Scabies is a highly prevalent parasitic disease that can have a significant impact on quality of life and even lead to serious complications such as streptococcal superinfection and associated kidney damage.1 In young children treatment consists of topical therapy, although this can be poorly tolerated and treatment adherence is often suboptimal.2 Ivermectin is a widely used antiparasitic that is effective for the treatment of scabies.1 Its use is approved in adults and children >15 kg, although recent studies suggest that it may also be safe for lower-weight infants.2,3
We describe 4 cases of children weighing less than 15 kg who had scabies refractory to topical therapy and were successfully treated with oral ivermectin (Table 1).
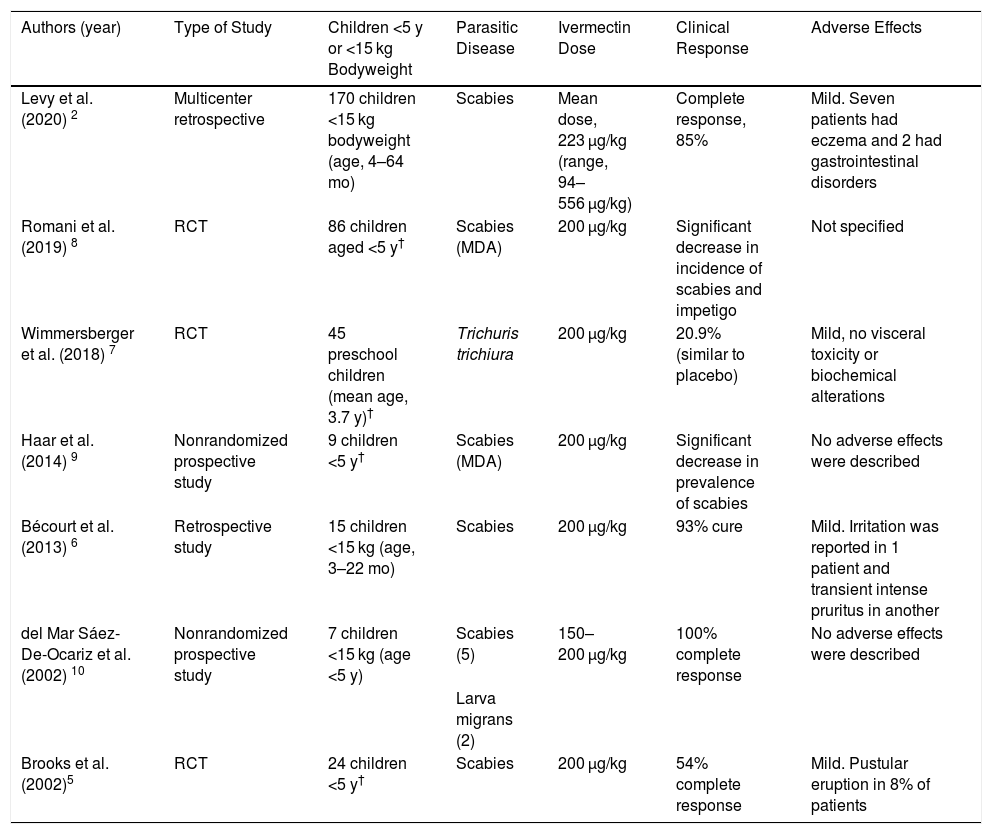
Clinical and Epidemiological Characteristics and Therapeutic Response to Oral Ivermectin in Scabies Patients Weighing <15 kg Refractory to Topical Treatment.
