Vitamin D (VD) deficiency has been associated with various tumors. However, the association between VD and skin cancer is controversial. Although in non-melanoma skin cancer, adequate or even high levels of VD can be associated with a higher risk of developing tumors, this could be biased by the direct association between sun exposure and VD levels. Regarding melanoma, results are contradictory. Most studies analyzed state that higher levels of VD could reduce the risk of melanoma, be associated with melanomas with better prognosis and with an enhanced antitumor response, and also with fewer adverse events associated with melanoma immunotherapy. However, prospective studies of adequate methodological quality are still needed to assess the association between VD levels and its supplementation and development/prognosis in skin cancer.
La deficiencia de vitamina D (VD) se ha relacionado con diferentes tumores. La asociación entre la VD y el cáncer cutáneo es controvertida. Para el cáncer cutáneo no melanoma, niveles adecuados o incluso elevados de VD podrían asociarse a un mayor riesgo tumoral, aunque este hecho podría estar sesgado por la asociación directa entre exposición solar y niveles de VD. En cuanto al melanoma, hay resultados contradictorios. La mayoría de los estudios analizados reportan que niveles más altos de VD podrían disminuir el riesgo de melanoma, podrían asociarse a melanomas con mejor pronóstico y podrían correlacionarse con una mejor respuesta antitumoral y un menor perfil de efectos adversos en la inmunoterapia del melanoma. Sin embargo, son necesarios estudios prospectivos de adecuada calidad metodológica que evalúen la asociación de los niveles de VD y su suplementación, y el desarrollo y pronóstico del cáncer cutáneo.
Vitamin D (VD) is a prohormone obtained mainly from exposure to ultraviolet B (UVB) radiation and diet (fig. 1).1 VD plays an essential role in bone health and immunity. Furthermore, VD has an antineoplastic effect by regulating cell proliferation, differentiation, migration, and apoptosis (table 1).2,3 VD has been associated with a lower risk of colorectal, breast, and prostate cancer.4 However, this association is controversial in the case of skin cancer (SC).5,6
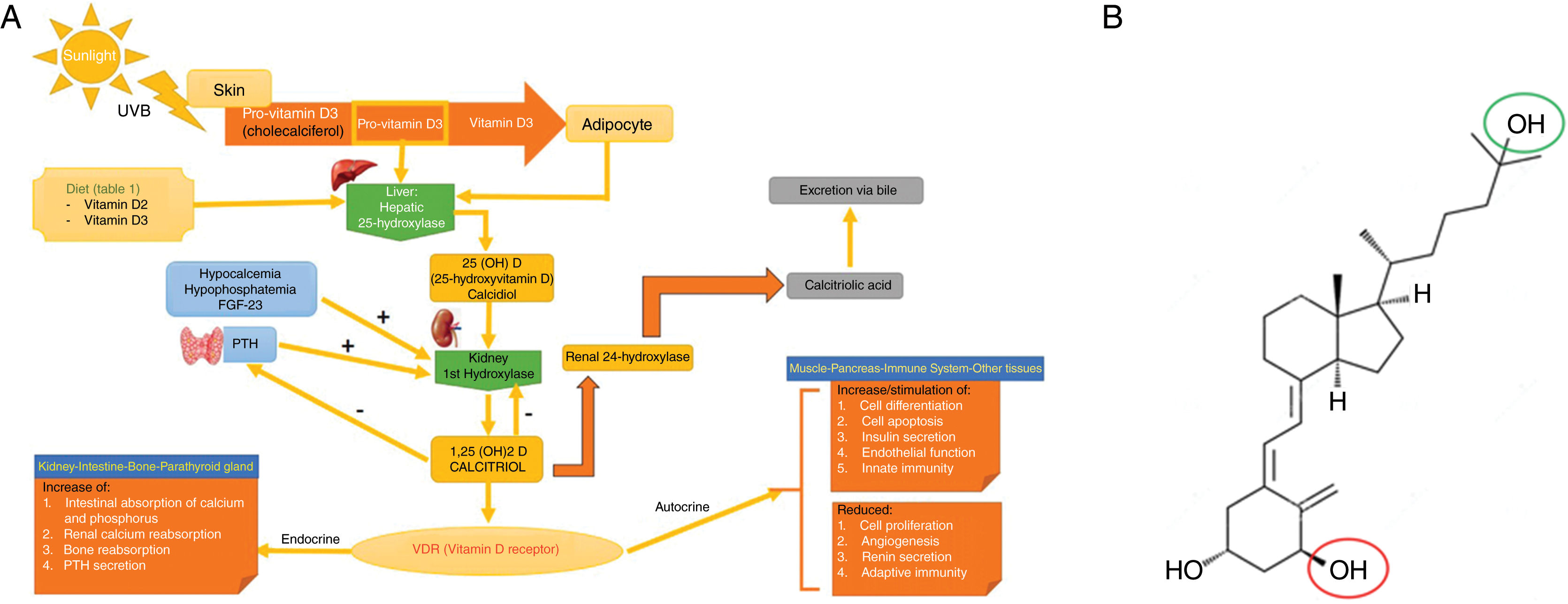
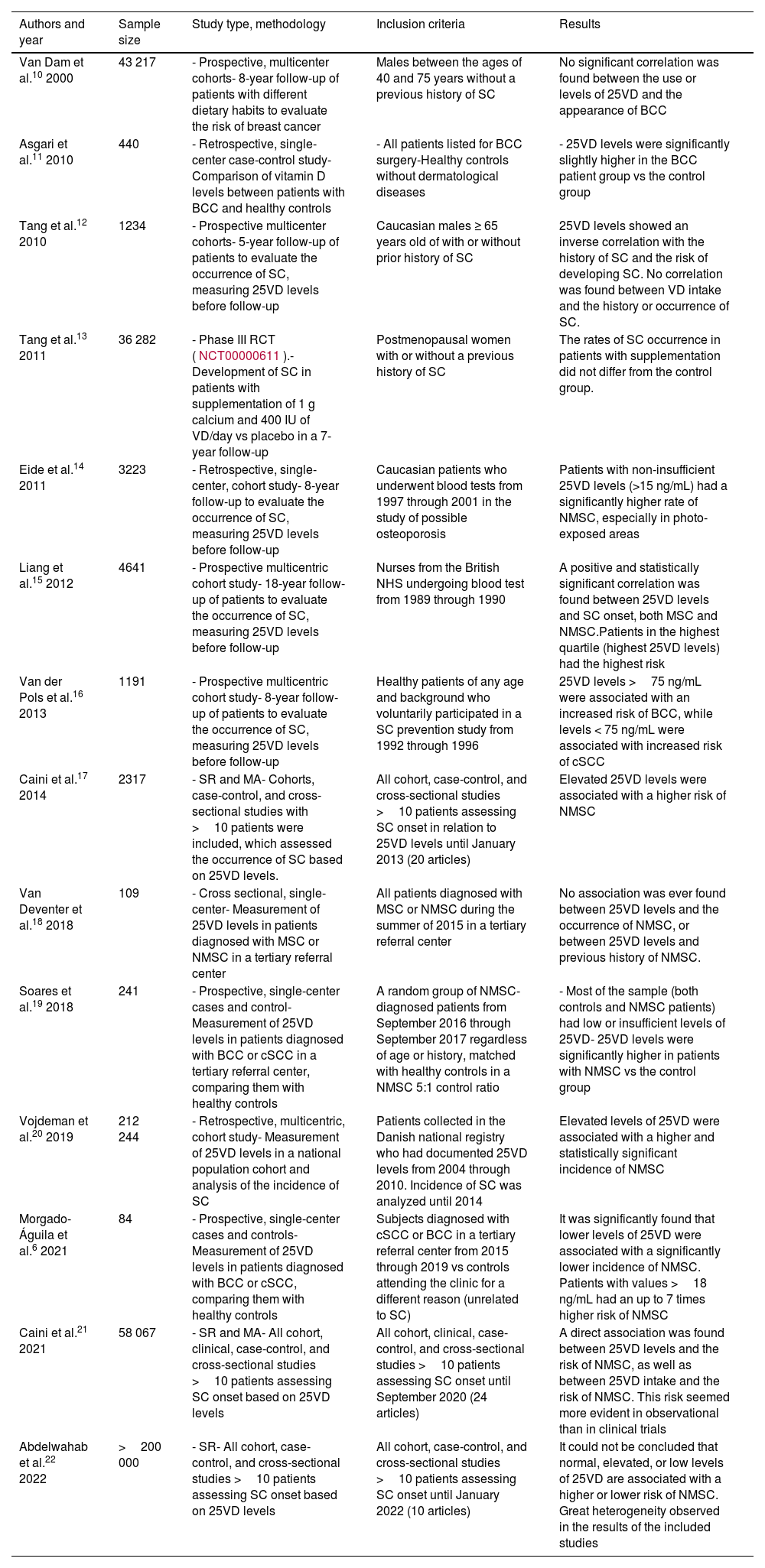
Vitamin D metabolism (panel A) and structure of activated vitamin D (calcitriol or 1,25-dihydroxycholecalciferol; C27H44O3) (panel B).
Panel A. Vitamin D metabolism. Vitamin D can be obtained through the diet, or by cutaneous conversion via UVB, which transforms provitamin D3 (synthesized at skin level) into previtamin D3. The first hepatic step converts it to calcidiol or 25-hydroxyvitamin D through hepatic 25-hydroxylase. The second step, which occurs at renal level, converts it into the active metabolite, 1,25-dihydroxycholecalciferol or calcitriol through renal 1-hydroxylase. This mechanism is regulated, among others, by serum levels of calcium, phosphorus, and parathyroid hormone. Calcitriol acts through intracellular vitamin D receptors (VDR), which activate the transcription and translation process of messenger RNA by synthesizing vitamin D-dependent proteins, with their corresponding activity based on the target cell. The excretion of vitamin D through bile is mediated by the activity of renal 24-hydroxylase, which converts calcitriol into calcitroic acid.
