Shingles is the cutaneous expression of the reactivation of latent varicella zoster virus infection in sensory ganglia. It presents as vesicles in the corresponding dermatome. The condition is called disseminated herpes zoster (DHZ) when more than 2 contiguous dermatomes are affected, more than 20 vesicles are observed outside the initial dermatome, or involvement is systemic. DHZ is rare and most frequently occurs in immunocompromised patients.
ObjectivesTo describe the epidemiology, predisposing factors, clinical presentation, laboratory findings, and clinical course of patients with DHZ, and to compare the findings in immunocompromised and immunocompetent patients.
MethodologyWe analyzed a retrospective case series of adults hospitalized between February 2010 and October 2015.
ResultsForty-one patients with virologically confirmed manifestations of DHZ were included. Stress as a trigger factor was detected in 39% and immunodepression in 58.5%. Immunocompromised patients were younger than the immunocompetent patients (mean ages, 60.5 vs 82 years, P<.01). The 8 immunocompetent patients with no detectable trigger factors were older (mean age, 85 years). In 95% of cases, DHZ was initially limited to a single dermatome and then spread to other dermatomes or became disseminated. Thrombocytopenia was detected in 56% of cases. Complication rates were similar in immunocompromised and immunocompetent patients (54% vs 59%, P>.01). Six patients died; there was no difference in mortality between the 2 groups.
ConclusionThis study provides evidence on the relationship between DHZ, the presence of underlying immunodepression, and complications. Immunosenescence may play an important role in the onset of this disease in older immunocompetent patients.
El herpes zoster es la expresión cutánea de la reactivación de una infección latente en ganglios sensitivos por el virus varicela zoster que se presenta con vesículas en el dermatoma correspondiente. Cuando compromete más de 2 dermatomas contiguos, presenta más de 20 vesículas por fuera del dermatoma inicial o tiene compromiso sistémico se denomina herpes zoster diseminado (HZD). Esta entidad es infrecuente y se suele presentar en pacientes inmunodeprimidos.
ObjetivosDescribir la epidemiología, factores predisponentes, clínica, laboratorio y evolución clínica de pacientes con HZD. Comparar hallazgos entre inmunodeprimidos e inmunocompetentes.
MetodologíaEstudio de una serie de casos retrospectiva, en adultos ingresados entre febrero de 2010 y octubre de 2015.
ResultadosSe incluyeron 41 pacientes con cuadro clínico de HZD y confirmación virológica. Se detectaron factores de estrés desencadenantes en el 39% y de inmunodepresión en el 58,5%. Los inmunodeprimidos fueron más jóvenes que los inmunocompetentes (60,5 vs 82 años, p<0,01). En 8 casos sin inmunodepresión ni desencadenantes se halló mayor edad (85 años). En el 95% de los casos el HZD comenzó en dermatomas definidos, y luego se extendió a otros o se generalizó. El 56% de los casos presentó trombocitopenia asociada. Las complicaciones afectaron por igual a inmunodeprimidos e inmunocompetentes (54% vs 59%, p>0,01). Fallecieron 6 pacientes, sin diferencias de mortalidad entre ambos grupos.
ConclusiónEste estudio aporta evidencia sobre la relación entre el HZD, la presencia de inmunosupresión subyacente y las posibles complicaciones. En pacientes inmunocompetentes, por inmunosenescencia, la edad podría desempeñar un papel importante en la aparición de esta enfermedad.
