioendotheliomatosis.19
Cold agglutinin diseaseDefinition: Cold agglutinin disease develops when the agglutinins react with the surface of RBCs at low temperatures. These cold-sensitive antibodies may be mono- or polyclonal.
Clinical presentation: Manifestations may be more or less severe depending on the degree of complement activation. They range from hemolytic anemia due to intravascular hemolysis (which is usually self-limited) to signs of acral ischemia (acrocyanosis). Other findings include hemoglobin in urine and fatigue attributable to anemia.
Histopathology: Vessels are replete with RBCs, especially in the superficial dermis. Thrombi formed of fibrin and neutrophils are also found19 (Fig. 5B). If ischemia develops, a wedge-shaped area of necrosis can be seen in the dermis or epidermis (Fig. 5C). There may be reactive vascular proliferation, with dilated vessels and intraluminal capillaries. Vascular proliferation may take a glomerular pattern in such cases.19
Infection-Induced ThrombosisEcthyma gangrenosumDefinition: Ecthyma gangrenosum is a potentially lethal, deep skin infection that most often occurs as a complication of infection by Pseudomonas aeruginosa in immunocompromised individuals, especially in conjunction with neutropenia. Primary ecthyma gangrenosum occurs less often and may or may not be associated with secondary septicemia. P aeruginosa produces toxins and enzymes (eg, exotoxin A, elastase, and phospholipase C) that destroy vascular and cutaneous tissues.20 However, even though P aeruginosa is the most common culprit, identical clinical pictures have been described in infections by other microorganisms.21,22
Clinical presentation: Lesions, which may be single or multiple, progress rapidly over a period of 2 to 5 days. They begin as erythematous macules that rapidly become hemorrhagic bullae, which soon burst and form characteristic gangrenous ulcers with hard, blackish centers encircled by an erythematous border.23 These ulcers are usually found in the axillae or anogenital region, or on the trunk or extremities. Rarely, they may form on the face (Fig. 6). Lesions secondary to sepsis are usually multiple and worsen the prognosis. Single lesions, which are usually the result of a primary skin infection, are typically found in intertriginous areas. The prognosis in these cases is less adverse. Early diagnosis and start of antibiotic therapy are of vital importance.24,25
Photographs taken during an autopsy of a patient with severe neutropenia and ecthyma gangrenosum secondary to sepsis due to Pseudomonas aeruginosa. The lesions shown are in various stages of the disease: A, Initial erythematous macules on the legs. B, A more advanced lesion with a blackish center and an erythematous border. C, Necrotic bullae on the face.
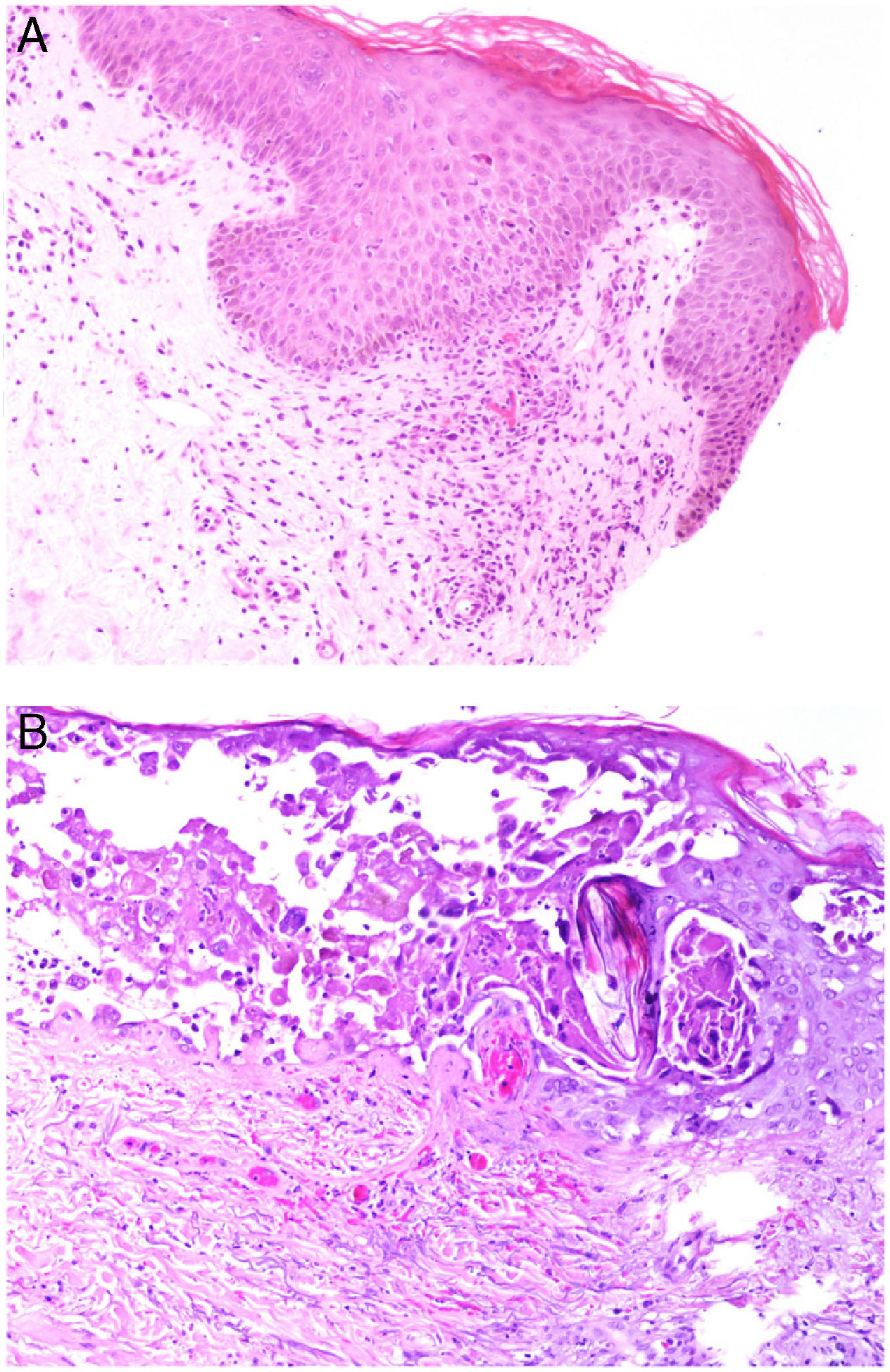
Histopathology: Findings may include a subepidermal hemorrhagic bulla (Fig. 7A) or epidermal necrosis with ulceration and a fibrinous purulent base. Hemorrhagic and necrotic areas can be seen in the dermis and hypodermis; a finding of an inflammatory infiltrate is not common due to neutropenia in many patients.26
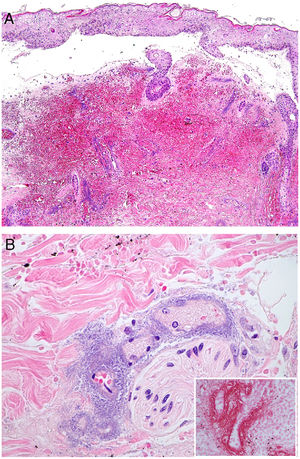
A, In ecthyma gangrenosum, cutaneous infarction with epidermal separation, subepithelial edema, and marked extravasation of red blood cells in the dermis. Also evident is the basophilic aspect of vessel walls in the dermis. Hematoxylin-eosin (H&E), magnification ×10. B, Vessel walls appear hazy because they have been replaced by basophilic material, which destroys the wall; scant inflammatory infiltrate. The detail shows that the material consists of gram-negative bacilli corresponding to Pseudomonas aeruginosa. H&E, magnification ×40.