Panel B. Structure of activated vitamin D. Note that vitamin D must undergo 2 different hydroxylations to be activated: activation #1 (green circle) occurs in the liver forming 25-hydroxycholecalciferol, or calcidiol; activation #2 (red circle) occurs in the kidney forming 1,25-dihydroxycholecalciferol or calcitriol, which is the active form.
Source: own elaboration and data obtained with permission from Navarro-Triviño et al.5
Role of vitamin D in immunosurveillance and immunosenescence.
| Biochemical action of vitamin D | Implication at biochemical level | Possible clinical implications of vitamin D deficiency |
|---|---|---|
| Inhibition of TLR2, 4, and 9 expression and signaling | Regulation of the innate immune system | - Increased frequency of autoimmune diseases- Increased severity of autoimmune diseases- Increased frequency of certain infectious processes- Early development of cardiovascular disease: obesity and arteriosclerosis- Earlier and more pronounced aging (accelerated dermatoporosis, sarcopenia, etc.) |
| Reduction in production of TNF-α, IL-1, IL-6, and IL-23 | Reduction of some cytokines that enhance the activity of T cells, especially CD4+T cells | |
| Increased production of IL-4, IL-5, IL-10, and CCL2 | Increased production of anti-inflammatory cytokines, regulating the activity of T cells, especially Th17 and enhancing Th2 activity | |
| Inhibition of IL-6 production | Reduced Th17 activity | |
| Inhibition of IL-33 production | Reduced T cell and dendritic cell activity in some autoimmune diseases, such as Guillain-Barré syndrome, psoriasis, or rheumatoid arthritis | |
| Increased production of Fox P3+regulatory T cells | Reduced autoimmune T cell activity | |
| Increased lymphocyte co-stimulation via CD-28 molecule | Potential activity of cytotoxic T cells and activation of B cells under specific circumstances | |
| Reduction in generation of autoantibodies and ROS | Fewer autoimmune cascades and inflammation control | |
| Increased levels of cathelicidin LL-37 and cationic protein AMP-18 | Fewer infections and early and adequate control of them | |
| Correct activation of TLR1, 2, and 8 response | Fewer infections and early and adequate control of them | |
| Controlled increase in IL-1β and defensin, telomere shortening reduction, increase in Nrf2 levels, decrease in NF-kβ pathway, decrease in NLRP-3 levels, DNA damage reduction, increase in mitochondrial biogenesis | Regulation of cutaneous and systemic aging, prevention of early and accelerated aging | |
| Reduction of inflammation (especially T cell response), decrease in RAAS activation and ROS generation, and control of plasma LDL levels | Prevention of arteriosclerosis, obesity, metabolic syndrome, and early cardiovascular disease |
CCL, chemokine (C-C motif) ligand; CD, cluster of differentiation; IL, interleukin; NLRP-3, nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3; ROS, reactive oxygen species; Th, T helper lymphocyte (collaborator); TLR, toll-like receptor; TNF, tumor necrosis factor.
