Several studies support the hypothesis that scabies is on the rise in Spain. There are also concerns about the possible development of resistance to treatment and an increase in atypical presentations. The aims of this study were to describe the demographic and clinical characteristics of patients with scabies seen by dermatologists in Spain, to identify the possible emergence of atypical forms of scabies, and to explore the frequency of treatment failures and associated risk factors.
MethodsWe conducted an observational, cross-sectional, multicenter study of data collected prospectively in April and May 2023 using the CLINI-AEDVp platform created by the Spanish Academy of Dermatology and Venereology (AEDV).
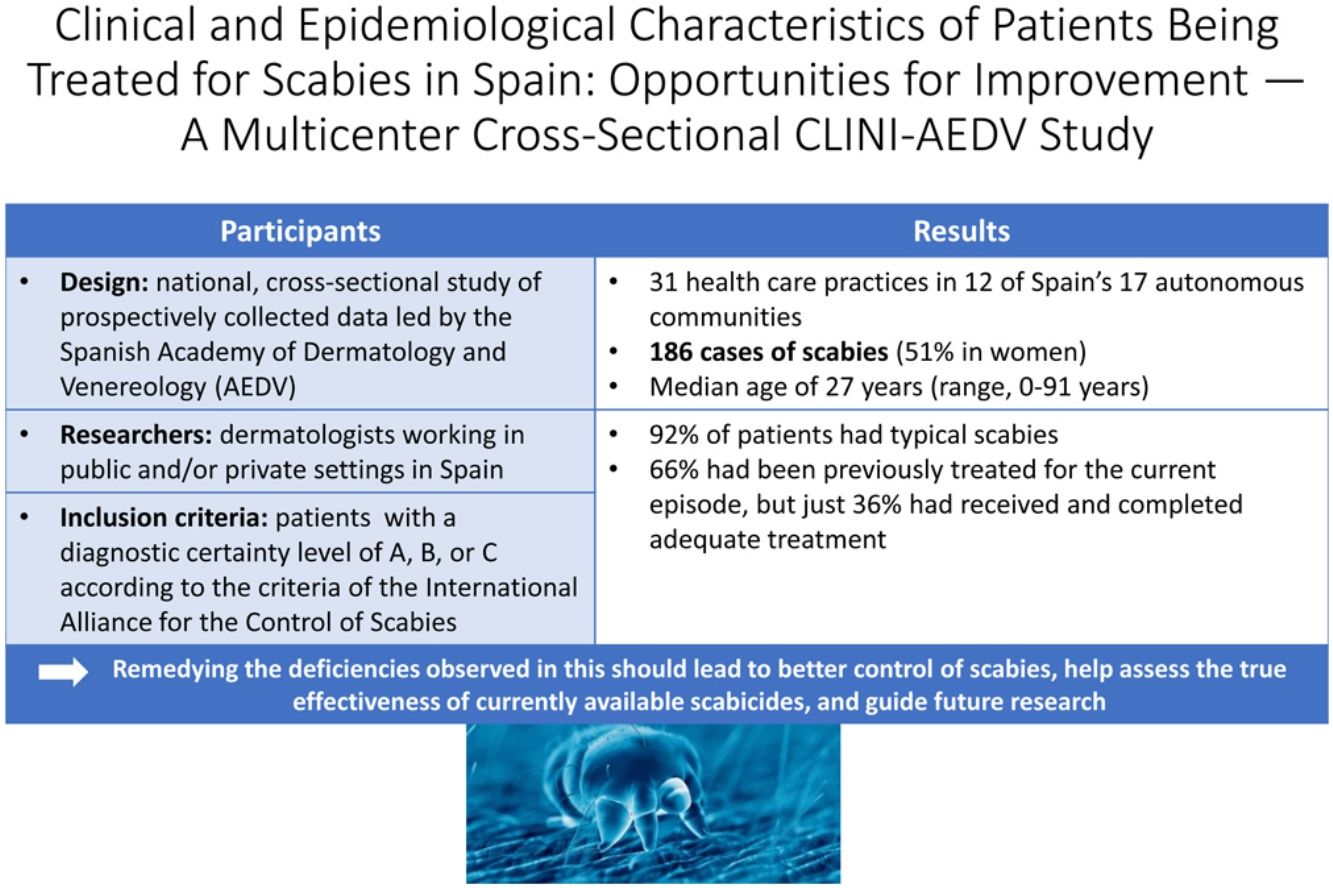
ResultsParticipating dermatologists from 31 hospitals in 15 of Spain's autonomous communities recorded 186 cases of active scabies (51% in women) during the study period. A diagnostic certainty level of A, B or C as per the International Alliance for the Control of Scabies Consensus Criteria was required for diagnosis. Overall, 92% of patients had clinical features of classic scabies and 66% had already been treated with a scabicide for the current episode. Of the treated patients, only 36% had received and completed adequate treatment (including the simultaneous treatment of all household members) and 50% had not received clear written recommendations.