A hazy basophilic infiltration in the vessel wall is a characteristic finding that reflects an accumulation of gram-negative bacteria (Fig. 7B). The bacilli affect mainly the vascular adventitia and media, but not the intima. Occasionally there is thrombosis. Some bacilli may also be seen dispersed among dermal collagen bundles or hypodermal fat cells.24,26
At low magnification, the basophilic appearance of small vessels can be confused with calciphylaxis, which can have a similar clinical picture.27
Angioinvasive fungal infections of the skinDefinition: Angioinvasive fungi cause vascular occlusion due to thrombosis, followed by ischemia and/or necrosis; besides the skin, many organs (especially the brain and the lungs) can be affected.28 The main angioinvasive fungi are Aspergillus, Fusarium and Candida species, or Rhizopus, Mucor, and Lichtheimia species in the order Mucorales.29
These fungi infect patients with neutropenia (due to blood neoplasms or chemotherapy, or after organ transplantation). While systemic aspergillosis and fusariosis are diagnosed nearly exclusively in such patients, they may also harbor candidiasis and invasive infections by the 3 Mucorales fungi. Other populations at risk are low-birth-weight infants (due to immaturity of the epidermis and intestinal mucosa); patients on total parenteral nutrition, wide-spectrum antibiotics, or transfusions; patients with burns; and intravenous drug users. Mucorales fungi have a particular tendency to infect patients with poorly controlled diabetes, especially those with ketoacidosis since a low plasma pH interferes with the phagocytic function of neutrophils.
Clinical presentation:Aspergillosis species infection: Cutaneous signs appear in 5% of patients with systemic aspergillosis. Angioinvasion rapidly leads to necrosis and bleeding. The resulting lesions are similar to those of ecthyma gangrenosum: necrotic papules and nodules and/or deep subcutaneous nodules with or without a central eschar. Primary cutaneous aspergillosis is less common and less aggressive than infection-induced secondary forms, in which mortality is high. Primary forms present as macules, papules, or erythematous-violaceous plaques that may progress to hemorrhagic bullae and ulcers.
Fusarium species infection: Cutaneous lesions appear in 75% of immunocompromised patients.30 These fungi are the most common cause of blood infections with cutaneous manifestations in the form of bullae or erythematous-violaceous, necrotic, or target lesions. Three well-defined forms have been described: 1) multiple subcutaneous nodules; 2) lesions similar to ecthyma gangrenosum; and 3) painful erythematous macules-papules with ulceration and/or blackish scarring that appear preferentially on the arms and legs. The course of systemic infections includes fever and the development of lung infiltrates and/or sinusitis. Mortality is 40% in fusariosis with skin involvement.
Candida species infection: Fever, muscle pain, and varying degrees of visceral organ involvement have been described. Cutaneous lesions are present in 13% to 38% of patients. They include papules, pustules, and nodules as well as nonspecific lesions. An erythematous halo is often seen, sometimes surrounding a bulla or pale center. Folliculitis has been described in drug addicts.
Mucorales fungal infections: There is skin involvement in 20% to 25% of patients. Primary cutaneous forms (associated with surgery, burns, or injuries) are more common than forms secondary to other diseases. Primary forms manifest as necrotic indurated eschars and erythema. They may produce target or purpuric lesions. Secondary forms may manifest as erythematous macules, nodules, or necrosis similar to ecthyma gangrenosum. Lung and/or paranasal sinus infections may spread to the orbit or the brain. Mortality is high at 45% and is even higher when there is systemic disease.
Histopathology: Dermal and/or subcutaneous microabscesses can be observed. Depending on the patient’s immune state, there may be variable presence of neutrophils, macrophages (with or without multinucleated cells), and necrosis. Fungi can be seen in vessel walls and lumina, habitually with thrombosis and bleeding.