| Patient | Sex/age, m | Weight, kg | Comorbidities | Clinical presentation | Diagnosis | Prior treatments | Oral ivermectin dose | Concomitant topical treatment | Formulation* | Clinical response | Adverse effects |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F/16 | 8 | No | Nodular scabies (multiple nodules on the face, trunk, and extremities) | Clinical and dermoscopic (presence of multiple furrows with delta wing sign) | Topical 5% permethrin (8 applications at weekly intervals) | 2 mg (0.25 mg/kg) on days 0 and 7† | No | Suspension formulated in the hospital pharmacy using 3-mg tablets | Resolution of clinical picture at 3 wk | No |
| 2 | M/13 | 13 | Atopic dermatitis treated with oral corticosteroids (indicated by pediatrician) | Norwegian scabies | Clinical, confirmed by direct examination (Muller test) | Topical 5% permethrin (2 applications 1 wk apart) | 3 mg (0.23 mg/kg) on days 0 and 7† | Petrolatum | Crushed 3-mg tablet | Resolution of clinical picture at 3 wk | No |
| 3 | M/10 | 11 | No | Classical scabies (vesicular/pustular lesions on palms and soles) | Clinical and dermoscopic (presence of multiple furrows with delta wing sign) | Topical 5% permethrin (3 cycles of 2 applications at weekly intervals) | 3 mg (0.27 mg/kg) on days 0 and 7† | No | Crushed 3-mg tablet | Resolution of clinical picture at 3 wk | No |
| 4 | F/20 | 12 | No | Classical scabies (vesicular/pustular lesions on palms and soles) | Clinical and dermoscopic (presence of multiple furrows with delta wing sign) | Topical 5% permethrin (6 applications 1 week apart) | 3 mg (0.25 mg/kg) on days 0 and 7† | No | Crushed 3-mg tablet | Resolution of clinical picture at 3 wk | No |
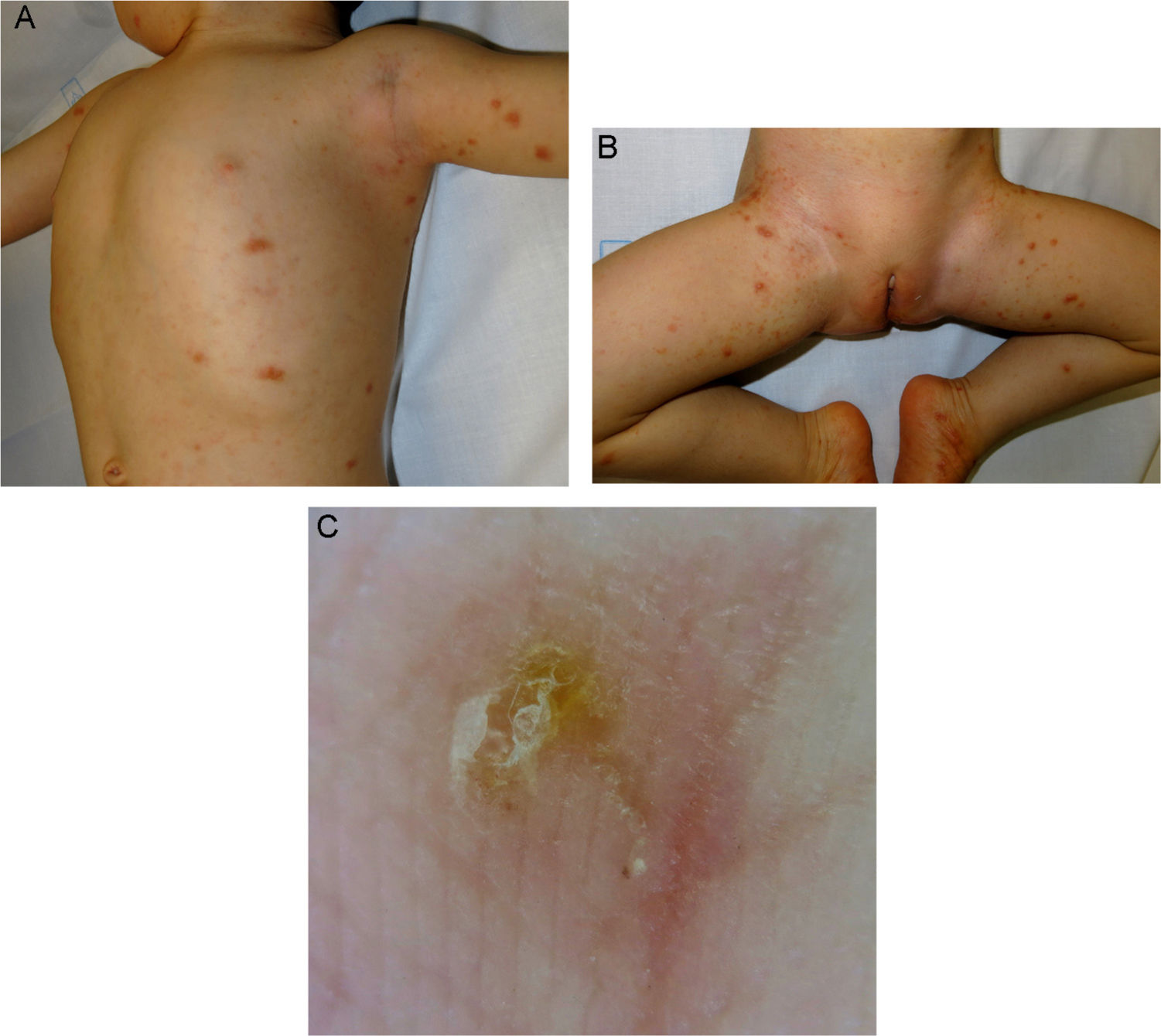
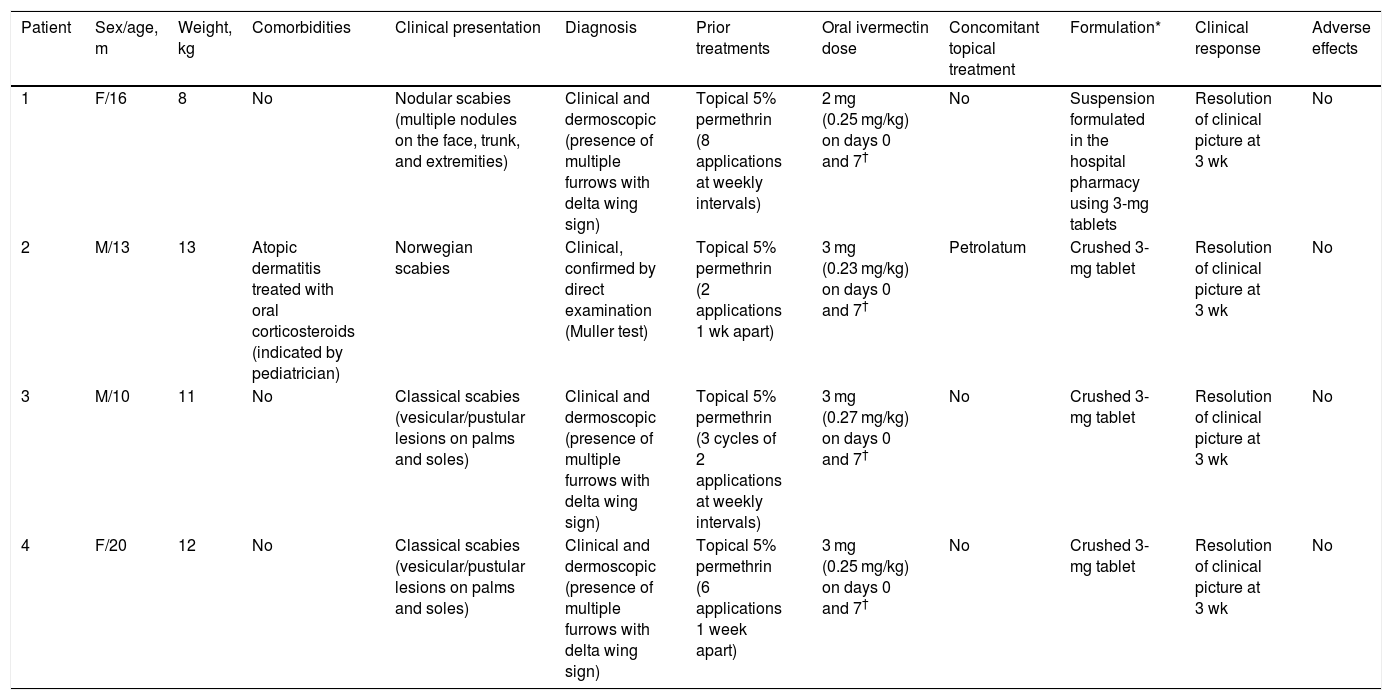