ConclusionsIn a high proportion of scabies cases, the patient has already received treatment. In those cases, we observe several remediable shortcomings that could explain why some of these treatments fail. Remedying these deficiencies should lead to better control of scabies and an improved assessment of the actual effectiveness of currently available scabicides.
Diversos trabajos apoyan la hipótesis de que en España se está produciendo un aumento de incidencia de la escabiosis, y existen dudas sobre el posible desarrollo de resistencias y el incremento de formas clínicas atípicas. Los objetivos de este estudio fueron caracterizar el perfil demográfico y clínico de los pacientes de escabiosis atendidos por dermatólogos en España, identificar la posible aparición de escabiosis atípicas, así como describir la frecuencia y los posibles factores de riesgo de los fracasos terapéuticos previos.
MétodosRealizamos un estudio observacional, transversal, multicéntrico con recogida de datos prospectiva, en abril y mayo de 2023 dentro de la plataforma CLINI-AEDVp de la Academia Española de Dermatología y Venereología.
ResultadosSe reclutaron 186 casos de escabiosis activa (51% mujeres) en 31 centros participantes de 15 comunidades autónomas. Se requirió un nivel A, B o C de los criterios de consenso de la International Alliance for the Control of Scabies (IACS) para el diagnóstico. El 92% de los pacientes presentaron formas clínicas típicas de escabiosis y un 66% había recibido tratamiento escabicida previo para el episodio en curso. De los pacientes previamente tratados, solo un 36% había recibido y cumplimentado una pauta terapéutica adecuada que incluyera el tratamiento simultáneo de convivientes, y un 50% careció de un documento escrito y claro con las recomendaciones.
ConclusionesUna elevada proporción de los casos de escabiosis atendidos actualmente ha recibido tratamiento previo. En estos se observan defectos corregibles que pueden justificar parte de los fracasos terapéuticos. Trabajar en la mejora de las deficiencias encontradas ayudará a un mejor control de la enfermedad y a evaluar la efectividad actual de los escabicidas disponibles.
Scabies is an infectious disease caused by the Sarcoptes scabiei var. hominis mite, an obligate parasite that spends its 14-day life cycle in human skin. Transmission usually requires skin-to-skin contact. Despite the genomic similarities of animal and human mites, scabies is not considered a zoonotic disease.1,2 It affects people across all age groups, latitudes, and socioeconomic conditions, and constitutes a significant global public health problem.3 Its incidence varies over time, yet there is a lack of consensus on the existence of seasonal variations.4,5 Scabies can present with a range of clinical features, but typical manifestations constitute what is known as classic or typical scabies.6,7
In its 2021-2030 roadmap for neglected tropical diseases, the World Health Organization (WHO) identified universal health coverage for scabies and tools for estimating its burden as priority actions.8 Nonetheless, many gaps remain in our understanding of the epidemiology of scabies, as in most countries, including Spain, notification of outbreaks is only mandatory in institutional settings.9–13
Various studies support the hypothesis that scabies is on the rise in Spain and that the mite is developing resistance to first-line scabicides.14–16 Outside the realm of scientific publications, a number of health care practitioners have reported concerns about this possible increase,17,18 dermatologists from the Spanish Academy of Dermatology and Venereology (AEDV) have shared the impression that they are facing an increased number of cases of atypical scabies.
In addition, increased scabicide prescriptions have been reported in Spain19,20 (particularly since the beginning of the COVID-19 pandemic) and other countries.21,22 Multiple factors could be driving the upward trend in scabies diagnoses, including changes to social habits (e.g., increase in the number of sexual partners), population aging, more patients with immobility and/or immunosuppression, and a growing tolerance of scabies mites to scabicides.7,23,24
In order to help better understand the situation of scabies in Spain, the AEDV, in collaboration with the Epidemiology and Health Promotion Working Group, launched a study to describe the demographic and clinical characteristics of patients with scabies seen by dermatologists in Spain. Secondary objectives were to identify the possible appearance of atypical scabies and explore the frequency of treatment failures and associated risk factors.
MethodsStudy DesignThis was a cross-sectional observational study of data collected prospectively using the CLINI-AEDVp platform created by the AEDV.25 The study was approved by the Hospital Universitario Puerta de Hierro-Majadahonda Research Ethics Committee (file 18/2022).
Study PopulationThe study population included male and female patients of all ages diagnosed with scabies by a participating dermatologist, during an official appointment. A prior diagnosis of scabies was not an excluding factor.
