The “Impact of scalp pruritus in dermatological consultations in Spain: The SCALP-PR trial” was initiated to address the common yet often insufficiently examined issue of scalp pruritus in dermatology. This condition leads to an uncontrollable urge to scratch, affecting the patients’ quality of life and potentially causing scalp damage. This study aimed to explore the prevalence, patient profile, underlying conditions, and therapeutic approaches for scalp pruritus in Spain, and to assess the safety and efficacy profile, as well as the tolerability of a non-pharmacologic treatment.
MethodsFrom 2021 through 2022, 75 dermatologists enrolled a total of 359 patients in a study on scalp pruritus, approved by the Bellvitge University Hospital Research Ethics Committee, Barcelona, Spain. This evidence-based research combined a meta-analysis with observational study techniques focused on real-world evidence to examine the therapeutic impact on quality of life (QoL). Utilizing the Dermatology Life Quality Index (DLQI) for QoL assessments, the study evaluated the effectiveness of the topical product over 15 days. Data collection was conducted via an eCRF and analyzed with statistical methods to provide reliable insights into the management of scalp pruritus.
ResultsThe prevalence of scalp pruritus in Spain was found to be 6.9%, predominantly among women with a mean age of 52.5 years. The leading causes identified were seborrheic dermatitis and pruritus of undetermined etiology or sensitive scalp. Stress was noted as a key factor, with corticosteroids and hygienic measures being common therapies. The topical product demonstrated significant reductions in pruritus and scratching in more than 90% of patients after 15 days. Improvements were also seen in dermatological quality of life, with 87.1% of patients showing enhancements in DLQI scores. The product was well-received thanks to its cosmetic properties, with high ratings in texture, ease of application, and fragrance.
ConclusionThe topical product studied is a safe, effective, and cosmetically appealing treatment, improving scalp pruritus in various etiologies for most patients. The results highlight the need for patient-center treatments in dermatology, providing important insights for clinical practice and future research.
El estudio «Impacto del prurito del cuero cabelludo en las consultas dermatológicas en España: el estudio SCALP-PR» se inició para abordar el problema común, pero a menudo insuficientemente examinado, del prurito del cuero cabelludo en la dermatología. Esta entidad clínica conduce a un impulso incontrolable de rascarse, afectando la calidad de vida y potencialmente causando daño en el cuero cabelludo. Este estudio tuvo como objetivo explorar la prevalencia, el perfil del paciente, las patologías subyacentes y los enfoques terapéuticos para el prurito del cuero cabelludo en España, y evaluar la eficacia, la seguridad y la tolerabilidad de un tratamiento no farmacológico.
MétodosEn 2021-2022, 75 dermatólogos inscribieron a 359 pacientes para un estudio sobre prurito del cuero cabelludo, aprobado por el Comité de Ética del Hospital Universitario de Bellvitge, Barcelona. Esta investigación basada en la evidencia combinó metaanálisis con técnicas de estudio observacional, enfocándose en evidencia del mundo real para examinar los impactos del tratamiento en la calidad de vida (QoL). Utilizando el Índice de Calidad de vida en dermatología (DLQI) para las evaluaciones de QoL, el estudio evaluó la efectividad del producto tópico durante 15 días. La recolección de datos se realizó a través de un eCRF, analizada con métodos estadísticos para proporcionar percepciones confiables sobre el tratamiento del prurito del cuero cabelludo.
ResultadosLa prevalencia del prurito del cuero cabelludo en España se encontró en un 6,9%, predominantemente entre mujeres con una edad promedio de 52,5 años. Las principales causas identificadas fueron la dermatitis seborreica y el prurito de origen indeterminado o cuero cabelludo sensible. El estrés se señaló como un factor clave, siendo los corticosteroides y las medidas higiénicas los tratamientos comunes. El producto tópico demostró reducciones significativas en el prurito y el rascado en más del 90% de los pacientes después de 15 días. También se observaron mejoras en la calidad de vida dermatológica, con el 87,1% de los pacientes mostrando mejoras en los puntajes del DLQI. El producto fue bien recibido por sus propiedades cosméticas, con altas calificaciones en textura, facilidad de aplicación y fragancia.