Source: Fantini et al.3
Since 2009, 25-hydroxyvitamin D or colecalciferol (25VD) has been quantified to recognize VD levels. According to the National Academy of Medicine (NAM), 25VD levels >20 ng/mL are sufficient for optimal health, while the American Endocrine Society (AES) suggests levels >30 ng/mL; insufficient levels range between 20 ng/mL and 30 ng/mL, and deficient levels are those <20 ng/mL.5 In recent years, VD deficiency and insufficiency have been regarded as having “pandemic proportions.” Up to 70% of the European population may have insufficient levels, even in countries with high sun exposure, such as Mediterranean regions.7 Some authors attribute this phenomenon to reduced sunlight exposure and/or diets low in VD-rich foods.8 However, other authors consider the cutoff of 20 ng/mL or 30 ng/mL too high, which would explain why most of the European population has low VD levels without accompanying symptoms.8 Recently, considering the contradictory evidence on the utility of measuring and supplementing VD, the U.S. Preventive Services Task Force has ill-advised measuring and supplementing VD levels in healthy individuals, something that should only be done in patients at risk of osteoporosis or fractures.9
As previously noted, the relationship between VD and SC is controversial, and the evidence is often contradictory.5,6 In this article, we will be reviewing the association between VD and the development and prognosis of SC.
Materials and methodsWe conducted a narrative review of currently available literature. Searches were conducted in Spanish and English across Medline and Google Scholar from September through October 2023 using the terms “vitamin D,” “calcitriol,” “colecalciferol,” “skin cancer,” “melanoma,” “squamous cell carcinoma,” “basal cell carcinoma,” “immunotherapy,” “supplementation,” “response to targeted therapy,” and “response to immunotherapy.” The search included articles from January 1st, 2000 through September 1st, 2023. Prospective and retrospective studies with ≥ 10 patients, and systematic reviews (SRs) and meta-analyses (MAs), were included. Articles were screened based on their abstracts and selected based on their relevance. Two authors (MMP and DMC) conducted the search and selected the articles.
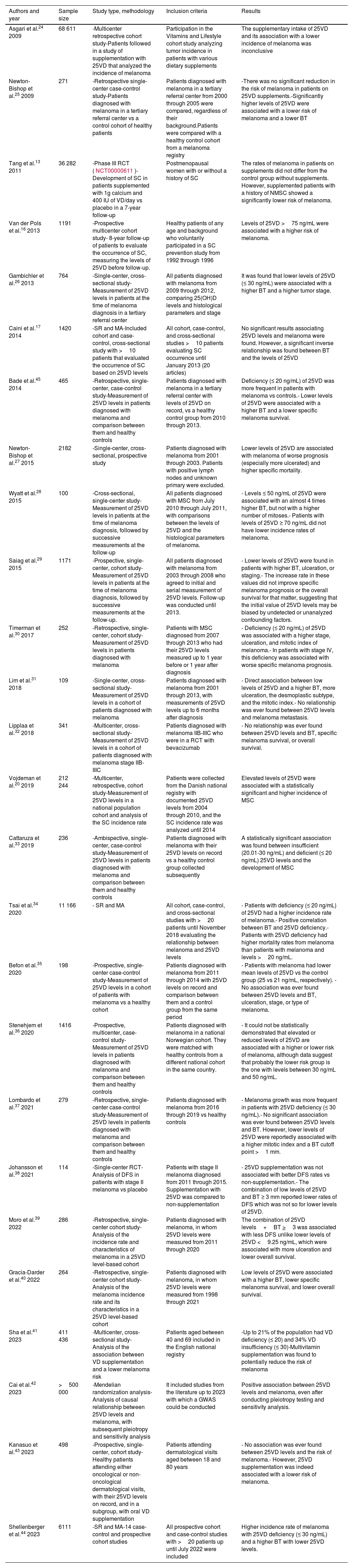
ResultsA. Vitamin D and non-melanoma skin cancerDevelopment of non-melanoma skin cancerMultiple studies have tried to elucidate the relationship between 25VD levels or VD supplementation and non-melanoma skin cancer (NMSC) (table 2).6,10–22 Most found that sufficient VD levels (≥ 20 ng/mL or ≥ 30 ng/mL depending on the studies) were associated with a higher risk of NMSC, especially basal cell carcinoma (BCC). Nonetheless, two recent SRs have provided contradictory results. Back in 2021, Caini et al. published 1 SR and 1 MA that included 24 studies: 3 prospective and 21 retrospective (58 067 patients overall). They found a direct association between 25VD levels and the risk of NMSC, and between a higher 25VD intake and the risk of NMSC. This risk seemed more evident in observational studies than in clinical trials.21 However, in 2022, in a different SR that included 10 studies and 204 000 patients, Abdelwahab et al. stated that it could not be concluded that there was an actual relationship between 25VD levels and the occurrence of BCC. They also said that this inconsistency was due to the lack of homogeneity of the included studies.22
Main studies on the association of vitamin D and non-melanoma skin cancer.