Primary infection by varicella zoster virus (VVZ) usually presents with varicella, a vesicular and pruriginous exanthem that extends down the body. It affects most people during infancy or adolescence, and evidence from serology studies shows that 98% of adults have been exposed to the virus.1,2
After primary infection, VVZ remains latent in the sensory ganglia of the dorsal root. Herpes zoster is an acute reactivation of this latent infection that affects 15% of immunocompetent patients and 50% of immunocompromised patients during their lifetime. Reported risk factors for and triggers of this condition include advanced age, physical and/or psychological stress, and the immunodepression caused by AIDS, transplantation, cancer, autoimmune diseases, and immunosuppressive treatment.1,2 Reactivation of VVZ leads to transient viremia that is rapidly controlled by the acquired immune system and usually presents with pain and vesicles in the affected dermatome. Viral particles occasionally reach skin at some distance from the affected dermatome to produce aberrant vesicles (<20).2–6 When viremia persists, it increases the probability of visceral involvement, and >20 vesicles can appear at sites outside the initial dermatome and/or affect >2 contiguous or noncontiguous dermatomes. In this case, the disease is known as disseminated herpes zoster (DHZ).1,7–9
The percentage of cases of DHZ reported in the literature varies, although it is believed to be as high as 40% in immunocompromised patients.2–6,10–12
DHZ is thought to present more often in immunocompromised patients, with severe clinical outcome and greater mortality, although it has been reported exceptionally in immunocompetent patients. The objectives of the present study were to describe the demographic and clinical characteristics, predisposing factors, clinical presentation, laboratory findings, and clinical course of patients with DHZ and to compare findings between immunocompromised and immunocompetent patients.
Materials and MethodsDesignRetrospective observational study of adult patients admitted to a tertiary hospital.
PopulationThe electronic clinical records of patients admitted with suspected DHZ between February 2010 and October 2015 were reviewed. The inclusion criteria were age >17 years, previous examination by a dermatologist, fulfillment of the clinical criteria for DHZ, and virological confirmation by detection of the antigen using direct immunofluorescence or polymerase chain reaction (PCR) based on a swab sample of the vesicles. Patients were followed for 3 months after diagnosis. Cases of suspected DHZ, where the presence of VVZ could not be confirmed, were excluded.
Measurements and Variables StudiedThe electronic clinical histories were reviewed. Demographic data and clinical characteristics were obtained from a secondary electronic database. Data were retrieved by 2 independent operators and reviewed by the principal investigator in order to detect erroneous values and missing information. The specific data recorded were age, sex, preexisting baseline comorbidities and comorbidities diagnosed after onset of DHZ, factors that triggered physical or psychological stress, previous immunosuppressive treatment, clinical characteristics at onset, and laboratory variables. Patients were considered to be immunocompromised if their underlying disease or treatment led to alteration of cellular and/or humoral immunity, namely, treated or untreated HIV infection, solid organ transplantation managed with immunosuppressive treatment, hematologic-oncologic disease or solid organ tumors treated or not treated with chemotherapy, diseases requiring treatment with more than 20mg of prednisone for 2 weeks (eg, autoimmune diseases or pulmonary fibrosis), and hypogammaglobulinemia (agammaglobulinemia). We also recorded outcome, associated symptoms, early and late complications (including postherpetic neuralgia at 3 months after the initial symptoms), and death during admission.
Statistical AnalysisThe baseline characteristics of the population were expressed using percentages for the categorical variables and the median (IQR) for continuous variables. The baseline characteristics of patients with a clearly defined predisposing cause of DHZ were compared with those of patients with no trigger. The Fisher exact test was used for categorical variables and the Mann-Whitney test for continuous variables. Statistical significance was set at P<.01.
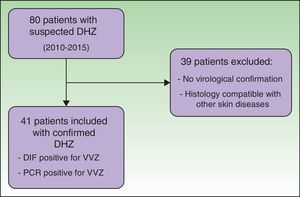
ResultsWe reviewed the clinical histories of 80 patients who had been admitted with suspected DHZ. During the study period, 41 patients fulfilled the inclusion criteria, with confirmation of VVZ by direct immunofluorescence assay or PCR (Fig. 1).
As for demographic characteristics (Table 1), 20 patients (48.78%) were women, and the median age was 70 (52-82) years.