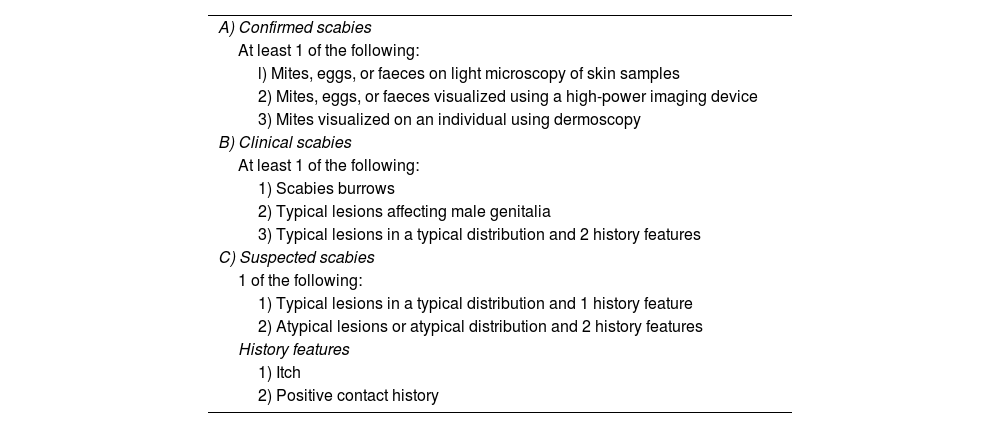
The researchers consecutively included all patients seen and diagnosed with scabies for the first time by any of the participating dermatologists. The diagnoses were made at any of the 3 certainty levels (A, B or C) established by the International Alliance for the Control of Scabies [IACS] (Table 1). Scabies was classified as typical when the patient had classic morphologic features (burrows and/or papules with a diameter of 2-3mm and/or nodules; and/or vesicles or pustules on the hands or feet of infants); and a minimum of 3 lesions in the same body area or within a maximum area of 20cm arranged in a typical distribution in adults, with any distribution in infants.7 Scabies was classified as atypical when any of the 3 criteria for typical scabies (morphology, minimum number of lesions, and body area) was not met.
Summary of the 2020 International Alliance for Scabies Control Consensus Criteria for the Diagnosis of Scabies.7
| A) Confirmed scabies |
| At least 1 of the following: |
| l) Mites, eggs, or faeces on light microscopy of skin samples |
| 2) Mites, eggs, or faeces visualized using a high-power imaging device |
| 3) Mites visualized on an individual using dermoscopy |
| B) Clinical scabies |
| At least 1 of the following: |
| 1) Scabies burrows |
| 2) Typical lesions affecting male genitalia |
| 3) Typical lesions in a typical distribution and 2 history features |
| C) Suspected scabies |
| 1 of the following: |
| 1) Typical lesions in a typical distribution and 1 history feature |
| 2) Atypical lesions or atypical distribution and 2 history features |
| History features |
| 1) Itch |
| 2) Positive contact history |
Diagnosis can be made at 1 of the 3 levels (A, B or C). A diagnosis of clinical or suspected scabies should only be made if other differential diagnoses are considered less likely than scabies.
Dermatologists were invited to participate in the study through the AEDV's dissemination channels and the social media networks of Spain's dermatology community. Patients were recruited by the participating dermatologists during routine practice in public or private settings. Informed patient consent was required for inclusion.
Data were collected via online case report forms on the REDCap (Research Electronic Data Capture) platform. The recruitment period was 8 weeks (April and May 2023).
Exclusion criteria were a previous diagnosis of scabies by the same dermatologist and visits to the medical center without an appointment or formal referral (e.g., people accompanying patients).
Measures and VariablesWe collected demographic, clinical, and epidemiological data and information on previous treatments.
Data AnalysisFor the descriptive analysis, we computed absolute values and percentages for qualitative variables, mean standard deviation (SD) for normally distributed continuous variables, and median (quartiles) for nonnormally distributed continuous variables. The χ2 test (or the Fisher exact test for categories with <5 counts) was used to compare categorical variables, the t test to compare means, and the Wilcoxon test to compare medians. Statistical analyses were performed using Stata (Version 16.0).
ResultsDuring the 2 months of recruitment, 44 dermatologists recorded 186 cases of scabies in 12 autonomous communities: 46 in Andalusia, 35 in Asturias, 25 in Catalonia, 21 in the Balearic Islands, 20 in Galicia, 13 in the Community of Madrid, 12 in the Valencian Community, 5 in the Canary Islands, 5 in La Rioja, 2 in the Basque Country, 1 in Aragon, and 1 in the Region of Murcia. The patients’ ages ranged from 0 to 91 years and 51% were female. Demographic, clinical, and epidemiological data are summarized in Table 2.
Demographic, Clinical, and Epidemiological Characteristics of Patients Diagnosed with Scabies by Dermatologists in Spain between April and May 2023.