| Authors and year | Sample size | Study type, methodology | Inclusion criteria | Results |
|---|---|---|---|---|
| Van Dam et al.10 2000 | 43 217 | - Prospective, multicenter cohorts- 8-year follow-up of patients with different dietary habits to evaluate the risk of breast cancer | Males between the ages of 40 and 75 years without a previous history of SC | No significant correlation was found between the use or levels of 25VD and the appearance of BCC |
| Asgari et al.11 2010 | 440 | - Retrospective, single-center case-control study- Comparison of vitamin D levels between patients with BCC and healthy controls | - All patients listed for BCC surgery-Healthy controls without dermatological diseases | - 25VD levels were significantly slightly higher in the BCC patient group vs the control group |
| Tang et al.12 2010 | 1234 | - Prospective multicenter cohorts- 5-year follow-up of patients to evaluate the occurrence of SC, measuring 25VD levels before follow-up | Caucasian males ≥ 65 years old of with or without prior history of SC | 25VD levels showed an inverse correlation with the history of SC and the risk of developing SC. No correlation was found between VD intake and the history or occurrence of SC. |
| Tang et al.13 2011 | 36 282 | - Phase III RCT (NCT00000611).- Development of SC in patients with supplementation of 1 g calcium and 400 IU of VD/day vs placebo in a 7-year follow-up | Postmenopausal women with or without a previous history of SC | The rates of SC occurrence in patients with supplementation did not differ from the control group. |
| Eide et al.14 2011 | 3223 | - Retrospective, single-center, cohort study- 8-year follow-up to evaluate the occurrence of SC, measuring 25VD levels before follow-up | Caucasian patients who underwent blood tests from 1997 through 2001 in the study of possible osteoporosis | Patients with non-insufficient 25VD levels (>15 ng/mL) had a significantly higher rate of NMSC, especially in photo-exposed areas |
| Liang et al.15 2012 | 4641 | - Prospective multicentric cohort study- 18-year follow-up of patients to evaluate the occurrence of SC, measuring 25VD levels before follow-up | Nurses from the British NHS undergoing blood test from 1989 through 1990 | A positive and statistically significant correlation was found between 25VD levels and SC onset, both MSC and NMSC.Patients in the highest quartile (highest 25VD levels) had the highest risk |
| Van der Pols et al.16 2013 | 1191 | - Prospective multicentric cohort study- 8-year follow-up of patients to evaluate the occurrence of SC, measuring 25VD levels before follow-up | Healthy patients of any age and background who voluntarily participated in a SC prevention study from 1992 through 1996 | 25VD levels >75 ng/mL were associated with an increased risk of BCC, while levels < 75 ng/mL were associated with increased risk of cSCC |
| Caini et al.17 2014 | 2317 | - SR and MA- Cohorts, case-control, and cross-sectional studies with >10 patients were included, which assessed the occurrence of SC based on 25VD levels. | All cohort, case-control, and cross-sectional studies >10 patients assessing SC onset in relation to 25VD levels until January 2013 (20 articles) | Elevated 25VD levels were associated with a higher risk of NMSC |
| Van Deventer et al.18 2018 | 109 | - Cross sectional, single-center- Measurement of 25VD levels in patients diagnosed with MSC or NMSC in a tertiary referral center | All patients diagnosed with MSC or NMSC during the summer of 2015 in a tertiary referral center | No association was ever found between 25VD levels and the occurrence of NMSC, or between 25VD levels and previous history of NMSC. |
| Soares et al.19 2018 | 241 | - Prospective, single-center cases and control- Measurement of 25VD levels in patients diagnosed with BCC or cSCC in a tertiary referral center, comparing them with healthy controls | A random group of NMSC-diagnosed patients from September 2016 through September 2017 regardless of age or history, matched with healthy controls in a NMSC 5:1 control ratio | - Most of the sample (both controls and NMSC patients) had low or insufficient levels of 25VD- 25VD levels were significantly higher in patients with NMSC vs the control group |
| Vojdeman et al.20 2019 | 212 244 | - Retrospective, multicentric, cohort study- Measurement of 25VD levels in a national population cohort and analysis of the incidence of SC | Patients collected in the Danish national registry who had documented 25VD levels from 2004 through 2010. Incidence of SC was analyzed until 2014 | Elevated levels of 25VD were associated with a higher and statistically significant incidence of NMSC |
| Morgado-Águila et al.6 2021 | 84 | - Prospective, single-center cases and controls- Measurement of 25VD levels in patients diagnosed with BCC or cSCC, comparing them with healthy controls | Subjects diagnosed with cSCC or BCC in a tertiary referral center from 2015 through 2019 vs controls attending the clinic for a different reason (unrelated to SC) | It was significantly found that lower levels of 25VD were associated with a significantly lower incidence of NMSC. Patients with values >18 ng/mL had an up to 7 times higher risk of NMSC |
| Caini et al.21 2021 | 58 067 | - SR and MA- All cohort, clinical, case-control, and cross-sectional studies >10 patients assessing SC onset based on 25VD levels | All cohort, clinical, case-control, and cross-sectional studies >10 patients assessing SC onset until September 2020 (24 articles) | A direct association was found between 25VD levels and the risk of NMSC, as well as between 25VD intake and the risk of NMSC. This risk seemed more evident in observational than in clinical trials |
| Abdelwahab et al.22 2022 | >200 000 | - SR- All cohort, case-control, and cross-sectional studies >10 patients assessing SC onset based on 25VD levels | All cohort, case-control, and cross-sectional studies >10 patients assessing SC onset until January 2022 (10 articles) | It could not be concluded that normal, elevated, or low levels of 25VD are associated with a higher or lower risk of NMSC. Great heterogeneity observed in the results of the included studies |
25(OH)D, 25-hydroxycholecalciferol; BCC, basal cell carcinoma; cSCC, cutaneous squamous cell carcinoma; MA, meta-analysis; MSC, melanoma skin cancer; NMSC, non-melanoma skin cancer; RCT, randomized clinical trial; SC, skin cancer; SCC, squamous cell carcinoma; SR, systematic review; VD, vitamin D (no subtype specification).