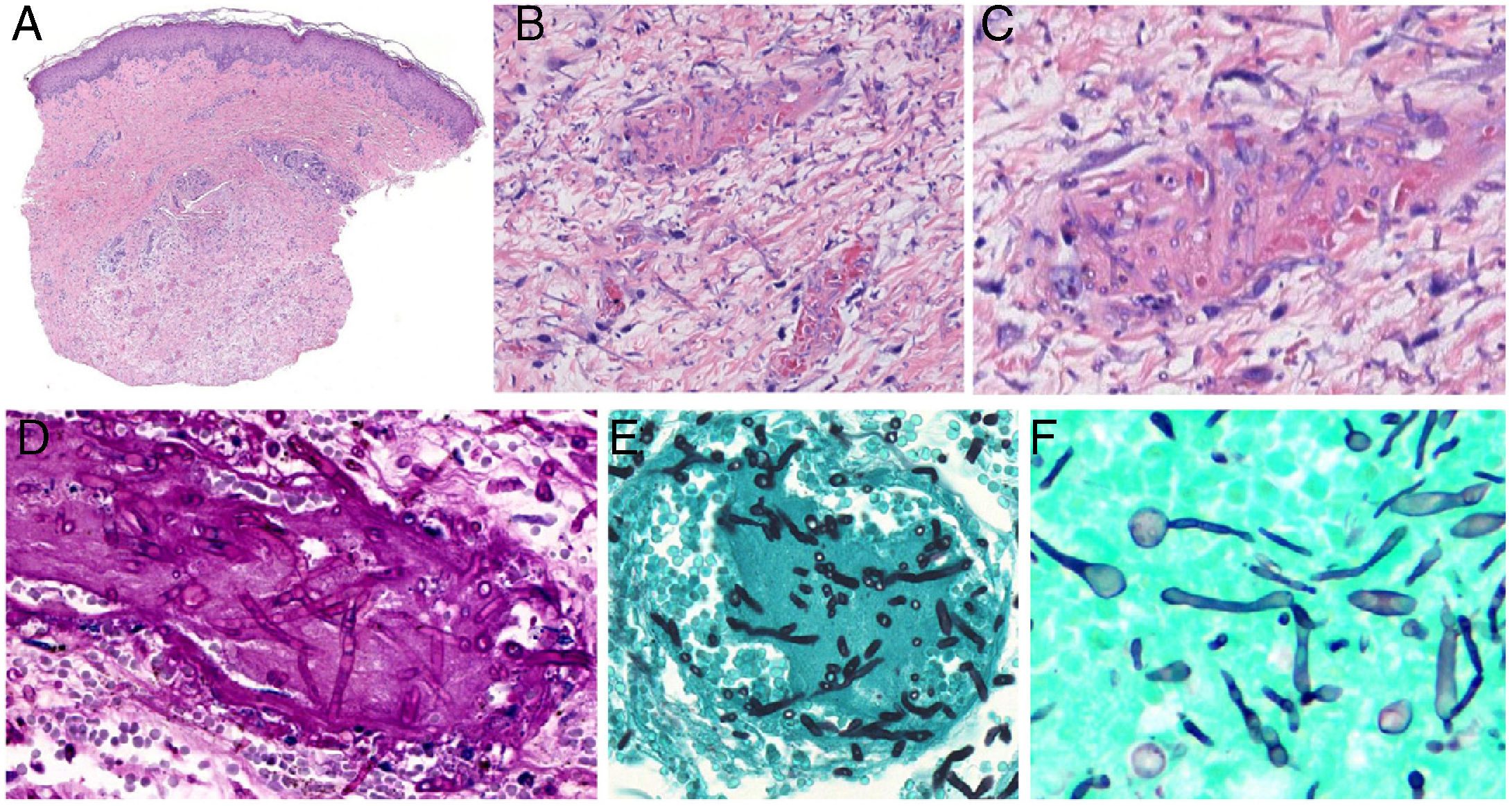
Aspergillosis: Septate hyphae branching at acute-angles (typically at 45º) are present. They measure 3 to 6μm. Inflammation is usually scant (Fig. 8, A-C).
Angioinvasive fungal infections of the skin. A–C, Angioinvasive cutaneous aspergillosis: branching septate hyphae occlude dermal vessels and cross the vessel wall; hardly any inflammatory component is present. Hematoxylin-eosin; magnifications ×20 (A), ×200 (B), and ×400 (C). D and E, Angioinvasive fusariosis: the hyphae are morphologically indistinguishable from those seen in aspergillosis. Periodic acid-Schiff, magnification ×400 (D); methenamine-silver, magnification ×400 (E). F, Invasive candidiasis: note the presence of yeasts and pseudomycelia. Methenamine-silver, magnification ×600.
Fusariosis: Fusarium species infection is histologically indistinguishable from aspergillosis: septate, nonpigmented hyphae branch at acute angles (Fig. 8 8, D-E). These findings can only be seen in cutaneous samples by using in-situ hybridization of polymerase chain reaction (PCR).
Candidiasis: These yeast infections, with or without germination, show mycelia or pseudomycelia (Fig. 8F). Half of cultures from biopsy samples are positive, as are blood cultures.
Mucorales infections: Thick hyphae (3 to 25μm in diameter) are observed in these infections. The hyphae are not septate, though they may appear so and are sometimes described as pseudoseptate. The fungal cell wall is thin, and PAS or silver stain uptake is weak. Therefore, fluorescence with calcofluor-white dye can be used. Perineural invasion can very often be seen in addition to angioinvasion.
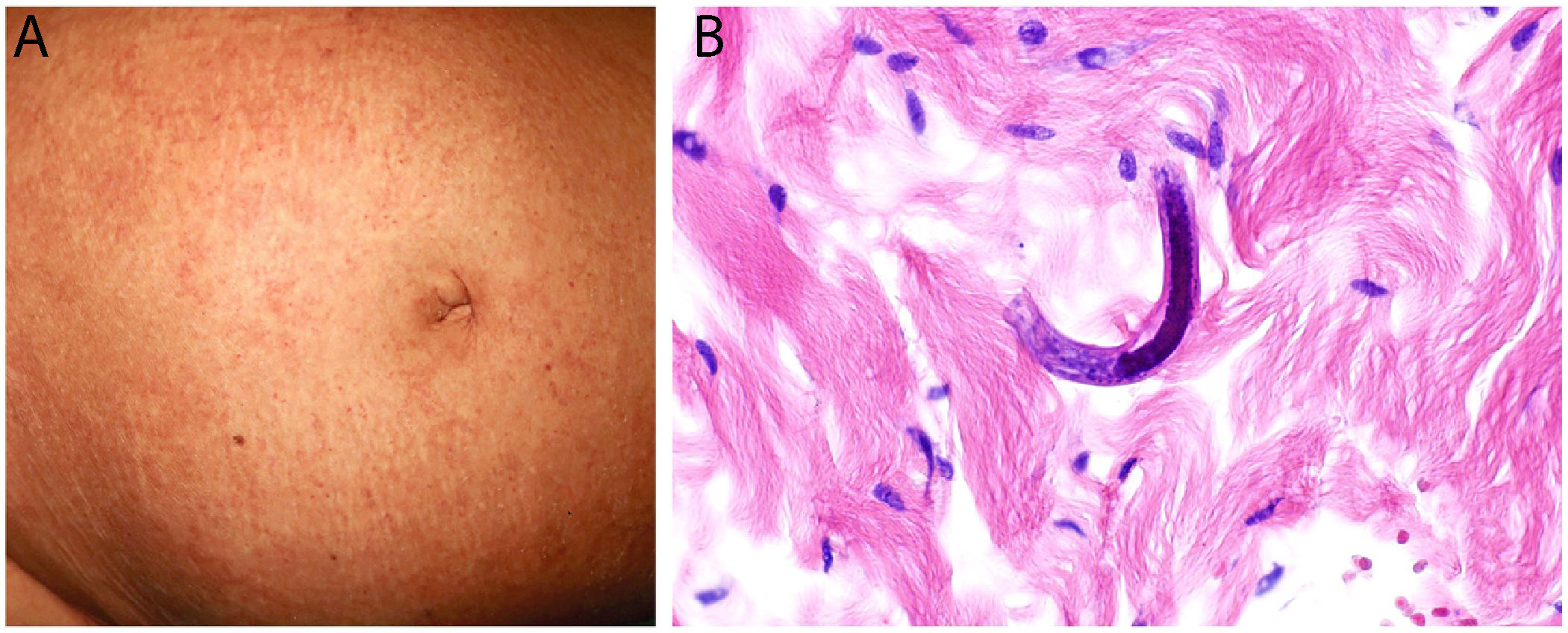
Disseminated strongyloidiasisDefinition: The culprit — the intestinal nematode Strongyloides stercoralis — is endemic in tropical and subtropical countries. This parasite enters humans by crossing the skin barrier from soil, where the filariform larvae reside. The larvae then migrate to the lungs and eventually take up position in the small intestine.31