| Variable | No. (%) |
|---|---|
| Patients (total) | 186 (100) |
| Type of center where patient was treated | |
| Public | 124 (67) |
| Private | 62 (33) |
| Demographic characteristics and personal history | |
| Age at diagnosis, in years | |
| Median age (IQR) | 27 (18-53) |
| Sex | |
| Female | 94 (51) |
| Past diagnosis of scabies (unrelated to current episode) | |
| Yes, years ago | 11 (6) |
| No, first time | 174 (94) |
| Not known | 1 (1) |
| Sexually transmitted infection in past year | |
| Yes | 3 (2) |
| No | 174 (94) |
| Not known | 9 (5) |
| Immunosuppression | |
| Yes | 7 (4) |
| No | 173 (93) |
| Not known | 6 (3) |
| Institutionalization | |
| Yes | 8 (4) |
| No | 177 (95) |
| Not known | 2 (1) |
| Contact with an animal with hair | |
| Yes | 63 (34) |
| No | 107 (58) |
| Not known | 16 (9) |
| Clinical and epidemiological characteristics of current scabies episode | |
| IACS diagnostic criteria | |
| Confirmed scabies | 86 (46) |
| Clinical scabies | 90 (48) |
| Suspected scabies | 10 (5) |
| Coliving units in past 3 months, No. | |
| 1 | 131 (70) |
| 2 | 38 (20) |
| ≥ 3 | 17 (9) |
| Total number of coinhabitants (including patient and adding all units) in past 3 months | |
| 1 | 16 (8) |
| 2 | 34 (18) |
| 3-4 | 81 (43) |
| ≥ 5 | 57 (31) |
| Number of people(including the patient) in coliving unit with itch in past 3 months | |
| 0 | 16 (9) |
| 1 | 69 (37) |
| 2 | 51 (28) |
| ≥ 3 | 48 (26) |
| Not reported | 2 (1) |
| Coinhabitants diagnosed with scabies in past 3 months | |
| 0 | 58 (31) |
| 1 | 68 (37) |
| ≥ 2 | 59 (32) |
| Not reported | 2 (1) |
| Number of intimate contacts | |
| 0 | 42 (22) |
| 1 | 100 (54) |
| ≥ 2 | 43 (23) |
| Not reported | 1 (1) |
| Probable source of infection (according to dermatologist) | |
| Relative (not counting sexual partner) | 59 (32) |
| Not known | 40 (21) |
| Sexual partner | 31 (17) |
| Circle of friends | 22 (12) |
| Working environment | 10 (5) |
| Care home | 9 (5) |
| Educational center | 5 (3) |
| Sporting activities | 3 (2) |
| Occasional accommodation | 3 (2) |
| Other | 4 (2) |
| Type of contacta | |
| Intimate | 40 (22) |
| Close | 83 (45) |
| Distant | 14 (7) |
| Not known | 49 (26) |
| Itch VAS score, mean (SD) | 7,7 (2,1) |
| Predominant elementary lesion | |
| Burrow | 85 (45) |
| Papule | 71 (38) |
| Scratch mark/excoriation | 15 (8) |
| Nodule | 9 (5) |
| Blister | 2 (1) |
| Erosion/ulcer | 1 (1) |
| Lichenification | 1 (1) |
| Wheal/dermographism | 1 (1) |
| Pustule | 1 (1) |
| Lesions, No. | |
| > 10 | 115 (62) |
| 3-10 | 64 (34) |
| 1-2 | 6 (3) |
| Not reported | 1 (1) |
| Typical scabies (IACS criteria) | |
| Yes | 172 (92) |
| No | 14 (8) |
| Previous treatment (reason) | |
| Yes (symptoms±coinhabitants) | 105 (56) |
| Yes (infected coinhabitants) | 16 (9) |
| No, no treatment | 65 (35) |
IACS, International Alliance for the Control of Scabies; VAS, visual analog scale.
Regarding clinical features, most patients (172, 92%) presented with classical clinical features, while the remaining 14 had atypical scabies, of which 11 (79%) had received previous treatment. In this group, in addition to having lesions in typical locations (hands, wrists, feet, breasts, armpits, buttocks, and genitals), 1 patient had head and neck lesions, 10 had trunk lesions, and 13 had lesions on nondistal parts of limbs. Atypical skin manifestations included extensive crusts (3 cases), fissures (2 cases), hives/dermographism (2 cases), and pustulosis (2 cases). Other less common atypical signs, with 1 case each, were extragenital nodules, eczema, and monomorphic papules in a follicular distribution.
Sixty-five percent of patients had been treated for the same episode of scabies in the 3 months prior to consultation, either because they were infected (57%) or the contact of a case (9%). Data on previous treatments are shown in Table 3. The most frequently used drugs were topical pyrethroids and oral ivermectin, and almost half of the patients (48%) had received at least 2 treatments. Of patients using topical pyrethroids, 88% followed the regimen correctly (at least 2 applications separated by 7-14 days for cases and at least 1 application for contacts), but only 59% were confident that they had applied the cream properly. Similar results were reported for oral ivermectin users, with 79% of patients being prescribed the appropriate dose and 64% following the regimen correctly.
Previous Treatments for Current Episode of Scabies.