ConclusiónEl producto tópico estudiado es un tratamiento seguro, efectivo y estéticamente atractivo, mejorando el prurito del cuero cabelludo en diversas etiologías para la gran mayoría de los pacientes. Los resultados destacan la necesidad de tratamientos centrados en el paciente en dermatología, proporcionando percepciones importantes para la práctica clínica y la investigación futura.
Scalp pruritus, a prevalent yet often under-explored concern in dermatology, prompted the initiation of the “Impact of scalp pruritus in dermatological consultations in Spain: The SCALP-PR trial.” This symptom triggers an uncontrollable need to scratch. This scratching, in turn, intensifies the inflammation and irritation, perpetuating a challenging cycle of pruritus and scratching.1,2 Such a cycle not only affects the patient's quality of life, but can also lead to scalp erosions, scaling, and infections, exacerbating the condition. Moreover, scalp pruritus, which can be associated with both dermatological and non-dermatological conditions, may present in both localized and generalized forms. Its complex etiology and management pose significant challenges for dermatologists, and if not properly treated, can severely impact patient well-being.3
Beyond its medical implications that stemming from cutaneous pathophysiology, scalp pruritus also poses a cosmetic inconvenience, primarily due to the discomfort it causes and its association with visible scaling, often referred to as dandruff. Patients may report varying degrees of this condition, from those exhibiting pruritus symptoms without visible signs to others with more severe signs and pronounced scaling. Scalp pruritus can be a distressing and frustrating condition for both patients and dermatologists.2
Delving into the pathophysiology of pruritus reveals four key mechanisms: pruritoceptive (triggered by activation of cutaneous afferent sensory fibers), neuropathic (resulting from direct neuronal damage), neurogenic (originating from nervous system mediators), and psychogenic (stemming from psychological or psychiatric sources). These mechanisms often interact with each other, and emerging evidence suggests a significant role of scalp microbiome in the pathogenesis of scalp pruritus.4
To further understand scalp pruritus, several key studies have been undertaken. A significant article underscored the diagnostic and therapeutic challenges of scalp pruritus, particularly in cases in which no visible lesions are evident.5 Other important studies of Experimental Dermatology have provided valuable insights into the neurobiology of scalp and hair follicles, with a focus on itch mediators and the unique neural structure of the scalp.2,6
Other research offered a comparative analysis between a non-corticosteroid anti-inflammatory/antifungal shampoo and 1% ketoconazole shampoo to treat mild-to-moderate seborrheic dermatitis of the scalp, demonstrating the potential of alternative treatments1 and several studies evaluated the effectiveness of a combination of 3% salicylic acid and 1% hydrocortisone in providing significant relief for scalp pruritus.7,8
Further research explored the antipruritic effects of emulsions containing Echinacea purpurea extract, highlighting its role in the management of both acute and chronic pruritus.2 Additionally, a comprehensive review in January 2019 delved into the pathogenesis, diagnosis, and management of scalp pruritus, emphasizing the importance of a thorough evaluation.9
Our study was primarily focused on evaluating the efficacy, perceived effectiveness, and tolerability of the topical product, Emolienta Scalp®, a non-pharmacologic treatment option, when used both as a primary and an adjunctive therapy. The topical product stands out in the treatment landscape due to its unique formulation that harnesses the benefits of non-pharmacologic components, making it a preferable choice for individuals seeking alternatives to conventional medicated therapies. This approach not only reduces the potential for adverse reactions commonly associated with pharmacological treatments but is also consistent with the growing preference for gentler treatment methods. The management of scalp pruritus involves a variety of approaches, including topical drugs, shampoos, and non-pharmacologic treatments.10–12 Some examples of topical drugs for scalp pruritus include glucocorticoids, calcineurin inhibitors, menthol or capsaicin.