Clinical presentation: The host experiences mild, nonspecific symptoms such as gastrointestinal complaints and eosinophilia. The most common skin sign is larva currens, a rapidly progressing serpiginous rash. Disseminated strongyloidiasis, which can develop in immunosuppressed individuals and is associated with high mortality, manifests as disseminated purpura, characteristically around the navel (Fig. 9A) and on thighs.31,32 The rash often traces a linear, digitiform pattern.33
Histopathology: Biopsied tissue reveals a perivascular, slightly eosinophilic inflammatory infiltrate. If larvae are not readily visible, several samples should be taken to rule out their presence. Visible larvae measure between 9 and 15μm in diameter and are usually found among dermal collagen bundles, although they can sometimes be seen in blood vessels32,34 (Fig. 9B).
Lucio phenomenonDefinition: Lucio phenomenon is a type-2 leprosy reaction, though some classify it as type 3.35 This phenomenon develops in the context of lepromatous leprosy, particularly in its diffuse form, and is known in Spanish as lepra bonita (pretty leprosy). It consists of a necrotizing vasculitis of small- and medium-caliber vessels in the superficial and deep reticular dermis of patients with diffuse lepromatous leprosy.36 Immune complexes are believed to appear on exposure to the release of large numbers of circulating antibodies against mycobacterial protein antigens. A new bacillus, Mycobacterium lepromatosis was recently isolated in these patients and is believed to be responsible for the phenomenon.37
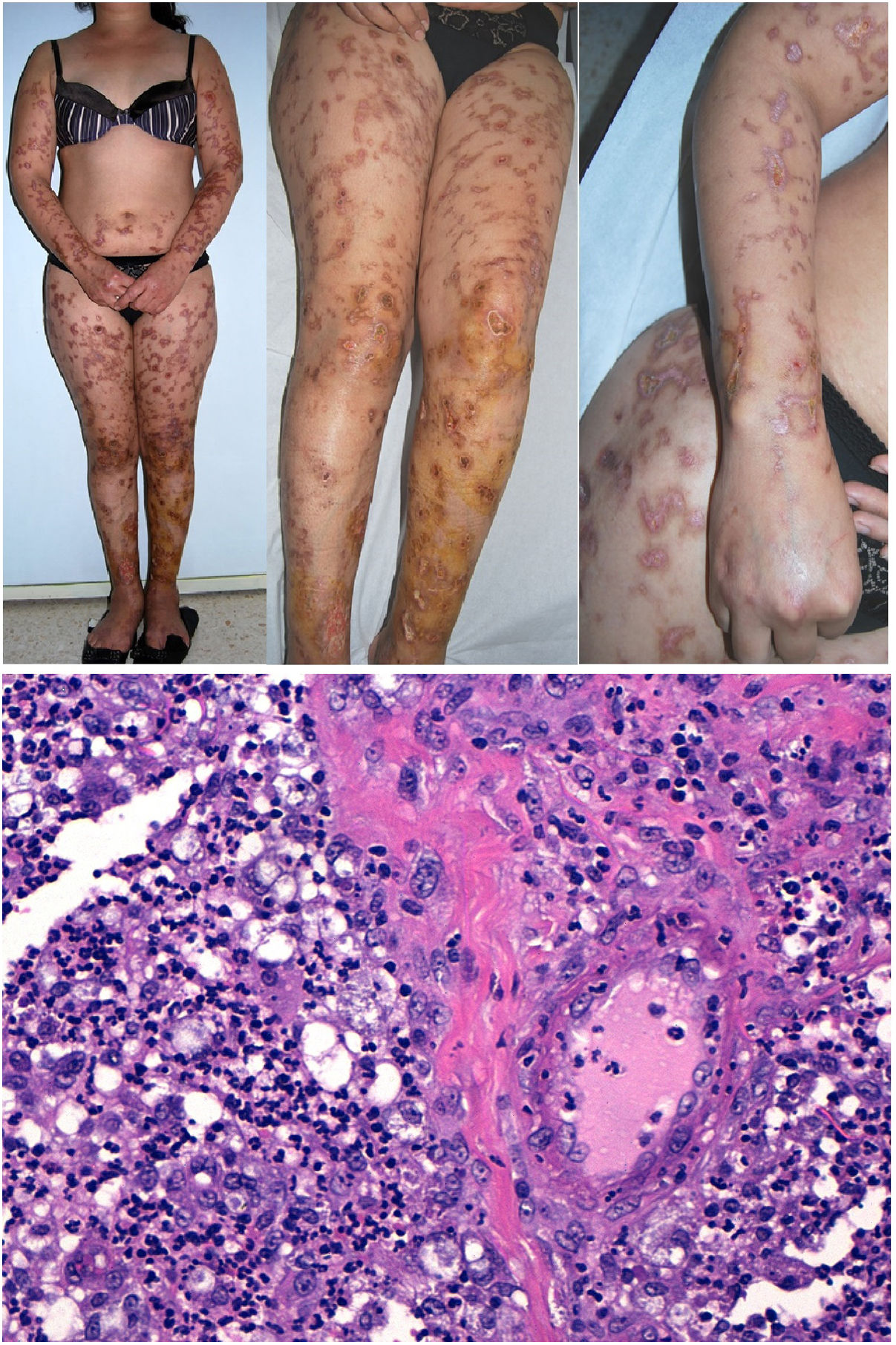