| Variable | No. (%) | |
|---|---|---|
| Patients (total) | 121 (100) | |
| Treatments received | Topical pyrethroids | 107 (88) |
| Oral ivermectin | 56 (46) | |
| Topical sulphur | 15 (12) | |
| Topical ivermectin | 10 (8) | |
| Benzyl benzoate | 1 (1) | |
| Lindane | 0 (0) | |
| Other | 1 (1) | |
| Topical pyrethroid+oral ivermectin | 46 (38) | |
| Details of treatment by treatment option | ||
| Topical pyrethroids | n=107 | |
| Applications, No. | 1, single application | 12 (11) |
| 2, separated by 7-14 days | 58 (54) | |
| 2, separated by <7 days | 3 (3) | |
| ≥3 | 31 (29) | |
| Not known/not reported | 4 (4) | |
| Correct administration | Yes, definitely | 64 (59) |
| Yes, but with some doubts | 29 (27) | |
| No, did not apply it to whole body | 9 (7) | |
| Not known | 5 (6) | |
| Oral ivermectin | n=58 | |
| Correct dose/patient weight | Yes | 44 (76) |
| No, infratherapeutic dose | 11 (19) | |
| Not known | 3 (5) | |
| Days treatment taken, No. | 1 | 12 (21) |
| 2, separated by 7-28 days | 37 (64) | |
| ≥ 3 | 6 (10) | |
| Not known/not reported | 3 (5) | |
| Topical sulphur | n=15 | |
| Regimen | Application for at least 3 consecutive days | 13 (87) |
| Other | 2 (13) | |
| Treatments used, No. | 1 | 63 (52) |
| 3 | 47 (39) | |
| 2 | 11 (9) | |
| Characteristics of all treatments provided (n=122) | ||
| Simultaneous treatment of coinhabitants | Yes, all | 57 (47) |
| No | 61 (50) | |
| Not known/not reported | 3 (2) | |
| Written treatment instructions | Yes | 61 (50) |
| No | 49 (40) | |
| Not known | 11 (10) | |
| Treatment doubts during application | Yes | 27 (22) |
| No | 79 (65) | |
| Not known | 15 (12) | |
| Reason for treatment failure (dermatologist opinion) | Lack of simultaneous treatment | 39 (32) |
| Treatment resistance | 25 (26) | |
| Various reasons/others | 17 (14) | |
| Incorrect application | 11 (9) | |
| Incorrect dose | 9 (7) | |
| Incorrect disinfection | 7 (6) | |
| Reinfection | 6 (5) | |
| Not known | 5 (4) | |
| Not reported | 2 (2) | |
Only 50% of previously treated patients claimed to have received written instructions on how to administer the treatment (Table 3). Of these, 18% mentioned that they had had doubts when applying the treatment, compared to 35% of patients who had not received written instructions (P=.04). A lack of written instructions was associated with a greater likelihood of incorrect treatment (inadequate regimen or application and/or failure to simultaneously treat cohabitants) (P<.001). Nevertheless, incorrect treatment was also observed in 51% of patients who had received written instructions.
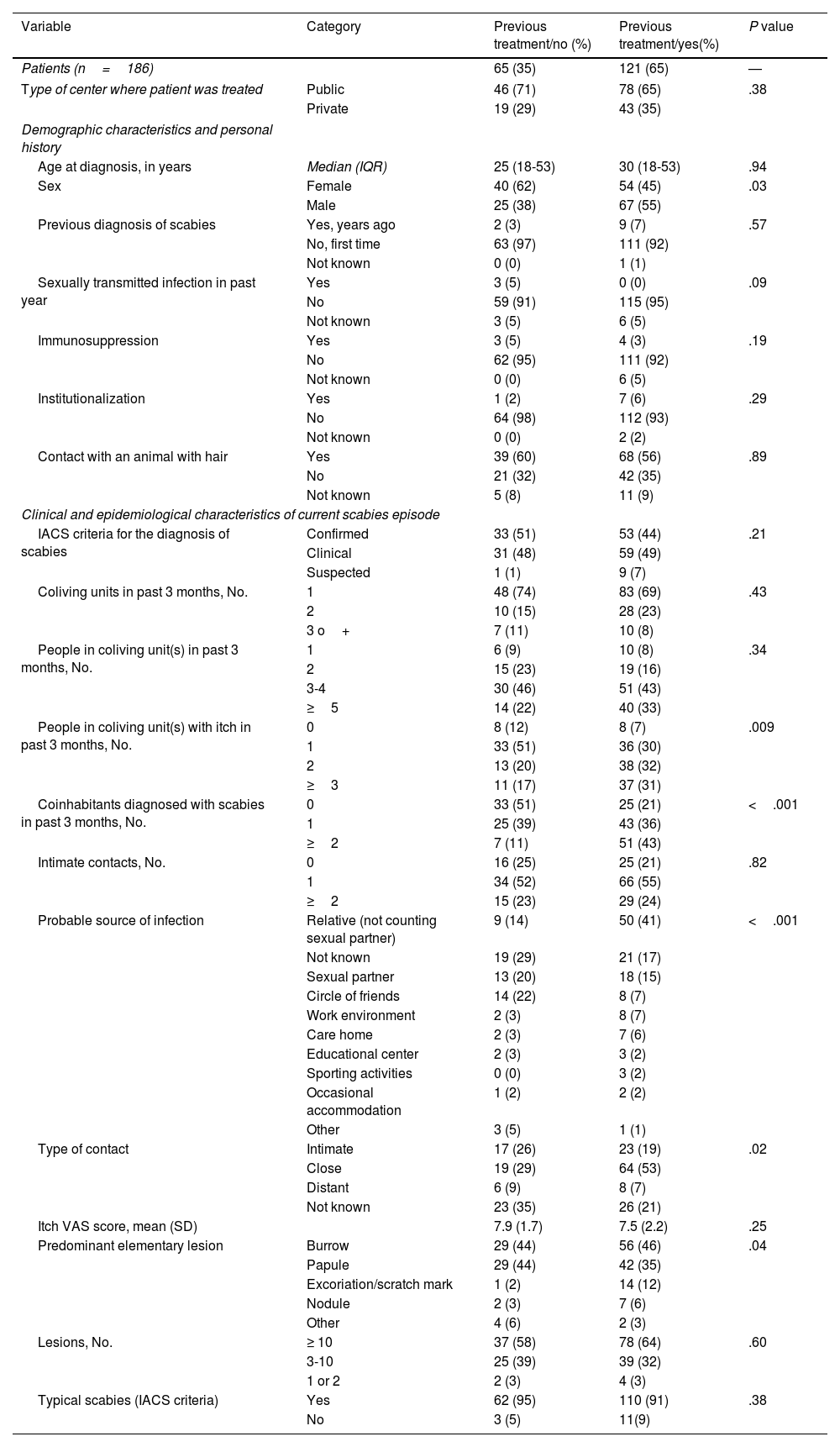
Significant differences were observed between previously treated and untreated patients for sex, number of cohabitants with itch, number of cohabitants diagnosed with scabies, probable source of infection, and suspected source of contact (Table 4).