The advantages of using a non-pharmacologic treatment like the topical product understudy include its suitability for long-term use, minimal risk of systemic side effects, and its potential for enhancing skin barrier function without the harsh impacts often seen with chemical-based products. Moreover, the product is specifically designed to address the multifactorial nature of scalp pruritus, providing relief through its soothing properties and aiding in the maintenance of scalp health.
In addition to assessing its therapeutic efficacy, our study also placed a significant emphasis on gauging patient satisfaction. This aspect is crucial as patient adherence and overall treatment experience are greatly influenced by their satisfaction with the treatment. By evaluating patient feedback, we aimed to understand better the real-world effectiveness of the topical product and its impact on improving the quality of life for individuals experiencing scalp pruritus.
MethodsA total of 75 dermatologists collaborated in this study, enrolling 359 patients. The research started in October–November 2021 and lasted until the first quarter of 2022. It received ethical approval from the Bellvitge University Hospital Research Ethics Committee, Barcelona, Spain and all participants gave their written informed consent prior to their involvement in the study.
In the selection of participants for this study, the inclusion criteria were established as follows: individuals must be of legal age and experience pruritus of the scalp, even if it is not the primary reason for consultation. Exclusion criteria were defined to omit any patients currently undergoing any form of treatment for scalp pruritus and those who did not sign the informed consent form.
Given the complex and diverse nature of scalp pruritus, along with a scarcity of comprehensive literature, there was a clear need for a more thorough and structured analysis of medical interventions. Our research methodology was anchored in evidence-based medicine, blending elements of meta-analysis and observational study approaches. Such studies, often referred to as real-world evidence, are instrumental in offering objective data about therapeutic interventions in real-world settings and on patient samples significantly larger than those typically available in clinical trials.
We conducted an open, observational study emphasizing the assessment of quality of life (QoL) at both the baseline visit – to understand the impact of scalp pruritus on QoL – and after 15 days of treatment to determine its improvement. The Dermatology Life Quality Index (DLQI)13 was chosen as the instrument to measure quality of life. The study was supplemented by a home-based questionnaire to assess pruritus progression after 15 days of treatment. The study methodology adhered to the STROBE guidelines for observational studies.14 The topical product, a fluid emollient gel specifically formulated for rapid and sustained relief of scalp pruritus, contains biosaccharide Gum-1, dipotassium glycyrrhizate, hyaluronic acid, and menthol. Three potential prescription protocols were recommended over a 15-day period: once daily, twice daily, and once daily within the first 7 days followed by every other day until completing the 15 days. Data collection was facilitated through an electronic case report form (eCRF) accessible on a secure and certified website, managed by an independent Contract Research Organization (CRO). The study sample was selected from patients attending dermatology consultations across Spain.
For data evaluation and analysis, various statistical methods were used. These included descriptive analysis, ANOVA, chi-square tests, Student t-tests and Wilcoxon tests, univariate analysis, and binary logistic regression, as well as the development of multivariate models. Effect size tables were used to identify significant relationships, while forest plots were used for the graphical representation of results.
This comprehensive methodological approach aimed to ensure the reliability and validity of our findings, contributing valuable insights into the treatment of scalp pruritus.