Clinical presentation: Mexico and Costa Rica account for nearly all reports of patients with Lucio phenomenon, although cases have appeared sporadically in various parts of the world in untreated or inadequately treated nonnodular diffuse lepromatous leprosy, especially if patients have multiple hemorrhagic ulcers on the chest or extremities.38 Ischemic skin lesions begin as macules and rapidly progress in irregular, lineal or serpiginous patterns. They have well-defined borders and leave atrophic pigmented scars (Fig. 10A). Therefore, the condition is sometimes referred to as “patchy leprosy.”39 Unlike erythema nodosum leprosy, Lucio phenomenon lesions do not respond to thalidomide.38
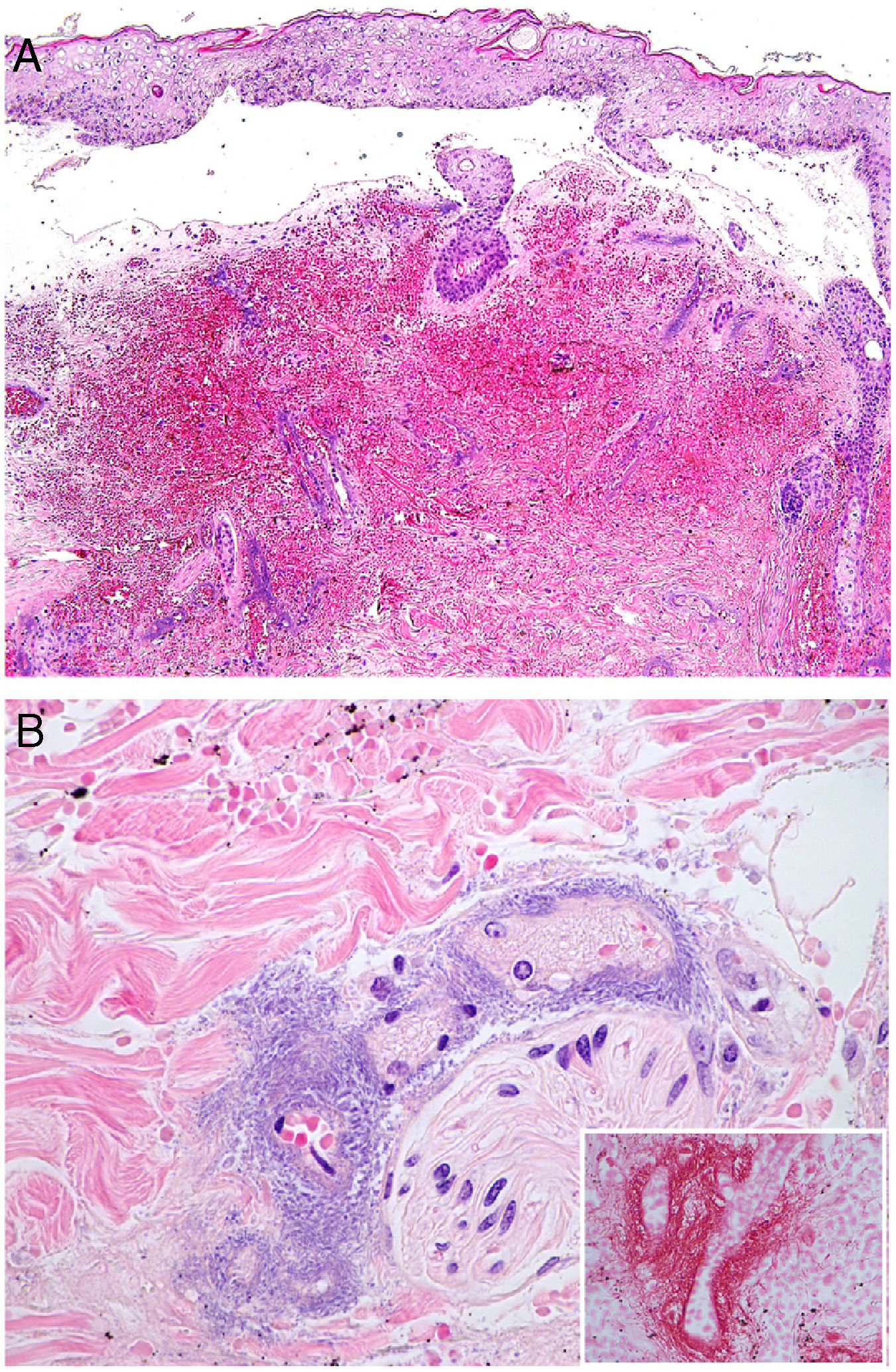
The top 3 photographs show the classic form of Lucio phenomenon progressing to pigmented scarring. The ulcers are irregular, lineal, or serpiginous and have well-defined borders as well as atrophic pigmented scars. (Photographs courtesy of Dr Andrés Sánz.) Below is a microphotograph of a tissue sample from a Lucio phenomenon lesion. Toward the right is a medium-sized vessel with fibrinoid necrosis and abundant polymorphonuclear neutrophils in the wall. Toward the left are foamy cells filled with mycobacteria (Virchow cells) and a neutrophilic dermal infiltrate. Hematoxylin-eosin; magnification ×200.
Histopathology: A frequent finding is a diffuse, lepromatous infiltration due to an abundance of foamy macrophages replete with M lepromatosis bacilli (Virchow cells) in the dermis, subcutaneous cells, and small nerves.36 This finding is accompanied by extensive necrotizing vasculitis involving capillaries and medium-sized arteries in the dermis and subcutaneous tissues (Fig. 10B); intravascular thrombi and bacilli also appear in some patients.40 The term Lucio phenomenon should be applied when the following criteria are met: 1) ulceration, 2) thrombosis and vasculitis, and 3) invasion of vessels by lepromatous mycobacteria.