Comparison of Patients Who Received and Did Not Receive Previous Scabicide Treatment for Current Episode.
| Variable | Category | Previous treatment/no (%) | Previous treatment/yes(%) | P value |
|---|---|---|---|---|
| Patients (n=186) | 65 (35) | 121 (65) | — | |
| Type of center where patient was treated | Public | 46 (71) | 78 (65) | .38 |
| Private | 19 (29) | 43 (35) | ||
| Demographic characteristics and personal history | ||||
| Age at diagnosis, in years | Median (IQR) | 25 (18-53) | 30 (18-53) | .94 |
| Sex | Female | 40 (62) | 54 (45) | .03 |
| Male | 25 (38) | 67 (55) | ||
| Previous diagnosis of scabies | Yes, years ago | 2 (3) | 9 (7) | .57 |
| No, first time | 63 (97) | 111 (92) | ||
| Not known | 0 (0) | 1 (1) | ||
| Sexually transmitted infection in past year | Yes | 3 (5) | 0 (0) | .09 |
| No | 59 (91) | 115 (95) | ||
| Not known | 3 (5) | 6 (5) | ||
| Immunosuppression | Yes | 3 (5) | 4 (3) | .19 |
| No | 62 (95) | 111 (92) | ||
| Not known | 0 (0) | 6 (5) | ||
| Institutionalization | Yes | 1 (2) | 7 (6) | .29 |
| No | 64 (98) | 112 (93) | ||
| Not known | 0 (0) | 2 (2) | ||
| Contact with an animal with hair | Yes | 39 (60) | 68 (56) | .89 |
| No | 21 (32) | 42 (35) | ||
| Not known | 5 (8) | 11 (9) | ||
| Clinical and epidemiological characteristics of current scabies episode | ||||
| IACS criteria for the diagnosis of scabies | Confirmed | 33 (51) | 53 (44) | .21 |
| Clinical | 31 (48) | 59 (49) | ||
| Suspected | 1 (1) | 9 (7) | ||
| Coliving units in past 3 months, No. | 1 | 48 (74) | 83 (69) | .43 |
| 2 | 10 (15) | 28 (23) | ||
| 3 o+ | 7 (11) | 10 (8) | ||
| People in coliving unit(s) in past 3 months, No. | 1 | 6 (9) | 10 (8) | .34 |
| 2 | 15 (23) | 19 (16) | ||
| 3-4 | 30 (46) | 51 (43) | ||
| ≥5 | 14 (22) | 40 (33) | ||
| People in coliving unit(s) with itch in past 3 months, No. | 0 | 8 (12) | 8 (7) | .009 |
| 1 | 33 (51) | 36 (30) | ||
| 2 | 13 (20) | 38 (32) | ||
| ≥3 | 11 (17) | 37 (31) | ||
| Coinhabitants diagnosed with scabies in past 3 months, No. | 0 | 33 (51) | 25 (21) | <.001 |
| 1 | 25 (39) | 43 (36) | ||
| ≥2 | 7 (11) | 51 (43) | ||
| Intimate contacts, No. | 0 | 16 (25) | 25 (21) | .82 |
| 1 | 34 (52) | 66 (55) | ||
| ≥2 | 15 (23) | 29 (24) | ||
| Probable source of infection | Relative (not counting sexual partner) | 9 (14) | 50 (41) | <.001 |
| Not known | 19 (29) | 21 (17) | ||
| Sexual partner | 13 (20) | 18 (15) | ||
| Circle of friends | 14 (22) | 8 (7) | ||
| Work environment | 2 (3) | 8 (7) | ||
| Care home | 2 (3) | 7 (6) | ||
| Educational center | 2 (3) | 3 (2) | ||
| Sporting activities | 0 (0) | 3 (2) | ||
| Occasional accommodation | 1 (2) | 2 (2) | ||
| Other | 3 (5) | 1 (1) | ||
| Type of contact | Intimate | 17 (26) | 23 (19) | .02 |
| Close | 19 (29) | 64 (53) | ||
| Distant | 6 (9) | 8 (7) | ||
| Not known | 23 (35) | 26 (21) | ||
| Itch VAS score, mean (SD) | 7.9 (1.7) | 7.5 (2.2) | .25 | |
| Predominant elementary lesion | Burrow | 29 (44) | 56 (46) | .04 |
| Papule | 29 (44) | 42 (35) | ||
| Excoriation/scratch mark | 1 (2) | 14 (12) | ||
| Nodule | 2 (3) | 7 (6) | ||
| Other | 4 (6) | 2 (3) | ||
| Lesions, No. | ≥ 10 | 37 (58) | 78 (64) | .60 |
| 3-10 | 25 (39) | 39 (32) | ||
| 1 or 2 | 2 (3) | 4 (3) | ||
| Typical scabies (IACS criteria) | Yes | 62 (95) | 110 (91) | .38 |
| No | 3 (5) | 11(9) | ||
Abbreviations: IACS, International Alliance for the Control of Scabies; VAS, visual analog scale.