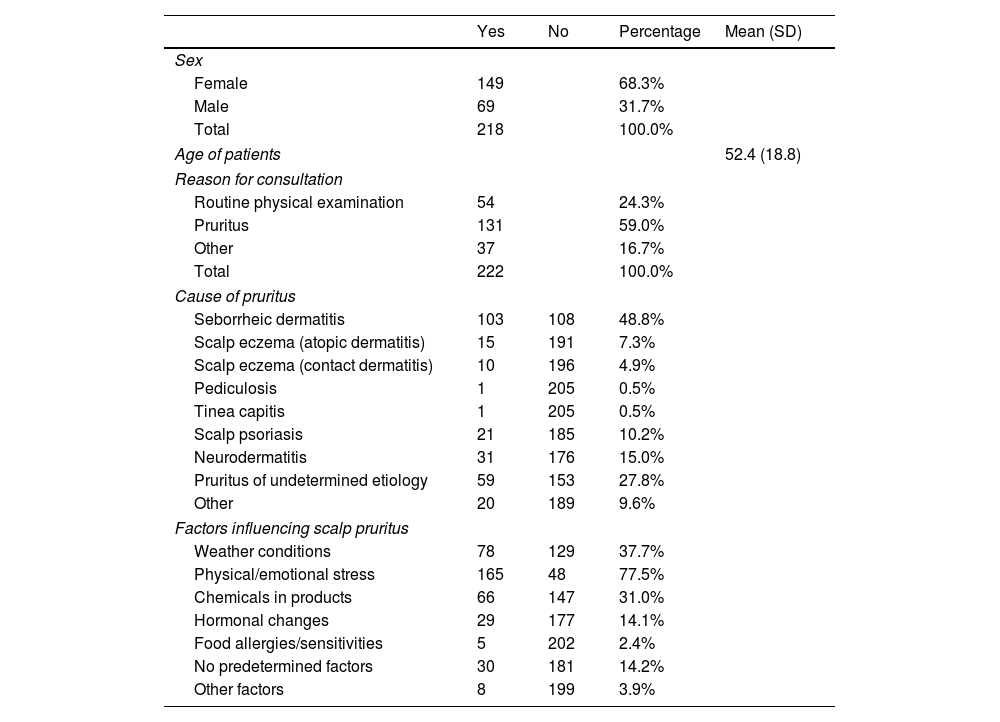
ResultsThe patient profile of patients with scalp pruritus participating in this observational study showed a gender distribution skewed towards women, accounting for 68.2% of participants, while men accounting for 31.8%. The mean age of these patients was 52.5 years, with a standard deviation of 18.9 years. Notably, over half of the patients (50.7%) were older than 52 years (Table 1).
Characteristics of the study sample.
| Yes | No | Percentage | Mean (SD) | |
|---|---|---|---|---|
| Sex | ||||
| Female | 149 | 68.3% | ||
| Male | 69 | 31.7% | ||
| Total | 218 | 100.0% | ||
| Age of patients | 52.4 (18.8) | |||
| Reason for consultation | ||||
| Routine physical examination | 54 | 24.3% | ||
| Pruritus | 131 | 59.0% | ||
| Other | 37 | 16.7% | ||
| Total | 222 | 100.0% | ||
| Cause of pruritus | ||||
| Seborrheic dermatitis | 103 | 108 | 48.8% | |
| Scalp eczema (atopic dermatitis) | 15 | 191 | 7.3% | |
| Scalp eczema (contact dermatitis) | 10 | 196 | 4.9% | |
| Pediculosis | 1 | 205 | 0.5% | |
| Tinea capitis | 1 | 205 | 0.5% | |
| Scalp psoriasis | 21 | 185 | 10.2% | |
| Neurodermatitis | 31 | 176 | 15.0% | |
| Pruritus of undetermined etiology | 59 | 153 | 27.8% | |
| Other | 20 | 189 | 9.6% | |
| Factors influencing scalp pruritus | ||||
| Weather conditions | 78 | 129 | 37.7% | |
| Physical/emotional stress | 165 | 48 | 77.5% | |
| Chemicals in products | 66 | 147 | 31.0% | |
| Hormonal changes | 29 | 177 | 14.1% | |
| Food allergies/sensitivities | 5 | 202 | 2.4% | |
| No predetermined factors | 30 | 181 | 14.2% | |
| Other factors | 8 | 199 | 3.9% | |
This table summarizes the study participants’ demographic and clinical characteristics, and includes gender distribution, mean age, reasons for consultation, causes of pruritus, and factors influencing scalp pruritus. Percentages reflect the proportion of participants within each category. For age, the mean and standard deviation are provided.
Note: Percentages may not total 100% due to rounding.