A randomized placebo-controlled trial (n=36 282) evaluated the effects of supplementation with 400 IU of VD and 1000mg of calcium on the risk of developing NMSC and melanoma in postmenopausal women. The mean follow-up was 7 years. Supplementation failed to reduce the overall incidence of NMSC or melanoma. In a subanalysis, the group of women with a history of NMSC who received supplementation had a lower risk of developing melanoma (hazard ratio, 0.43; 95%CI, 0.21-0.90) compared with the placebo group.13 One MA from 2020 that included prospective studies only (13 studies with, overall, more than 200 000 patients) also failed to reveal a higher risk of cutaneous squamous cell carcinoma or melanoma but did show a higher risk of basal cell carcinomas in individuals who were on VD supplements.23 Similar results were observed in the SR conducted by Caini et al.21
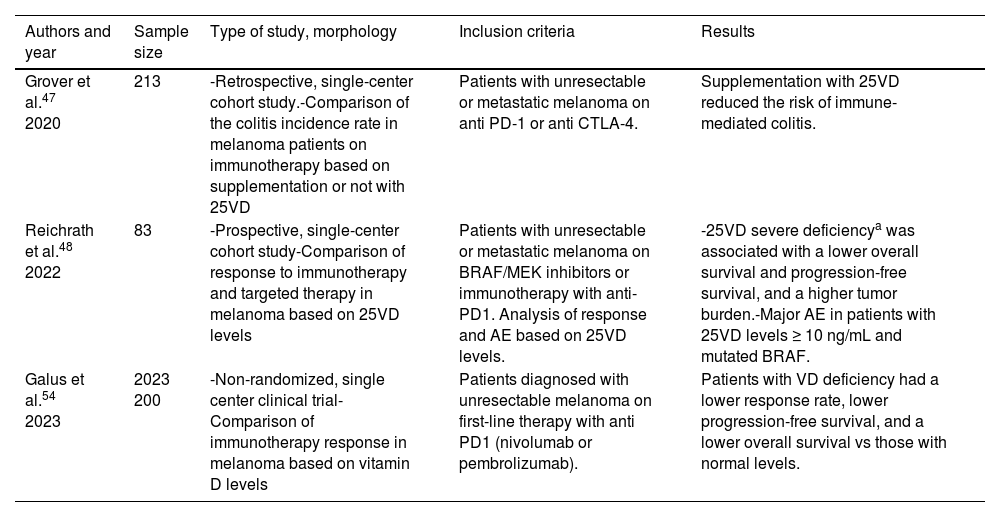
B. Vitamin D and melanomaDevelopment of melanomaNumerous studies have associated VD with the development or progression of melanoma (table 3).13,16,17,20,24–44 Certain reports link lower levels of VD with a higher incidence of melanoma.33–35,37,44,45 One 2023 SR and 1 MA, which collected 14 case-control studies and prospective cohorts for a total of 6111 patients confirmed a higher incidence of melanoma and a higher Breslow thickness (BT) in patients with VD insufficiency (≤ 30 ng/mL).44 However, other studies have not found a relationship between VD levels and the incidence of melanoma,13,17,36 such as the recent multicenter case-control study conducted by Stenehjem et al., with over 1000 patients, which failed to conclude that low or elevated levels of VD are associated with the incidence of melanoma, although they postulate that the lower incidence of melanoma reported may possibly be in patients with VD levels between 30 ng/mL and 50 ng/mL.36 Lastly, other studies showed that elevated levels of VD could be associated with a higher incidence of melanoma.20,42 Particularly relevant is the study conducted by Cai et al., which used a Mendelian randomization analysis, with a sample size of more than 500 000 patients, to eventually find a possible direct correlation between VD levels and melanoma. This analysis was confirmed after performing pleiotropy tests and sensitivity analysis.42
Main studies on the association of vitamin D and melanoma.
| Authors and year | Sample size | Study type, methodology | Inclusion criteria | Results |
|---|---|---|---|---|
| Asgari et al.24 2009 | 68 611 | -Multicenter retrospective cohort study-Patients followed in a study of supplementation with 25VD that analyzed the incidence of melanoma | Participation in the Vitamins and Lifestyle cohort study analyzing tumor incidence in patients with various dietary supplements | The supplementary intake of 25VD and its association with a lower incidence of melanoma was inconclusive |
| Newton-Bishop et al.25 2009 | 271 | -Retrospective single-center case-control study-Patients diagnosed with melanoma in a tertiary referral center vs a control cohort of healthy patients | Patients diagnosed with melanoma in a tertiary referral center from 2000 through 2005 were compared, regardless of their background.Patients were compared with a healthy control cohort from a melanoma registry | -There was no significant reduction in the risk of melanoma in patients on 25VD supplements.-Significantly higher levels of 25VD were associated with a lower risk of melanoma and a lower BT |
| Tang et al.13 2011 | 36 282 | -Phase III RCT (NCT00000611)-Development of SC in patients supplemented with 1g calcium and 400 IU of VD/day vs placebo in a 7-year follow-up | Postmenopausal women with or without a history of SC | The rates of melanoma in patients on supplements did not differ from the control group without supplements. However, supplemented patients with a history of NMSC showed a significantly lower risk of melanoma. |
| Van der Pols et al.16 2013 | 1191 | -Prospective multicenter cohort study- 8-year follow-up of patients to evaluate the occurrence of SC, measuring the levels of 25VD before follow-up. | Healthy patients of any age and background who voluntarily participated in a SC prevention study from 1992 through 1996 | Levels of 25VD >75 ng/mL were associated with a higher risk of melanoma. |
| Gambichler et al.26 2013 | 764 | -Single-center, cross-sectional study-Measurement of 25VD levels in patients at the time of melanoma diagnosis in a tertiary referral center | All patients diagnosed with melanoma from 2009 through 2012, comparing 25(OH)D levels and histological parameters and stage | It was found that lower levels of 25VD (≤ 30 ng/mL) were associated with a higher BT and a higher tumor stage. |
| Caini et al.17 2014 | 1420 | -SR and MA-Included cohort and case-control, cross-sectional study with >10 patients that evaluated the occurrence of SC based on 25VD levels | All cohort, case-control, and cross-sectional studies >10 patients evaluating SC occurrence until January 2013 (20 articles) | No significant results associating 25VD levels and melanoma were found. However, a significant inverse relationship was found between BT and the levels of 25VD |
| Bade et al.45 2014 | 465 | -Retrospective, single-center, case-control study-Measurement of 25VD levels in patients diagnosed with melanoma and comparison between them and healthy controls | Patients diagnosed with melanoma in a tertiary referral center with levels of 25VD on record, vs a healthy control group from 2010 through 2013. | Deficiency (≤ 20 ng/mL) of 25VD was more frequent in patients with melanoma vs controls.- Lower levels of 25VD were associated with a higher BT and a lower specific melanoma survival. |