The first patient (age, 16 mo; bodyweight, 8 kg) was referred to our department for nodular scabies. Mite furrows were evident on the feet and wrists. Dermoscopic examination revealed the presence of the delta wing sign (Fig. 1A–C). The patient received two 2-mg doses of ivermectin (250 µg/kg) separated by 7 days. The second patient (age, 13 mo; bodyweight, 13 kg) had a history of atopic dermatitis and was being treated with oral corticosteroids, which were prescribed by the attending pediatrician 2 months earlier. One month earlier the patient had presented with generalized, scaly erythematous lesions, and had been diagnosed with Norwegian scabies (possibly related to immunosuppression secondary to systemic corticosteroid therapy) and treated with topical permethrin on multiple occasions (Table 1). Direct examination of a scale scraping confirmed the presence of Sarcoptes scabiei. The patient received two 3-mg doses of ivermectin (230 µg/kg) separated by 7 days. The third and fourth patients (age, 10 mo and 20 mo; bodyweight, 11 kg and 12 kg) presented with classical scabies lesions, consisting of mite furrows in the skin folds and on the trunk and wrists, as well as itchy vesicles and pustules on the palms and soles. They received two 3-mg doses of ivermectin (270 µg/kg and 250 µg/kg, respectively) separated by 7 days. All 4 cases showed a complete response after 3 weeks, and no adverse effects were observed.
Treatment of scabies in children weighing <15 kg can be complex: topical treatments can be difficult to apply and can cause skin irritation and systemic adverse effects. Ivermectin is an antiparasitic included in the World Health Organization’s list of essential drugs, and its efficacy and tolerance for the treatment of scabies are similar to those of topical permethrin.1 The adverse effects of ivermectin are mostly mild and include dizziness, headache, gastrointestinal symptoms, paresthesia, and eczema.4