Significantly more patients (58.8%) sought consultation primarily for scalp pruritus. Additionally, a notable segment attended for routine dermatological check-ups (24.4%), and others presented with different dermatological conditions (16.7%). This diverse patient demographic underscores the varied clinical presentations and the need for tailored therapeutic approaches in managing scalp pruritus.
In terms of the underlying causes of scalp pruritus, seborrheic dermatitis emerged as the most common, affecting 48.8% of patients. This was followed by neurodermatitis, characterized by intensely pruritic, circumscribed lichenified plaques with a chronic course, arising from scratching and not involving immune mechanisms, affecting 15.0% of patients. Scalp psoriasis accounted for 10.2%, and a significant 27.8% of cases were attributed to undetermined etiologies, often categorized as sensitive scalp.
Other less prevalent causes, such as eczema, pediculosis, and tinea capitis, were observed in less than 10% of patients. This diverse range of etiologies highlights the need for a comprehensive diagnostic approach in the management of scalp pruritus, while considering both common and less frequent causes. The prevalence of seborrheic dermatitis as a primary cause also underscores the importance of focusing on effective treatment strategies for this condition.
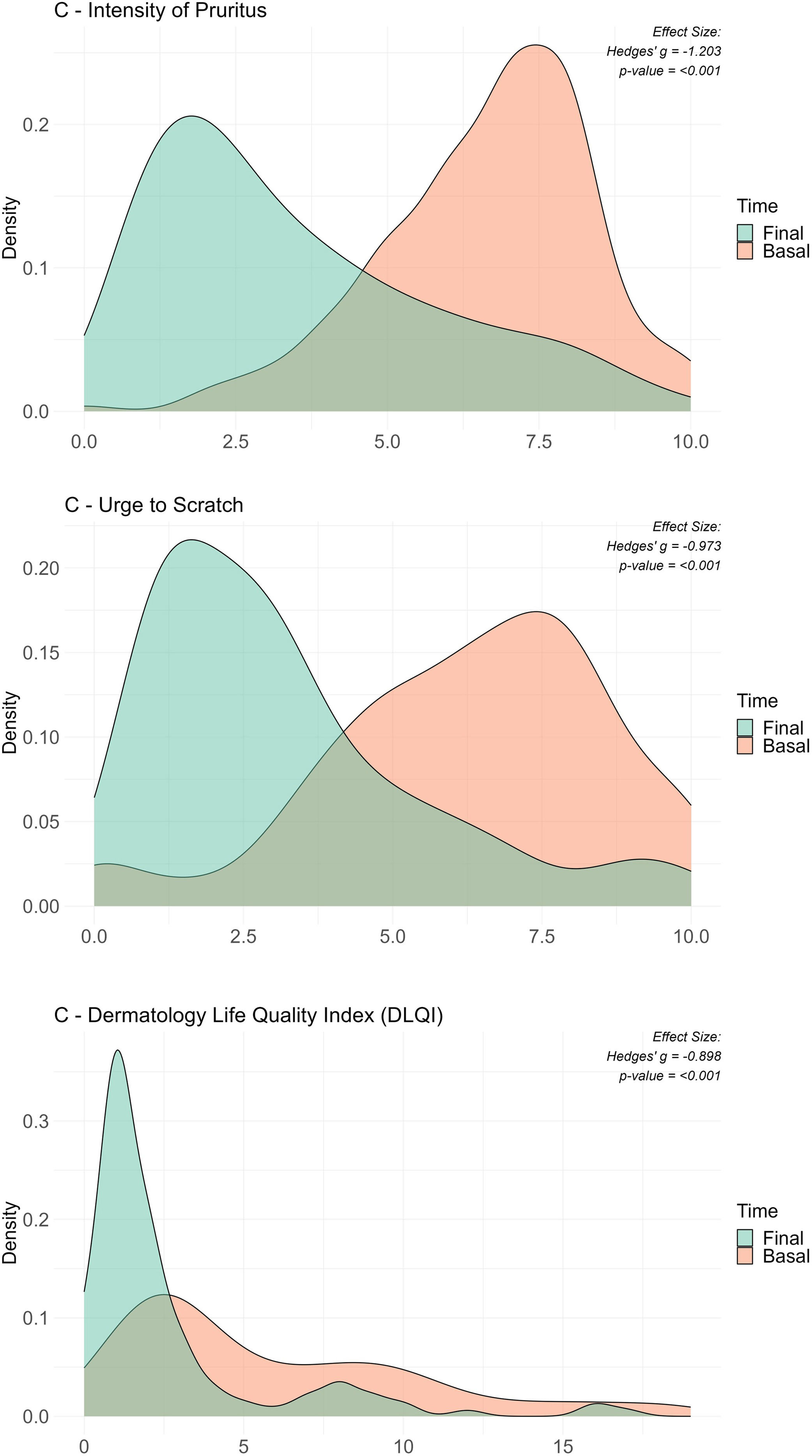
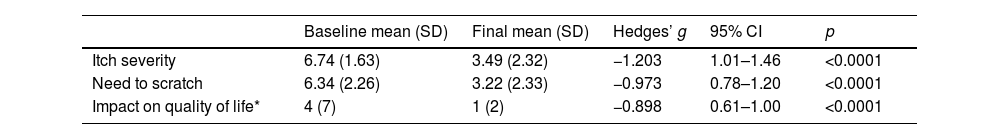
After 15 days of using the topical product, a significant reduction was observed in both the intensity of pruritus (from 6.74 on day 0 down to 3.49 on day 15; effect size: d=1.79; p<0.00001) and urge to scratch (from 6.34 on day 0 down to 3.22 on day 15; effect size: d=1.41; p<0.00001) (Table 2). Remarkably, 91.5% and 89.7% of patients experienced a reduction in these symptoms, respectively.
Evolution of itch severity, need to scratch and quality of life from baseline to final assessment.