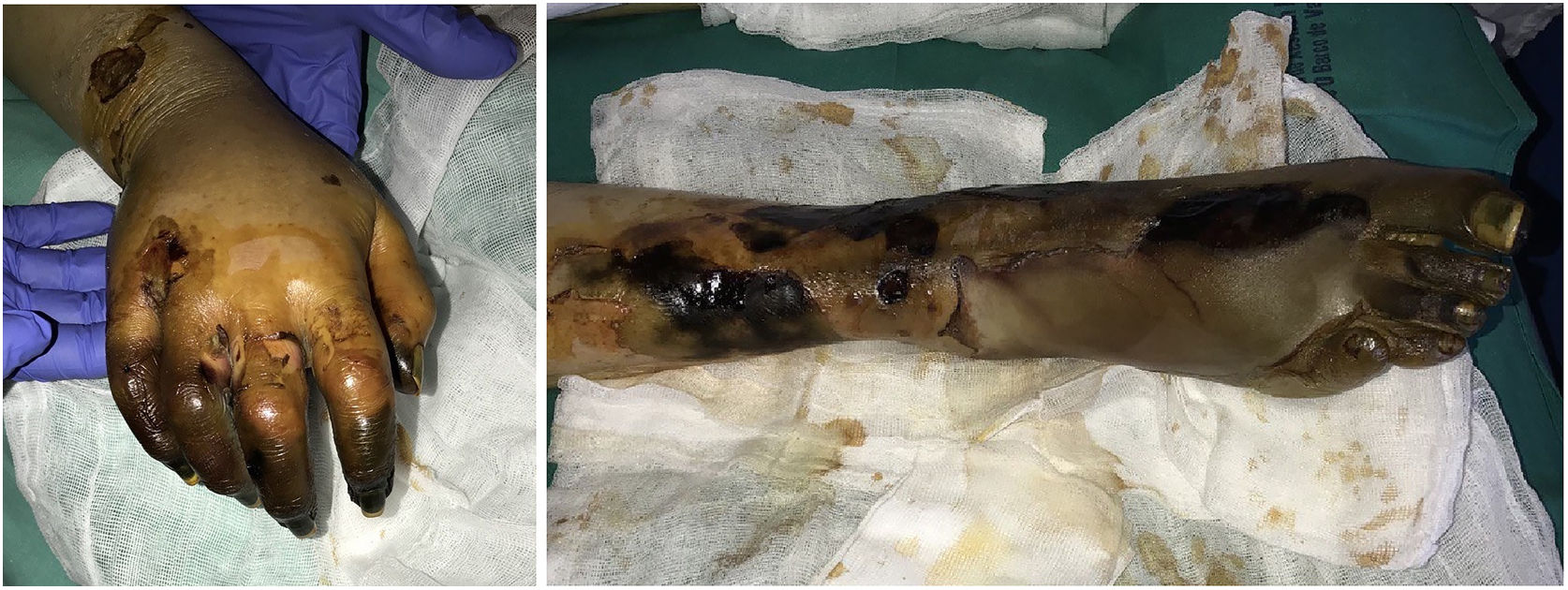
Purpura fulminansDefinition: This syndrome progresses rapidly to intravascular thrombosis, bleeding, and multiple infarcts.41 Disseminated intravascular coagulation and death may occur. A defect in protein C, an important regulator in the coagulation cascade, is responsible, inducing a prothrombotic state.42 The defect can be hereditary (as in neonatal purpura fulminans) or acquired (mainly in Neisseria meningitidis, streptococcal, and methicillin-resistant Staphylococcus aureus infections, but idiopathic cases have also been reported). Although the syndrome is usually seen in children, it can occur in adults.43
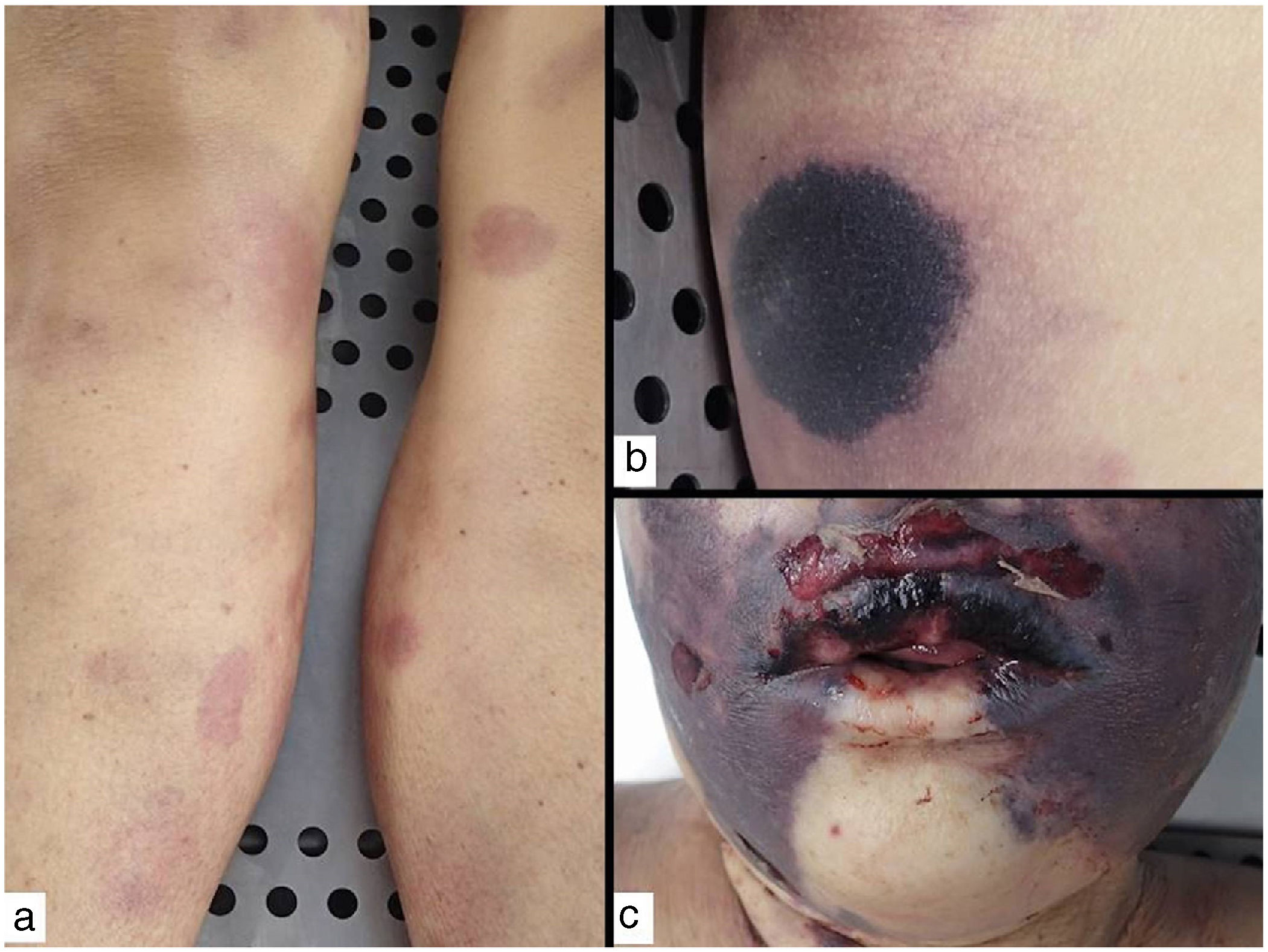
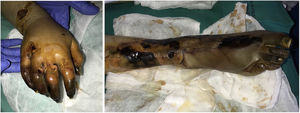
Clinical presentation: The 4 main clinical signs in all forms of purpura fulminans are purplish skin lesions, fever, hypotension, and disseminated intravascular coagulation if the condition is left untreated. The fulminant clinical picture establishes itself quickly. The process may start with erythema or petechiae and progress to ecchymosis; alternatively, painful indurated plaques with irregular borders may be present (Fig. 11). Vesicles, blisters, and necrotic areas can also develop, and if the patient survives, there will be scarring. Disseminated thrombosis is accompanied by drastic hypoperfusion of many areas of the skin (such as on the extremities and in acral areas) as well as of internal organs (such as the lungs, heart, and kidneys).