Baseline Characteristics of the Study Population.
| Covariates | Patients With Confirmed DHZ (n=41) | |
|---|---|---|
| Demographic characteristics | ||
| Female sex, No. (%) | 20 | 48.78 |
| Median (IQR) age, y | 70 | (52-82) |
| Predisposing factors and triggers | ||
| Predisposing factors, No. (%)a | 24 | 58.54 |
| Solid organ transplant | 7 | 17.07 |
| Hematologic neoplasm | 6 | 14.63 |
| Solid organ neoplasm | 4 | 9.76 |
| Untreated HIV infection | 4 | 9.76 |
| Autoimmune disease under treatment | 4 | 9.76 |
| Idiopathic pulmonary fibrosis under treatment | 1 | 2.44 |
| Immunosuppressive treatment (long-term corticosteroids or chemotherapy) | 17 | 41.46 |
| Stress factors No. (%)b | 16 | 39.02 |
| Medical cause | 12 | 75 |
| Surgical cause | 3 | 18.75 |
| Injury | 1 | 6.25 |
| Clinical features | ||
| Onset, specific dermatome, No. (%) | 39 | 95.12 |
| Onset, generalized, No. (%) | 2 | 4.88 |
| Progression to more than 2 contiguous dermatomes, No. (%) | 25 | 60.97 |
| Progression to disseminated disease, No. (%) | 14 | 34.14 |
| Symptoms on presentation, No. (%) | ||
| No symptoms | 15 | 36.59 |
| Pain | 21 | 51.22 |
| Pruritus | 3 | 7.32 |
| Burning sensation | 2 | 4.88 |
| Clinical presentation, No. (%) | ||
| Classic | 29 | 70.73 |
| Atypicalc: | 12 | 29.27 |
| Purulent vesicles | 6 | 14.63 |
| Hemorrhagic vesicles | 3 | 7.32 |
| Purulent and hemorrhagic vesicles | 2 | 4.88 |
| Necrosis | 1 | 2.44 |
| Mucosal involvement, No. (%) | 12 | 29.27 |
| Ocular involvement, No. (%) | 7 | 17.07 |
| Feverd, No. (%) | 15 | 36.59 |
| Laboratory valuese | ||
| Anemia, No. (%) | 10 | 24.39 |
| Leukocytosis, No. (%) | 6 | 14.63 |
| Leukopenia, No. (%) | 13 | 31.71 |
| Thrombocytopenia, No. (%) | 23 | 56.10 |
| Mild (100-150×103/μL) | 12 | 29.27 |
| Moderate (100-50×103/μL) | 8 | 19.51 |
| Severe (<50×103/μL) | 3 | 7.32 |
| Thrombocytosis, No. (%) | 1 | 2.44 |
| Alterations in liver values, No. (%) | 8 | 19.51 |
| Alterations in kidney function, No. (%) | 5 | 12.20 |
| Specific complications | ||
| No complications, No. (%) | 18 | 43.90 |
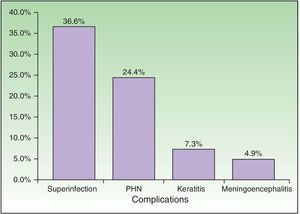
| Complications, No. (%) | 23 | 56.10 |
| Bacterial superinfection | 15 | 36.59 |
| Postherpetic neuralgia | 10 | 24.39 |
| Meningoencephalitis | 2 | 4.88 |
| Keratitis | 3 | 7.32 |
| Outcome | ||
| Cure | 35 | 85.37 |
| Death | 6 | 14.63 |
Abbreviations: DHZ, disseminated herpes zoster; IQR, interquartile range; HIV, human immunodeficiency virus.
Predisposing factors are understood to be preexisting diseases that cause immunodepression by themselves or as a result of treatment.
Stress factors are understood to be any medical, surgical, or injury-based cause that could trigger reactivation of VVZ.