| Baseline mean (SD) | Final mean (SD) | Hedges’ g | 95% CI | p | |
|---|---|---|---|---|---|
| Itch severity | 6.74 (1.63) | 3.49 (2.32) | −1.203 | 1.01–1.46 | <0.0001 |
| Need to scratch | 6.34 (2.26) | 3.22 (2.33) | −0.973 | 0.78–1.20 | <0.0001 |
| Impact on quality of life* | 4 (7) | 1 (2) | −0.898 | 0.61–1.00 | <0.0001 |
Note: Values for “Itch severity” and “Need to scratch” are expressed as the mean (SD); for “Impact on quality of life”, the median (IQR) is shown. Hedges’ g was used for effect size.
Furthermore, there was a notable improvement in dermatological quality of life, as measured by the Dermatology Life Quality Index (DLQI), with scores improving from 5.56 on day 0 down to 2.63 on day 15 (effect size: r=0.43; p<0.001) (Table 2). This improvement was observed in 87.1% of patients. A detailed evaluation of all items on the DLQI scale showed significant enhancements, the effect size ranged from r=0.12 to r=0.43. After treatment, half of the patients reported no impact on their quality of life (52.7%), and 33.6% experienced only minor effects.
Most patients experienced improvements across various dermatological assessments. Specifically, 85.5% reported a decrease in the intensity of pruritus (Fig. 1A), 82.4% less urge to scratch (Fig. 1B), and 77.9% showed betterment in their quality of life as indicated by the Dermatology Life Quality Index (DLQI) (Fig. 1C). Conversely, a minority of patients reported worsening symptoms, with 10.7% noting an increase in pruritus intensity, 12.2% an increased urge to scratch, and 9.2% a lower DLQI score. Ties, representing no change, were observed in 3.8% for pruritus intensity, 5.3% for the urge to scratch, and 13.0% for the DLQI. The statistical significance of these findings was confirmed by p-values <0.001 in the Wilcoxon Signed Ranks Test for all three measures, indicating highly significant improvements after treatment.