| Newton-Bishop et al.27 2015 | 2182 | -Single-center, cross-sectional, prospective study | Patients diagnosed with melanoma from 2001 through 2003. Patients with positive lymph nodes and unknown primary were excluded. | Lower levels of 25VD are associated with melanoma of worse prognosis (especially more ulcerated) and higher specific mortality. |
| Wyatt et al.28 2015 | 100 | -Cross-sectional, single-center study-Measurement of 25VD levels in patients at the time of melanoma diagnosis, followed by successive measurements at the follow-up | All patients diagnosed with MSC from July 2010 through July 2011, with comparisons between the levels of 25VD and the histological parameters of melanoma. | - Levels ≤ 50 ng/mL of 25VD were associated with an almost 4 times higher BT, but not with a higher number of mitoses.- Patients with levels of 25VD ≥ 70 ng/mL did not have lower incidence rates of melanoma. |
| Saiag et al.29 2015 | 1171 | -Prospective, single-center, cohort study-Measurement of 25VD levels in patients at the time of melanoma diagnosis, followed by successive measurements at the follow-up. | All patients diagnosed with melanoma from 2003 through 2008 who agreed to initial and serial measurement of 25VD levels. Follow-up was conducted until 2013. | - Lower levels of 25VD were found in patients with higher BT, ulceration, or staging.- The increase rate in these values did not improve specific melanoma prognosis or the overall survival for that matter, suggesting that the initial value of 25VD levels may be biased by undetected or unanalyzed confounding factors. |
| Timerman et al.30 2017 | 252 | -Retrospective, single-center, cohort study-Measurement of 25VD levels in patients diagnosed with melanoma | Patients with MSC diagnosed from 2007 through 2013 who had their 25VD levels measured up to 1 year before or 1 year after diagnosis | - Deficiency (≤ 20 ng/mL) of 25VD was associated with a higher stage, ulceration, and mitotic index of melanoma.- In patients with stage IV, this deficiency was associated with worse specific melanoma prognosis. |
| Lim et al.31 2018 | 109 | -Single-center, cross-sectional study-Measurement of 25VD levels in a cohort of patients diagnosed with melanoma | Patients diagnosed with melanoma from 2001 through 2013, with measurements of 25VD levels up to 6 months after diagnosis | - Direct association between low levels of 25VD and a higher BT, more ulceration, the desmoplastic subtype, and the mitotic index.- No relationship was ever found between 25VD levels and melanoma metastasis. |
| Lipplaa et al.32 2018 | 341 | -Multicenter, cross-sectional study-Measurement of 25VD levels in a cohort of patients diagnosed with melanoma stage IIB-IIIC | Patients diagnosed with melanoma IIB-IIIC who were in a RCT with bevacizumab | - No relationship was ever found between 25VD levels and BT, specific melanoma survival, or overall survival. |
| Vojdeman et al.20 2019 | 212 244 | -Multicenter, retrospective, cohort study-Measurement of 25VD levels in a national population cohort and analysis of the SC incidence rate | Patients were collected from the Danish national registry with documented 25VD levels from 2004 through 2010, and the SC incidence rate was analyzed until 2014 | Elevated levels of 25VD were associated with a statistically significant and higher incidence of MSC |
| Cattaruza et al.33 2019 | 236 | -Ambispective, single-center, case-control study-Measurement of 25VD levels in patients diagnosed with melanoma and comparison between them and healthy controls | Patients diagnosed with melanoma with their 25VD levels on record vs a healthy control group collected subsequently | A statistically significant association was found between insufficient (20.01-30 ng/mL) and deficient (≤ 20 ng/mL) 25VD levels and the development of MSC |
| Tsai et al.34 2020 | 11 166 | - SR and MA | All cohort, case-control, and cross-sectional studies with >20 patients until November 2018 evaluating the relationship between melanoma and 25VD levels | - Patients with deficiency (≤ 20 ng/mL) of 25VD had a higher incidence rate of melanoma.- Positive correlation between BT and 25VD deficiency.- Patients with 25VD deficiency had higher mortality rates from melanoma than patients with melanoma and levels >20 ng/mL. |
| Befon et al.35 2020 | 198 | -Prospective, single-center case-control study-Measurement of 25VD levels in a cohort of patients with melanoma vs a healthy cohort | Patients diagnosed with melanoma from 2011 through 2014 with 25VD levels on record and comparison between them and a control group from the same period | - Patients with melanoma had lower mean levels of 25VD vs the control group (25 vs 21 ng/mL, respectively). - No association was ever found between 25VD levels and BT, ulceration, stage, or type of melanoma. |
| Stenehjem et al.36 2020 | 1416 | -Prospective, multicenter, case-control study-Measurement of 25VD levels in patients diagnosed with melanoma and comparison between them and healthy controls | Patients diagnosed with melanoma in a national Norwegian cohort. They were matched with healthy controls from a different national cohort in the same country. | - It could not be statistically demonstrated that elevated or reduced levels of 25VD are associated with a higher or lower risk of melanoma, although data suggest that probably the lower risk group is the one with levels between 30 ng/mL and 50 ng/mL. |
| Lombardo et al.37 2021 | 279 | -Retrospective, single-center case-control study-Measurement of 25VD levels in patients diagnosed with melanoma and comparison between them and healthy controls | Patients diagnosed with melanoma from 2016 through 2019 vs healthy controls | - Melanoma growth was more frequent in patients with 25VD deficiency (≤ 30 ng/mL).- No significant association was ever found between 25VD levels and BT. However, lower levels of 25VD were reportedly associated with a higher mitotic index and a BT cutoff point >1 mm. |