We have described the characteristics of patients with scabies currently seen by dermatologists in Spain and offer insights into the probable causes of the purported increase in cases and treatment failures. Typical scabies was the most common form, observed in 92% of patients. More than half of these (65%) had been previously treated for the current episode, and their treatment was associated with remediable shortcomings that may explain, at least in part, the treatment failures observed and guide actions to improve disease control at both the individual and community level.
The distribution of cases showed no predilection for sex, and the age range was very wide; the population, however, was predominantly younger (Table 1), supporting previous findings for Spain and other countries.13,26 Most patients had no history of immunosuppression, sexually transmitted infections, or previous episodes of scabies, indicating that if there is indeed an upward trend, it is not linked to comorbidities or any of the other clinical risk factors studied.
Level of diagnostic certainty was higher than reported elsewhere,14–16 with 94% of diagnoses classified as IACS level A or B. Most patients reported noncomplex personal circumstances, such as single-family dwellings (70%), noninstitutional settings (95%), and an absence of multiple sexual partners. A high proportion of patients (74%), however, lived with 3 or more people, or with a person who had been diagnosed with scabies (69%). The most likely source of infection was the home environment or a sexual partner (49% of cases). In 66% of cases, the patient had intimate or very close contact with the infected person. These findings suggest that shared living conditions and close or habitual relationships are the main sources of scabies infection, transmission, and persistence.
Ninety-two percent of patients had typical scabies and presented with classical clinical manifestations (intense itch, burrows, and papules), indicating that atypical manifestations in scabies are still rare.
The high number of previously treated patients with active scabies in this series (66%) is in line with several recent reports suggesting that both incidence and treatment failures are on the rise.4,13,21,22 According to the authors of a systematic review, it is difficult to draw conclusions on why different treatments fail due to the limitations of the studies available.27 We explored some of these reasons and obtained some novel insights. In some cases, previous treatments may have failed because of prescription errors (inappropriate doses and/or regimens in 14% of cases) while, in others, the most likely cause seems to be poor treatment adherence, as hypothesized in other studies.26 Our findings also support the idea that poor understanding among patients is one of the reasons for poor adherence (50% of patients had doubts when applying the treatment). In addition, a recent publication described a mutation in the voltage-sensitive sodium channel in S. scabiei var. hominis associated with permethrin tolerance that could partly explain some of the treatment failures.28 The authors suggested that longer exposure to permethrin or the combined use of permethrin and ivermectin may be necessary to achieve complete response. Thirty-eight percent of patients with previously failed treatment in our series had been treated with both permethrin and ivermectin, but it was not specified whether these drugs had been used concomitantly or successively. Other potential contributors to treatment failures are drug costs and difficulties applying treatment properly.29
It is striking that approximately half of the previously treated patients in our series acknowledged that not all their cohabitants had been treated simultaneously (51%) and that they had not received written instructions on how to apply treatment properly (50%). The recruiting dermatologists were of the opinion that nontreatment of cohabitants was responsible for one-third of treatment failures. Notably, 32% of patients in whom drug resistance was suspected as a reason for failure had received an inappropriate dose or lived with people who had not been treated. Regardless, assuming that the scabies mites had been correctly eliminated from fomites, just 36% of previously treated patients had received written instructions and completed treatment correctly. The above considerations constitute a crack in the system and make it impossible to determine whether a given treatment was effective or not. They also indicate that better information and monitoring may improve scabies control.