Histopathology: Thrombi, present in small blood vessels, especially at the dermal-epidermal junction, are mixed, containing fibrin, platelets, and white blood cells.44 Endothelial cells may appear swollen. The associated perivascular infiltrate is usually scant. Neutrophils may be present in acute infectious purpura fulminans, but they are absent in idiopathic and neonatal forms.45 The clinical picture is accompanied by a greater or lesser degree of RBC extravasation and generally progresses to dermal edema. In end stages, dermal-epidermal necrosis develops with or without concomitant blistering.
Viral infection-induced thrombosisDefinition: Viruses can cause thrombotic states by means of direct or indirect mechanisms, including direct cutaneous inoculation of open reading frames (ORFs), dissemination to the skin from other locations (as in herpes zoster infection), or generalized dissemination through vascular endothelia (as in morbilliform rashes such as in measles). Thrombosis might also occur secondary to an immune reaction to a virus.46
Clinical presentation: The presentations vary greatly according to the virus involved.
The varicella zoster virus, in the human herpesvirus alpha subfamily, causes chickenpox as the primary infection; herpes zoster occurs when the virus is reactivated. Systemic vascular disease involving mainly small-caliber arteries can develop, leading to stroke or infarction in other parts of the body, or even to granulomatous aortitis.47
Measles is caused by a virus of the family Paramyxoviridae. It produces a characteristic exanthem.
Dengue fever is caused by a virus in the family Flaviviridae. It is transmitted by mosquitos of the genus Aedes, particularly by Aedes aegypti. Between 50% and 82% of patients with this infection develop a morbilliform rash compared to “white islands in a red sea.” Hemorrhagic signs such as petechiae, epistaxis, and gingival bleeding may also appear. Besides fever, patients experience pain (typically very intense and located behind the eye) as well as gastrointestinal complaints. Palpable lymph nodes are also common and hepatosplenomegaly may even be felt.
Zika virus infection is also transmitted by mosquitos. The clinical picture encompasses fever, joint pain, conjunctivitis, and fatigue. The usual skin manifestation consists of a generalized pruritic, symmetric rash of macules and papules on the face, neck, trunk, palms, and soles; it may be more pronounced in proximal areas of the extremities.48 Less usual is a morbilliform rash. Timing is highly characteristic: the rash appears 24 to 48hours after the other systemic symptoms in 90% of patients.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, which is of great relevance today as the cause of coronavirus disease 2019 (COVID-19), can cause thrombotic phenomena. Two clinical scenarios of cutaneous thrombosis have been reported: periodic acral thrombosis (Fig. 13, upper photograph) and necrotic and livedoid lesions.
Histopathology: Measles: The epidermis shows parakeratosis (subject to viral tropism for the acrosyringium), spongiosis, variable degrees of vacuolar degeneration, and apoptotic keratinocytes (Fig. 12A). The dermis harbors a predominantly perivascular lymphocytic infiltrate. Fibrin thrombi and leukocytoclasia is seen in approximately a third of cases; more common is RBC extravasation, which is present in up to two-thirds of patients.49 A relatively characteristic histopathologic finding is giant syncytial cells in the epidermis. There is a monoclonal antibody against the nucleoprotein of the measles virus.49
A, Measles: slight focal parakeratosis and lymphocytic exocytosis, along with apoptotic keratinocytes. Polymorphonuclear tropism for the epithelium is evident in the basement membrane. The underlying papillary dermis harbors a mixed inflammatory infiltrate, vessels containing thrombi, and a few extravasated red blood cells (RBCs). Hematoxylin-eosin (H&E); magnification ×100. B, Chickenpox: cell acantholysis, cells with chromatin margination, and multinucleated cells in the intraepidermal vesical. Dilated capillaries with fibrin thrombi and extravasated RBCs are evident in the underlying dermal layer. H&E, magnification ×100.
Varicella-zoster: Frank leukocytoclastic vasculitis has been described in some cases of varicella-zoster infection,50 but it is more common to find fibrin thrombi in the superficial dermal vasculature. They are sometimes surrounded by an inflammatory lymphocytic infiltrate and are usually located under the affected intradermal vesicle, which also usually displays cell abnormalities that are characteristic of this diagnosis51 (Fig. 12B). Both herpes simplex and herpes zoster viruses can be detected by immunohistochemistry.52
Dengue virus infection: Findings are relatively nonspecific: edema, mixed perivascular infiltrates and endothelial damage, and RBC extravasation; thrombosis also develops, but more rarely.53,54
Zika virus infection: A range of findings have been reported: nonspecific lymphocytic perivascular infiltrates, spongiosis, exocytosis, apoptotic keratinocytes, slight edema, RBC extravasation, and even a regular psoriasiform hyperplasia with a perivascular lichenoid infiltrate in post-Zika psoriasiform lesions.48 Even though thrombi are not mentioned among the histopathologic features of this infection, infected cells secrete the nonstructural 1 protein (NS1), which can bind to the surface of endothelial cells and alter permeability in skin, lung, umbilical and cerebral tissues.55 This process occurs in Zika and other diseases caused by viruses of the Flavivirus family (dengue virus, West Nile virus, yellow fever, and Japanese encephalitis).