| Johansson et al.38 2021 | 114 | -Single-center RCT-Analysis of DFS in patients with stage II melanoma vs placebo | Patients with stage II melanoma diagnosed from 2011 through 2015. Supplementation with 25VD was compared to non-supplementation | - 25VD supplementation was not associated with better DFS rates vs non-supplementation.- The combination of low levels of 25VD and BT ≥ 3 mm reported lower rates of DFS which was not so for lower levels of 25VD. |
| Moro et al.39 2022 | 286 | -Retrospective, single-center cohort study-Analysis of the incidence rate and characteristics of melanoma in a 25VD level-based cohort | Patients diagnosed with melanoma, in whom 25VD levels were measured from 2011 through 2020 | The combination of 25VD levels+BT >3 was associated with less DFS unlike lower levels of 25VD <9.25 ng/mL, which were associated with more ulceration and lower overall survival. |
| Gracia-Darder et al.40 2022 | 264 | -Retrospective, single-center cohort study-Analysis of the melanoma incidence rate and its characteristics in a 25VD level-based cohort | Patients diagnosed with melanoma, in whom 25VD levels were measured from 1998 through 2021 | Low levels of 25VD were associated with a higher BT, lower specific melanoma survival, and lower overall survival. |
| Sha et al.41 2023 | 411 436 | -Multicenter, cross-sectional study-Analysis of the association between VD supplementation and a lower melanoma risk | Patients aged between 40 and 69 included in the English national registry | -Up to 21% of the population had VD deficiency (≤ 20) and 34% VD insufficiency (≤ 30)-Multivitamin supplementation was found to potentially reduce the risk of melanoma |
| Cai et al.42 2023 | >500 000 | -Mendelian randomization analysis-Analysis of causal relationship between 25VD levels and melanoma, with subsequent pleiotropy and sensitivity analysis | It included studies from the literature up to 2023 with which a GWAS could be conducted | Positive association between 25VD levels and melanoma, even after conducting pleiotropy testing and sensitivity analysis. |
| Kanasuo et al.43 2023 | 498 | -Prospective, single-center, cohort study-Healthy patients attending either oncological or non-oncological dermatological visits, with their 25VD levels on record, and in a subgroup, with oral VD supplementation | Patients attending dermatological visits aged between 18 and 80 years | - No association was ever found between 25VD levels and the risk of melanoma.- However, 25VD supplementation was indeed associated with a lower risk of melanoma. |
| Shellenberger et al.44 2023 | 6111 | -SR and MA-14 case-control and prospective cohort studies | All prospective cohort and case-control studies with >20 patients up until July 2022 were included | Higher incidence rate of melanoma with 25VD deficiency (≤ 30 ng/mL) and a higher BT with lower 25VD levels. |
25VD, 25-hydroxycholecalciferol; BT, Breslow thickness; DFS, disease-free survival; GWAS, Genome Wide Association Studies; MA, meta-analysis; MSC, melanoma skin cancer; NMSC, non-melanoma skin cancer; RCT, randomized clinical trial; SC, skin cancer; SR, systematic review.
Several studies have described that low levels of VD were associated with melanomas of worse prognosis (table 3).17,25–31,34,37–40,45,46 For example, a Spanish study from 2019 with 204 individuals with melanoma found that lower levels of VD were associated with tumor ulceration and a higher mitotic index.46 One 2020 SR and 1 MA, with more than 10 000 patients, studied the relationship between 25VD levels and the risk of melanoma—prognostic factors included—and concluded that patients with 25VD deficiency (≤ 20 ng/mL) had a higher incidence rate, as well as a higher mortality specifically associated with melanoma. Similarly, a direct correlation was found between BT and 25VD deficiency.34
Regarding VD supplementation, a clinical trial with 114 stage II melanoma patients (80% of them with insufficient VD levels) confirmed that VD supplementation was not associated with higher disease-free survival rates compared with non-supplementation. At the 12-month follow-up, patients with low 25VD levels and a BT ≥ 3mm had lower disease-free survival, which was not the case of individuals with lower 25VD levels alone.38
Vitamin D supplementation and melanoma riskMultiple studies have analyzed the incidence of melanoma in patients with VD supplementation with contradictory results.24,25,38,41,43 A recent cross-sectional study (n=498) revealed that individuals with oral VD supplementation had a lower risk of developing melanoma (0.447 [p=0.016; 95%CI, 0.231-0.862]).43 However, a clinical trial with more than 30 000 postmenopausal women,13 and a recent MA,23 both previously discussed, did not find a decrease in melanoma rates in patients on VD supplements.
Vitamin D and response to advanced therapyIn 2020, Grover et al. conducted a retrospective study in patients with metastatic melanoma on anti-PD-1 or anti-CTLA-4. A total of 17% of these patients developed immune-mediated colitis. Prior VD supplementation showed a significant association with a lower risk of colitis. These results were later confirmed in an independent cohort (table 4).47 A prospective study with 83 patients with metastatic melanoma on BRAF or MEK inhibitors, or immunotherapy, revealed that 25VD deficiency (< 10 ng/mL) was associated with a significantly lower overall and progression-free survival.48 Recently, Galus et al. published the results of a clinical trial (n=200), showing that patients with 25VD deficiency on anti-PD-1 treatment showed lower objective response rates (36.2% vs 56.0%), lower disease-free survival (5.75 months vs 11.25 months), and lower overall survival compared with patients with normal 25VD levels.44
Main studies on vitamin D and response to advanced therapy in melanoma.