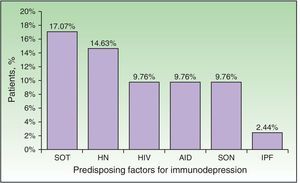
Immunodepression was recorded in 24 patients (58.54%). Some patients had more than 1 comorbid condition that affected their immune response (Fig. 2). The most frequent causes of immunodepression were solid organ transplant (7 patients [17.07%]) and hematologic-oncologic diseases (6 patients [14.63%]), whereas autoimmune diseases, treatment of solid tumors, and HIV infection affected 4 patients each (9.76%). One patient was receiving corticosteroids for treatment of idiopathic pulmonary fibrosis. A total of 17 patients (41.46%) were receiving corticosteroids or chemotherapy. The search for immunodepression arising from DHZ revealed 1 case of HIV, 1 neuroendocrine tumor of the ileum, and 1 case of lymphoplasmacytic lymphoma. Stress as a trigger factor (severe infection, major surgery, or injury) was detected in 16 cases (39.02%). Immunocompromised patients were significantly younger than immunocompetent patients (median, 60.5 [43.5-70] years vs 82 [75-87] years; P<.01) (Table 2). Although triggers were less common in immunocompromised patients than in immunocompetent patients (7 [29.17%] vs 9 [52.94%]), the difference was not significant. No frank immunodepression or stress as a trigger was detected in 8 cases (19.51%), although median age was higher in immunocompromised patients with stress factors than in immunocompetent patients with stress factors (85 vs 77 years).
Comparison Between Immunocompromised and Immunocompetent Patients.
| Immunocompromised | Immunocompetent | P Value | |
|---|---|---|---|
| Demographic characteristics | |||
| Female sex, No. (%) | 12 (50) | 8 (47.06) | .85 |
| Median (IQR) age, y | 60.5 (43.5-70) | 82 (75-87) | .0001 |
| Stress factors, No. (%) | 7 (29.17) | 9 (52.94) | .12 |
| Atypical clinical presentation, No. (%) | 8 (33.33) | 4 (23.53) | .49 |
| Complications, No. (%) | 13 (54.17) | 10 (58.82) | .77 |
| Bacterial superinfection | 10 (41.67) | 5 (29.41) | .42 |
| Postherpetic neuralgia | 5 (20. 83) | 5 (29.41) | .53 |
| Meningoencephalitis | 1 (4.17) | 1 (5.88) | .80 |
| Keratitis | 1 (4.17) | 2 (11.76) | .36 |
| Death, No. (%) | 3 (12.5) | 3 (17.65) | .65 |
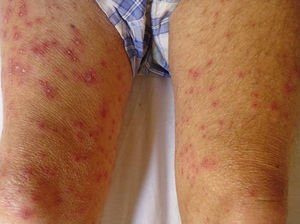
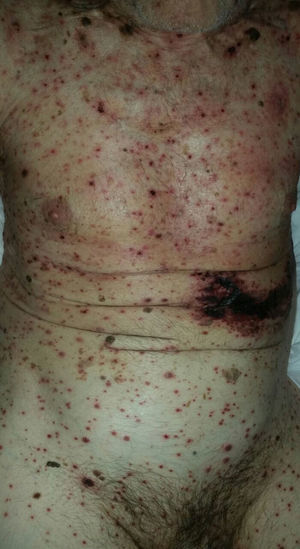
As for clinical outcomes (Fig. 3), the initial clinical manifestations were disseminated vesicles in 2 patients, whereas in 39 patients (95.12%), onset of DHZ affected specific dermatomes before spreading to contiguous dermatomes (25 cases) and becoming disseminated (14 cases). Associated symptoms were observed in 26 patients (63.41%). Pain was the most frequent manifestation, affecting half of all cases. Less frequently, symptoms were associated with burning sensation, paresthesia, and pruritus. The classic clinical presentation took the form of clusters of vesicles with serous content on erythematous plaques. The vesicles eventually ruptured, leaving serous and hemorrhagic crusts or erosions in 29 patients (70.73%). On the other hand, in 12 patients—4 immunocompetent (23.53%) and 8 immunocompromised (33.33%)—we observed atypical manifestations such as purulent or bloody vesicles. The only immunocompromised patient who presented cutaneous necrosis during the course of the disease died (Fig. 4). However, analysis of the frequency of atypical clinical presentation revealed no statistically significant differences between the groups (P>.01). Mucosal involvement was recorded in 12 patients (29.27%) and ocular involvement in 7 (17.07%). Fever, which was not attributable to bacterial superinfection or other septic foci, was recorded in 15 patients.