Comparative density plots for key variables before and after the intervention. This figure shows three density plots illustrating the distributions before and after the intervention for three variables that have shown significant changes from baseline to the final visit. The variables analyzed are (a) intensity of pruritus, (b) urge to scratch, and (c) Dermatology Life Quality Index (DLQI), with baseline and final distributions overlaid to highlight shifts. Each plot includes the effect size measured by Hedges’ g to quantify the standardized mean difference, alongside the p-value from Wilcoxon tests for repeated measures. Despite the initial analysis using Wilcoxon tests, Hedges’ g was chosen to better reflect the size and direction of distribution changes, indicating effect sizes in terms of standard deviation. This visual representation emphasizes the significant impact of the intervention on patient-reported outcomes and quality of life related to dermatological conditions.
Analysis of various factors using logistic regression models revealed that the presence of hormonal factors negatively influenced pruritus improvement. In contrast, patients who rated the absorption speed of the gel highly, and those whose dermatologist used corticosteroids showed more substantial improvement in their pruritus. Neurodermatitis as a cause of pruritus and prescribing the fluid gel by dermatologists to more than 20% of patients were associated with improved relief from the urge to scratch.
The 15-day application regimen of once daily was associated with a higher likelihood of symptom improvement. Regarding gender differences, pruritus of undetermined etiology or sensitive scalp was more common in women (33.6%) vs men (16.2%) (p=0.009). Pruritus associated with chemical factors present in soaps and cosmetics was also more frequent in women (37.6%) vs men (19.1%) (p=0.007), as was the need for scratching during the initial visit, being higher in women (6.5 on a 0–10 on the Visual Analogue Scale) vs men (5.8; p<0.05).
The cosmetic assessment of the topical product revealed overwhelmingly positive responses from the participants, focusing on various attributes like texture, ease of extension, absorption speed, and fragrance. In terms of texture, a significant majority (80.9%) rated it as ‘quite pleasant’ or ‘very pleasant’, while only a minority found it ‘regular’ or less. The ease of spreading the product also received high marks, with 81.7% of respondents describing it as ‘quite easy’ or ‘very easy’ to spread. The product absorption speed was another highlight, with approximately 70% of participants noting that it absorbed ‘quite quickly’ or ‘very quickly’, and about a quarter of them describing it as ‘Regular’. The fragrance of the topical product was also well-received, with more than 70% enjoying it as ‘quite pleasant’ or ‘very pleasant’.
The product soothing effect, skin comfort, hydration, reduction in tightness, and reduction in roughness were equally notable. A significant 78.7% of users found the product ‘quite’ or ‘very soothing’. Similarly, skin comfort and well-being were positively rated, with 76.7% feeling a ‘considerable’ or ‘high’ level of comfort. In terms of hydration, 71.8% observed ‘considerable’ or ‘high’ increases in skin hydration. Moreover, 64.9% experienced a ‘considerable’ or ‘high’ reduction in skin tightness, and around 57% reported a decrease in skin roughness. When asked about overall satisfaction with the fluid gel, 80.9% liked it ‘quite a bit’ or ‘very much’, and a significant 84.3% of participants indicated they would continue using the topical product.
DiscussionThe present study originated with the goal of addressing the frequently encountered yet often under-examined issue of scalp pruritus within dermatology. Through this research, we have gained insights into the prevalence and patient profile of those seeking dermatological consultation for scalp pruritus in Spain. We were able to identify the most common underlying conditions often associated with scalp pruritus, the accompanying factors contributing to its onset, and the therapeutic measures, including products and protocols, used by dermatologists for the management of this entity. The study also evaluated the efficacy, safety, and tolerability of the topical product.
We found that the prevalence of scalp pruritus in dermatological consultations in Spain stands at 6.9%. There is a slight predominance among women, with a mean age of 52.5 years. Seborrheic dermatitis is identified as the leading cause of scalp pruritus, followed by pruritus of undetermined etiology or sensitive scalp, which is the second most common diagnosis.3
Stress emerged as the most significant factor influencing the onset of pruritus, with corticosteroids and hygienic measures being the most widely used therapeutic practices. The topical product demonstrated a significant reduction in both pruritus and the urge to scratch in more than 90% of patients after 15 days of treatment. Treatment regimens deviating from once-daily application over 15 days were associated with a lower likelihood of improvement in the need for scratching.