| Authors and year | Sample size | Type of study, morphology | Inclusion criteria | Results |
|---|---|---|---|---|
| Grover et al.47 2020 | 213 | -Retrospective, single-center cohort study.-Comparison of the colitis incidence rate in melanoma patients on immunotherapy based on supplementation or not with 25VD | Patients with unresectable or metastatic melanoma on anti PD-1 or anti CTLA-4. | Supplementation with 25VD reduced the risk of immune-mediated colitis. |
| Reichrath et al.48 2022 | 83 | -Prospective, single-center cohort study-Comparison of response to immunotherapy and targeted therapy in melanoma based on 25VD levels | Patients with unresectable or metastatic melanoma on BRAF/MEK inhibitors or immunotherapy with anti-PD1. Analysis of response and AE based on 25VD levels. | -25VD severe deficiencya was associated with a lower overall survival and progression-free survival, and a higher tumor burden.-Major AE in patients with 25VD levels ≥ 10 ng/mL and mutated BRAF. |
| Galus et al.54 2023 | 2023 200 | -Non-randomized, single center clinical trial-Comparison of immunotherapy response in melanoma based on vitamin D levels | Patients diagnosed with unresectable melanoma on first-line therapy with anti PD1 (nivolumab or pembrolizumab). | Patients with VD deficiency had a lower response rate, lower progression-free survival, and a lower overall survival vs those with normal levels. |
AE, adverse events; BRAF, B-rapidly accelerated fibrosarcoma; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; MEK, mitogen-activated protein kinase; PD, programmed cell death protein; 25VD, 25-hydroxycholecalciferol.
The relationship between the use of topical sunscreens and its impact on VD levels was also reviewed. In this regard, a 2019 SR concluded that regular use of topical sunscreens does not impact VD levels in healthy individuals.49 Similarly, a recent study with more than 3000 patients found no association between the use of photoprotection measures, including the application of topical sunscreens, and decreased bone mass density or increased risk of osteoporotic fractures.50 In conclusion, the regular use of topical sunscreens does not impact VD levels, bone mass density, or increase the risk of osteoporotic fractures in healthy individuals.49,50
DiscussionThe association between VD levels and skin cancer is as controversial as the association between VD supplementation and decreased SC, or its prognosis. Currently, there are no established criteria or recommendations in the main guidelines on the management of SC, and we lack standardized recommendations on the measurement or supplementation of VD in patients at risk or suffering from SC.
Regarding NMSC, numerous studies have reported a direct association between BCC and 25VD levels.6,11,12,14–17,19–21 This relationship has also been reported in cutaneous squamous cell carcinoma.6,12,14,15,17,19–21 This is surprising, given the growing evidence supporting VD supplementation vs other types of cancer, such as colorectal or lung cancer.51 This may possibly be explained by the direct relationship between 25VD levels and sun exposure, a crucial risk factor for the development of NMSC.21
As for the association between melanoma and VD levels, the analyzed studies differ on whether there is a direct, or inverse association, or no association whatsoever. Although most studies report that low levels of VD or VD supplementation could reduce the risk of melanoma,13,17,25–31,33–35,37–41,43–45 we found inconclusive studies,24,32,36 and even some that positively correlate VD levels with the risk of melanoma or worse prognosis.16,20,42
The role of vitamin D receptor (VDR) and melanoma has been studied recently: some studies suggest that certain polymorphisms in the VDR gene could be associated with a higher risk of melanoma.52 A recent retrospective study (703 melanomas) stated that higher levels of VDR expression independently protected vs melanoma-related death, both primary and metastatic. Tumors with high rates of VDR expression showed increased anti-tumor immune activity and reduced proliferative pathways, particularly Wnt-β-catenin signaling. In this study, VD deficiency was associated with lower survival in primary melanoma, especially when VDR levels were low. These findings suggest a causal relationship between VD-VDR signaling and melanoma survival.53
For the relationship between VD levels or supplementation and immunotherapy in melanoma, there are studies showing that low VD levels would correlate with a lower antitumor response to immunotherapy48,54 or even a higher rate of adverse effects.47 Currently, VD supplementation in melanoma patients has a grade B recommendation (probably an effective recommendation), with a level evidence IIb (cohort studies and case-control studies), although current evidence is still scarce.5 As a matter of fact, the National Comprehensive Cancer Network (NCCN) guidelines do not take a stance on this matter,55 nor does the European Society for Medical Oncology (ESMO) guidelines,56 or the 8th edition of the American Joint Committee on Cancer (AJCC).57 New studies are needed to evaluate the value of VD supplementation in melanoma patients.
A recent retrospective study with 663 patients evaluated whether melanoma patients were at increased risk of developing other non-cutaneous cancers after a previous diagnosis of melanoma. A total of 34 non-cutaneous tumors were found, and the multivariate analysis concluded that age older than 60 years (HR, 3.4; 95%CI, 1.5-7.6) and nodular subtype of melanoma (HR, 2.2; 95%CI, 1-4.8) were directly associated with the appearance of other non-cutaneous tumors, but not VD levels.58
LimitationsOur review has multiple limitations: it is a narrative–not a systematic one–review; many studies included are retrospective and/or use several methodologies to measure variables and outcomes, which complicates data comparison. These factors hinder the generalization of findings and conclusions, underscoring the need for properly designed prospective studies with prolonged follow-up.
ConclusionsThe association between VD and SC is a complex one. Adequate or even elevated levels of VD could be associated with a higher risk of NMSC, especially BCC. As for melanoma, the relationship is uncertain, with contradictory results. However, most studies state that higher levels of VD or VD supplementation could reduce the risk of melanoma and are associated with melanomas of better prognosis. Similarly, elevated levels of VD or VD supplementation could be associated with a better antitumor response and fewer adverse effects with melanoma immunotherapy. In conclusion, to date, there is still insufficient evidence to recommend VD supplementation in patients at risk of NMSC or melanoma. However, normal levels of VD could be associated with better therapeutic responses and prognosis in melanoma. Prospective studies of high methodological quality are required to clarify the role of VD and its supplementation in SC and develop clinical guidelines accordingly.
Conflicts of interestNone declared.
We wish to thank Dr. Francisco José Navarro Triviño for letting us use his scheme shown in Figure 1.