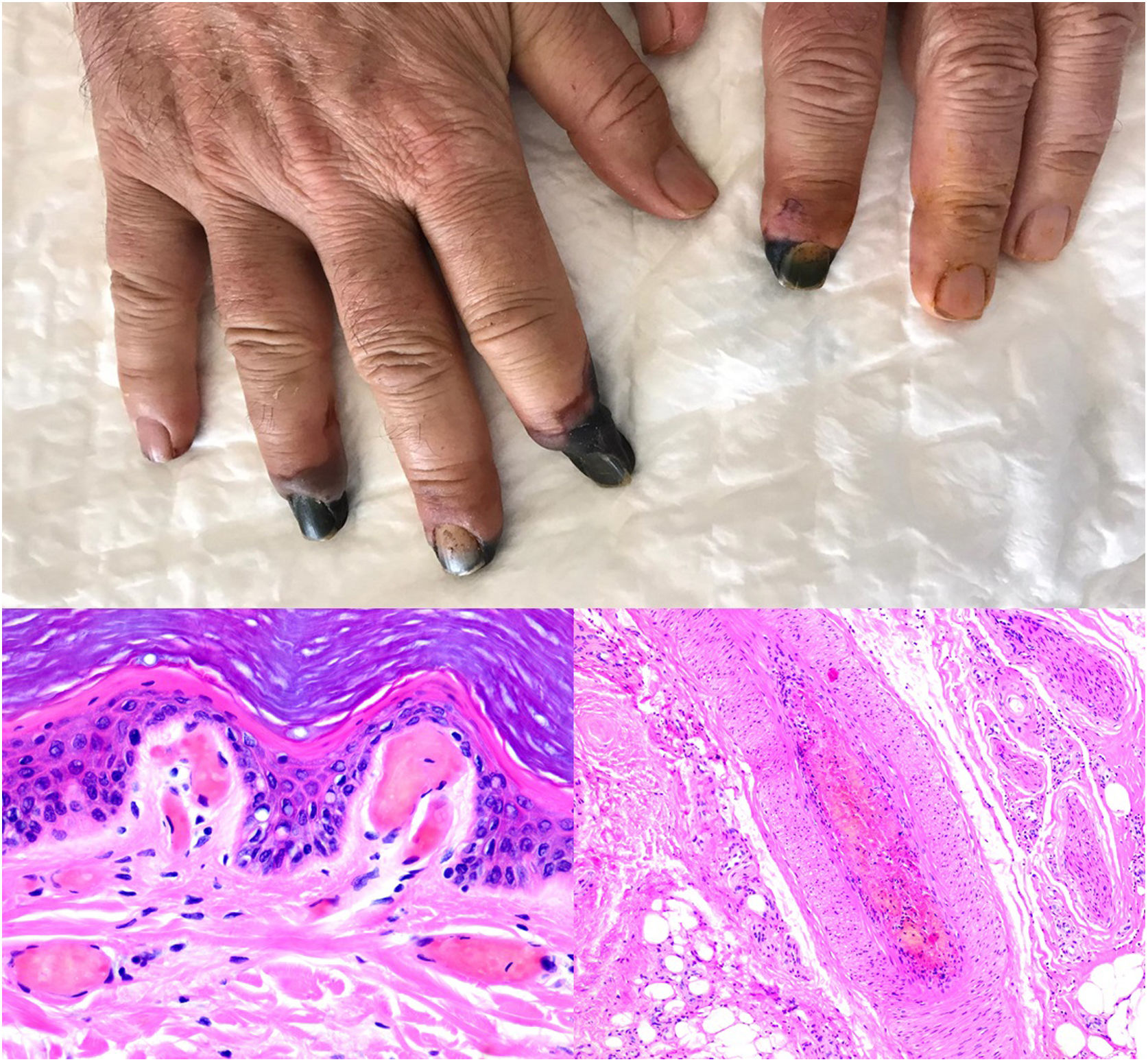
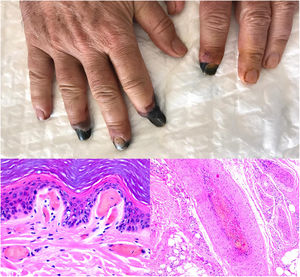
SARS-CoV-2 infection: A moderate to intense, superficial and deep perniosis-type perivascular infiltrate can be identified, associated with endothelial inflammation, RBC extravasation, and to a lesser degree, fibrin thrombi56 (Fig. 13, lower right microphotograph). These findings are more common in patients with less severe systemic symptoms.57 Acral livedoid and ischemic lesions (Fig. 13, upper photograph). are found more often in patients with severe clinical pictures that require hospital admission. In such patients hyaline thrombi are seen in both the papillary dermis and large arteries located at the dermal-hypodermal junction58 (Fig. 13, lower left microphotograph). Necrosis has also been observed in eccrine glands. Even though reverse transcriptase PCR assays to detect COVID-19 are usually negative, even in the presence of both types of histopathologic findings, some authors have reported immunohistochemical positivity for a spike protein on SARS-CoV-2 in eccrine glands in the context of perniosis-type lesions.59
Thrombosis due to coronavirus disease 2019. Upper photograph: The shape of the distal portion of several fingers has become sharper and the tips have taken on a blackish color. Proximal to these ischemic areas, a brownish-gray macula has a residual retiform appearance. Detail of arterial thrombosis (right microphotograph). Hyaline thrombi inside dilated vessels in the papillary dermis (left microphotograph).
The authors declare that they have no conflicts of interest.
Please cite this article as: Beato Merino MJ, Diago A, Fernandez-Flores A, et al. Dermatopatología de la oclusión intraluminal vascular: parte I (trombos). Actas Dermosifiliogr. 2021;112:1–13.