Another notable finding in our study was that 50% of patients who had received written instructions did not complete treatment properly, highlighting the need to improve the chain of scabies care provision. Efforts must be made to ensure that dermatologists take the necessary time to corroborate that both patients and cohabitants correctly understand both the recommended treatment and control measures and that infected patients receive the necessary support in identifying and notifying contacts. Rapid access to treatment is also important. Considering the high percentage of young people affected, apart from leaflets and brochures with clear graphics, it seems necessary to explore the use of alternative educational material for patients and their contacts, such as game-based tools, social media posts, videos, and other online resources.30,31 These improvements cannot be implemented during patient visits due to time constraints.32 They require the collaboration of the different institutions involved and the allocation of sufficient economic and human resources.
Observation of a history of prior treatment for the current episode in patients with a history of symptoms suggests that these cases were treatment failures. Ideally, to properly assess risk factors for treatment failure, it would be necessary to compare responders and nonresponders, but this was not possible in our study due to its cross-sectional design. We consider that previously treated patients in our series represent treatment failures, but the group of patients being treated for the first time included responders and nonresponders (treatment failures). The 2 groups are more similar than they should be. If differences were found in the comparisons conducted (Table 4), they would most likely reveal true differences between responders and nonresponders, although it should be noted that absence of differences could be real or due to bias.
On comparing previously treated and untreated patients, we observed more men in the former group. Perhaps women took more care to apply the cream correctly and to eliminate mites from contaminated material. As expected, the previous treatment group contained a higher proportion of cohabitants with itch or a diagnosis of scabies (P<.01). Likewise, there were also differences regarding probable sources of infection (P<.001), which in the previous treatment group, were related to “obligatory” coliving conditions (family, partner, and residence in a care home). In the group of patients treated for the first time, by contrast, the most frequent source was unknown or a more sporadic contact, such as a friend (Table 4). Logically, scabies is more difficult to eliminate when it is not possible to avoid contact with the source of infection, as occurs in the family environment or home. We observed no associations with contact with hair-bearing animals, suggesting that transmission routes have remained unchanged and that human scabies is still transmitted from humans to humans (i.e., it is not a zoonotic disease).33
This study had several limitations. Due to its descriptive, cross-sectional design, it did not generate prospective data on new treatments, limiting group comparisons on treatment response. In addition, the population may not be representative of the general population. On the one hand, it included patients who were referred to the dermatology department or had access to private practices run by the participating dermatologists, while on the other, nonresponders and patients with complex cases would be more likely to be seen in these types of health care settings. Although 12 of Spain's 17 autonomous communities are represented, the researchers were not evenly distributed across the country. Nonetheless, there are no grounds to suspect geographic variations in the characteristics of scabies.
Our study also has a number of strengths. The data were collected prospectively in 31 public and private health care settings across a wide geographic area. The level of diagnostic certainty was also high, with all diagnoses made by dermatologists and the majority classified as IACS level A or B. Our findings depict the clinical and epidemiological characteristics of patients recently diagnosed with scabies in Spain by dermatologists, adding robustness to our study and highlighting the opportunity and need for improved care. The proportions of IACS diagnostic certainty levels A and B were similar, but it would be desirable to increase that of level A diagnoses (microbiologic confirmation). This could be achieved by ensuring on-site access to the necessary diagnostic tools, increasing time spent with each patient, and improving the training of health personnel in entodermatoscopy and/or other diagnostic tools.7,34,35
We trust that this study will guide other prospective, longitudinal studies that will add to the body of evidence on the causes of treatment failures and the real-life effectiveness of currently available treatments.
ConclusionsMost patients diagnosed with scabies in this series were immunocompetent and had classic clinical presentations and standard living conditions. A high proportion had been unsuccessfully treated with a scabicide prior to the current episode. Many had been treated with inadequate doses or regimens, had misapplied the treatment, and/or had not received sufficient information in writing on how to correctly treat their scabies. These findings highlight the need for improvement, and for health services to place greater emphasis on treatment and control measures targeting both patients and their contacts, ensuring that they correctly understand the treatment and are provided with clear, easy-to-understand instructions. Further studies are needed to evaluate the effectiveness of new treatment options, such as combined treatments, longer exposure to permethrin, and use of new drugs or drugs that are not currently considered first-line treatments. Work on remedying the deficiencies observed will help determine the clinical effectiveness of currently available scabicides and inform necessary research.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We thank the members of the CLINI-AEDV Scientific Committee (Drs. Vicente García-Patos, Mar Llamas, Antonio Torrelo, Francisco Allegue and Gastón Roustan) for their helpful comments during the evaluation of the study proposal.
Ángel Aguado-García (Clínica Belaneve, Alicante); Antonio José Durán Romero (Hospital Universitario Puerta del Mar, Cádiz); Francisco Javier García Martínez (Clínica Universidad de Navarra, Madrid); Javier Fernández-Vela (Hospital General de Granollers, Barcelona); Lucía Pérez Varela, María Luisa Fernández Díaz y Pedro Mercader García (Hospital Universitario Morales Meseguer, Murcia); Ramón Grimalt (Clínica Grimalt); Sandra Mateo Suárez (Clínica Mateo Suárez, Santiago de Compostela), Miguel Ángel Descalzo Gallego (Unidad de Invesigación, Academia Española de Dermatología y Venereología) y Francisco Allegue (Complexo Hospitalario Universitario CHUVI, Vigo).