A recent retrospective multicenter study of 170 children aged 4–64 months, weighing 4–14 kg (31 < 7.5 kg), who were treated with oral ivermectin (mean dose, 223 µg/kg; range, 94–556 µg/kg), reported a therapeutic response in 85% of participants, with minimal adverse effects. Seven patients had eczema and 2 had mild gastrointestinal disturbances. A dose >200 µg/kg and a dose interval <10 days were associated with a higher probability of remission. Although ivermectin was only available in tablet form, the authors reported no difficulties in administering the drug.2 A systematic review of the literature published in 20183 found 9 studies with a total of 61 patients aged <5 years who were treated with ivermectin for various parasites, 24 of whom weighed <15 kg (no information was available on the weight of the other patients, although based on their age many likely weighed <15 kg), and reported a good therapeutic response among scabies patients, with no significant adverse effects. Of particular note is a randomized clinical trial by Brooks et al.5 of 45 scabies patients aged 1 to 5 years who were treated with a dose of 200 µg/kg. Although the response rate (54%) was lower than that reported in children who received 2 doses,1,2 no moderate or serious adverse effects were observed. Bécourt et al.6 conducted a retrospective study with 15 children weighing <15 kg (age, 3–22 mo) with scabies who were treated with 2 doses of oral ivermectin. A complete response was observed in 93% of patients, and adverse effects were both minimal and transient. A randomized clinical trial (not included in the aforementioned systematic review) evaluated the efficacy of oral ivermectin (200 µg/kg) for the treatment of the parasite Trichuris trichiura in 45 preschool children (mean age, 3.7 y).7 The treatment was not effective, but was well tolerated, resulting in only mild adverse effects (Table 2). Laboratory studies performed at baseline and 72 hours after ivermectin treatment revealed no instances of anemia, plaquetopenia, neutropenia, renal failure, or liver function alterations.