Of all the laboratory findings that could be attributed to DHZ, the most outstanding was thrombocytopenia, which was observed in 23 patients (56.10%). In almost half of these patients (11 [47%]), thrombocytopenia reached moderate or severe values (<100×103/μL and 50×103/μL, respectively), although no cases of hemorrhage were recorded. Other findings included leukopenia (13 patients [31.71%]), anemia (10 patients [24.39%]), and asymptomatic alterations in liver biochemistry tests (8 patients [19.51%]).
Complications were recorded in 23 patients (56.10%) (Fig. 5) and affected immunocompromised and immunocompetent patients equally (54.17% vs 58.82%). Minimal differences were detected when both groups were compared, although these were not statistically significant. The most common complication was bacterial superinfection (15 patients [36.59%]), which was more frequent in immunocompromised patients (10 patients [41.67%]) than in immunocompetent patients (5 [29.41%]). The second most common complication was postherpetic neuralgia (10 patients [24.39%]), which was more common in immunocompetent patients (5 [29.41%]) than in immunocompromised patients (5 [20.83%]). Three patients had keratitis, and 2 patients had meningoencephalitis with isolation of VVZ in cerebrospinal fluid by PCR. With respect to outcome, 35 patients (85.37%) were cured with systemic antiviral treatment.
All patients were prescribed acylovir 10 to 15mg/kg tid administered intravenously or adjusted for renal function—at least until new lesions ceased to appear—before switching to oral treatment. Treatment led to remission of symptoms in 35 cases (85.37%). Six patients (14.63%) died. The 2 patients with meningoencephalitis died (1 immunocompetent and 1 immunocompromised). One immunocompromised patient died with suspected nervous system involvement, although this was not confirmed, as no lumbar puncture was performed; the remaining 3 patients died of multiple intercurrent conditions. No significant differences in mortality were recorded between immunocompetent patients and immunocompromised patients (17.65% vs 12.50%).
DiscussionOur study included only patients with DHZ, who comprise the largest population with this disease reported to date. We determined the epidemiology, clinical manifestations, and outcome of patients with DHZ and compared our findings between immunocompetent and immunocompromised patients.
Consistent with findings reported elsewhere, our analysis of epidemiological data revealed no differences in the frequency of DHZ between the sexes.10–12 The incidence of classic herpes zoster, which affects only 1 dermatome, has been shown to increase with age: more than 66% of patients with this disease are aged >50 years. We reported the same finding in the patients with DHZ we studied.1,3–5,7,10,11,13–16 This association seems to be related to senescence, or aging, of the immune system. Several studies have shown that the cellular immune response by lymphocytes stimulated in vitro through exposure to VVZ is diminished with age. This phenomenon is known as immunosenescence.2,3,17
In the study carried out by Merselis et al.12 between 1932 and 1962—the only one to analyze DHZ in both immunocompromised and immunocompetent individuals—of the 17 patients studied, 11 (65%) were considered immunocompromised because of diseases known at the time. We found immunodepression-related diseases in 24 patients (58.54%). Consistent with the literature, the causes of immunodepression we identified compromised cellular immunity, with no cases involving alterations of humoral immunity.2–4,6,12,16,18–24 DHZ was the first manifestation of an underlying immunodepressive systemic disease in 3 patients, thus highlighting the importance of assessing predisposing comorbid conditions in patients with DHZ.