Significant improvements were seen in dermatological quality of life (DLQI) in 87.1% of patients. All items on the DLQI scale showed substantial improvement, with most effect sizes being large. The topical product is a safe product, as no adverse events or reactions were reported in any of the studies (prospective/retrospective).
These results in Wilcoxon's tests suggest that most patients experienced improvement in the intensity of pruritus, the urge to scratch, and their quality of life as measured by the DLQI after the intervention, with statistically significant changes seen across all measures.
The findings regarding the product cosmetic properties reveal a high level of patient satisfaction, crucial for treatment adherence in dermatological care. The product was well-received in terms of texture, ease of application, absorption rate, and fragrance, aspects that enhance user experience and encourage continued use.
Notably, the product was effective in providing a soothing effect, improving skin comfort, hydration, and reducing skin tightness and roughness. These results suggest that it not only alleviates symptoms of scalp pruritus, but also contributes to overall scalp health. The willingness of most participants to continue using the product underscores its acceptability and potential as a preferred treatment option in dermatology.
In conclusion, our study has expanded the understanding of scalp itching by determining a prevalence of 6.9% in dermatological consultations in Spain, as well as characterizing the patients who experience it. Furthermore, it has demonstrated that the topical product is safe, highly effective, and cosmetically appealing, improving scalp pruritus of any etiology in 9 out of 10 patients. This study significantly contributes to our understanding of scalp pruritus, providing valuable data for both clinical practice and future research in this area.
FundingThis study was generously funded by Laboratorios Viñas S.A., which facilitated the extensive involvement of more than 60 dermatologists across Spain. The financial support provided by Laboratorios Viñas S.A. was instrumental in the execution of this study and the drafting of this article.
Conflict of interestsThe authors state that they have no conflict of interests.
Declaration of AIDuring the preparation of this work, the author(s) used OpenAI ChatGPT to assist with the drafting of the manuscript, enhance language clarity, and improve readability. After utilizing this tool, the author(s) thoroughly reviewed and edited the content as necessary and take full responsibility for the content of the publication.
Aitor De Vicente Aguirre, Alba Calleja Algarra, Alba Gómez Zubiaur, Álvaro Iglesias Puzas, Andrea Pérez González, Aniza Giacaman Contreras, Anna Agustí Mejias, Anna Badell Giralt, Blanca Díaz Ley, Carolina Alexandra Domínguez Mahamud, Clara Matas Nadal, Daniel Ramos Rodríguez, Daniela Subiabre Ferrer, Elena Macías del Toro, Eloy Tarín Vicente, Emili Masferrer i Niubò, Enrique Rodríguez Lomba, Esperanza Manrique Silva, Fátima Tous Romero, Fernando Gallardo Hernández, Francisco José Navarro Triviño, Gemma Martín Ezquerra, Gloria Abad, Iria Montero Pérez, Javier Sabater Abad, Jesús Molinero Caturla, José Antonio Pérez Caballero, José Blasco Melguizo, José Neila Iglesias, Leire Mitxelena Elosegi, Loredana Cannatella, Mª Cristina García, Manuel Sánchez Regaña, María Garayar Cantero, María Luz Nedgrin Diaz, María Pousa Martínez, Marta Bandini, Olane Guergue Díaz de Cerio, Pilar Gómez Centeno, Priti Melwani Melwani, Rafael Aguayo Ortiz, Raquel Pérez Mesonero, Roger Rovira López, Sabrina Kindem Gómez, Sergio Alique García, Tania Marusia Capusán, Vanessa Piquero Casals, Vicente Leils Dosil, Víctor González Delgado, and Xavier Bosch Amate.