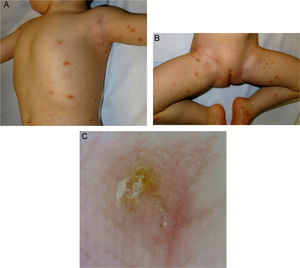
Studies of Oral Ivermectin for the Treatment of Parasitic Diseases in Children Weighing <15 kg*.
| Authors (year) | Type of Study | Children <5 y or <15 kg Bodyweight | Parasitic Disease | Ivermectin Dose | Clinical Response | Adverse Effects |
|---|---|---|---|---|---|---|
| Levy et al. (2020) 2 | Multicenter retrospective | 170 children <15 kg bodyweight (age, 4–64 mo) | Scabies | Mean dose, 223 µg/kg (range, 94–556 µg/kg) | Complete response, 85% | Mild. Seven patients had eczema and 2 had gastrointestinal disorders |
| Romani et al. (2019) 8 | RCT | 86 children aged <5 y† | Scabies (MDA) | 200 µg/kg | Significant decrease in incidence of scabies and impetigo | Not specified |
| Wimmersberger et al. (2018) 7 | RCT | 45 preschool children (mean age, 3.7 y)† | Trichuris trichiura | 200 µg/kg | 20.9% (similar to placebo) | Mild, no visceral toxicity or biochemical alterations |
| Haar et al. (2014) 9 | Nonrandomized prospective study | 9 children <5 y† | Scabies (MDA) | 200 µg/kg | Significant decrease in prevalence of scabies | No adverse effects were described |
| Bécourt et al. (2013) 6 | Retrospective study | 15 children <15 kg (age, 3–22 mo) | Scabies | 200 µg/kg | 93% cure | Mild. Irritation was reported in 1 patient and transient intense pruritus in another |
| del Mar Sáez-De-Ocariz et al. (2002) 10 | Nonrandomized prospective study | 7 children <15 kg (age <5 y) | Scabies (5) | 150–200 µg/kg | 100% complete response | No adverse effects were described |
| Larva migrans (2) | ||||||
| Brooks et al. (2002)5 | RCT | 24 children <5 y† | Scabies | 200 µg/kg | 54% complete response | Mild. Pustular eruption in 8% of patients |
Abbreviations: MDA, mass drug administration (i.e. treatment of all individuals in a community regardless of their status [scabies patients or contacts thereof]; RCT, randomized clinical trial.
Several recent studies evaluating mass drug administration (MDA), which involves the treatment of all individuals in a community regardless of their status (scabies patients or contacts thereof) have reported encouraging findings in patients with distinct infections and infestations. One clinical trial compared 3 scabies treatment arms: oral ivermectin MDA (including 86 children <5 y), topical permethrin MDA, and standard topical permethrin therapy of scabies patients and their contacts. Evaluation of the 823 participants after 24 months revealed that the prevalence of scabies and impetigo were significantly lower in the oral ivermectin MDA arm. The incidence of adverse effects was not reported.8
Since mid-2021, oral ivermectin has been commercially available in Spain (Ivergalen, Galenicum Derma) at a dose of 12-mg (four 3-mg tablets), facilitating prescribing of this drug.
Oral ivermectin appears to be well tolerated in children weighing <15 kg with scabies. Randomized clinical trials are a priority given the high prevalence of various parasites at this age and the good results obtained with MDA of this drug.
FundingThis work has not received any type of funding.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Morgado-Carrasco D, Piquero-Casals J, Creus-Vila L, Fustà-Novell X. Ivermectina oral como tratamiento de la escabiosis refractaria en ni˜nos menores de 15 kg. Descripción de cuatro casos y revisión de la literature. Actas Dermosifiliogr. 2022;113:99–103.