Cases of DHZ in immunocompetent patients are exceptional in the literature, and no studies have evaluated the risk of dissemination of herpes zoster in this population.13–15,25–27 The high percentage of cases of DHZ in immunocompetent patients that we found in our study suggests the possibility of dissemination in any patient with herpes zoster. The statistically significant difference in age between immunocompetent and immunocompromised patients suggests that age per se could be a risk factor for DHZ.11,28
In most of the cases of DHZ reported in the literature and in 95% of patients in the present study, the disease initially affected a specific dermatome before spreading to contiguous dermatomes or becoming disseminated.28 The vesicles that are typical of this disease can progress with suppuration, hemorrhagic content, or necrosis. In contrast with our observations on age, we found no significant differences between immunocompetent and immunocompromised patients with respect to clinical manifestations. Only 1 immunocompromised patient had necrosis of cutaneous lesions, which was associated with a poor prognosis due to a fatal outcome.11,20,21,24
The laboratory alterations caused by VVZ were reported during primary infection, and we found that they are also frequent in DHZ. Thrombocytopenia affected more than half of the patients and in some cases reached critical values. Leukopenia, anemia, and altered liver biochemistry values were frequent. Therefore, it is important to monitor these laboratory parameters and to evaluate the mechanisms underlying the alterations owing to their potential clinical repercussions.2,3
In a study of 1669 patients with classic nondisseminated herpes zoster, complications were recorded in 28% of cases, with a significant difference between immunocompromised and immunocompetent patients (20% vs 9%).5 In contrast, among the patients with DHZ in our study, in whom VVZ had spread across the skin, the percentage who presented other, additional complications was much higher, and the complications affected both immunocompetent and immunocompromised patients equally. The incidence of postherpetic neuralgia is 10%-20% in patients with herpes zoster and increases with age. We recorded similar data for DHZ.5,10 Postherpetic neuralgia is caused by inflammation and necrosis of the sensory ganglia, thus explaining the greater number of cases of this complication in immunocompetent patients, who maintain the possibility of a more intense immune response. However, the difference between immunocompetent and immunocompromised patients was not significant. The most severe complication we observed was meningoencephalitis, which was fatal in 2 cases (1 immunocompromised and 1 immunocompetent). In contrast with findings from the literature, no other complications (eg, motor involvement, Ramsay-Hunt syndrome, or vasculitis) were detected.22,23,26,28
All patients with DHZ received treatment with intravenous acyclovir. Studies in immunocompromised patients show that the main risk factor for dissemination of VVZ is the time between onset of symptoms and treatment, and a longer delay until initiation of treatment increases the risk.11,25,29 Therefore, in these cases it is necessary to start intravenous antiviral therapy early.
Our review of the literature revealed that the mortality of DHZ was 5%-15% in non–HIV-infected immunocompromised patients and 26% in HIV-infected patients.28 These findings were similar to ours. Of note, there were no differences in mortality between immunocompetent and immunocompromised patients, thus indicating that this outcome could affect all patients.
In conclusion, our study provides information on demographic characteristics, underlying immunosuppression, triggering factors, clinical manifestations, laboratory alterations, and outcome in patients with DHZ. We show that, in the present study population, the frequency of DHZ was similar in immunocompetent and immunocompromised patients. In addition, comparison of findings for both groups revealed significant differences in age, although no significant differences were found for clinical manifestations, outcome, or mortality. This finding highlights the important role of age in immunocompetent patients with respect to onset of the disease owing to immunosenescence. It also shows that, in both immunocompetent and immunocompromised patients, onset of herpes zoster can take the form of disseminated disease. Therefore, the disease should be suspected in all patients irrespective of immune status, and both diagnosis and treatment should be early. Future studies could be performed to evaluate whether the vaccine that has been shown to prevent reactivation of VVZ and its potential complications could also be used to reduce the risk of dissemination by improving specific cellular immunity.3,14,22,24,30
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purposes of this study.
Confidentiality of dataThe authors declare that they have followed their institutional protocols on publication of patient data.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Bollea-Garlatti ML, Bollea-Garlatti LA, Vacas AS, Torre AC, Kowalczuk AM, Galimberti RL, et al. Características clínicas y evolutivas de una población con herpes zoster diseminado: un estudio de cohorte retrospectiva. Actas Dermosifiliogr. 2017;108:145–152.