In the field of vascular anomalies, distinguishing between vascular malformations and tumors has become crucial for a correct therapeutic approach. However, the differential diagnosis between these two groups is not always well explained in classical texts, mainly because many vascular malformations are still known with old names that suggest a tumoral nature. Also, genetic and pathogenic knowledge of these entities has greatly increased in recent decades, so researchers and clinicians now have a better understanding of vascular malformations. In this paper, we present the main histopathological tips to recognize and identify a vascular malformation as such. We also contextualize such information in the clinical and pathogenic knowledge for a better understanding of these entities.
En el campo de las anomalías vasculares, distinguir entre malformaciones vasculares y tumores vasculares se ha vuelto esencial para un enfoque terapéutico correcto. Sin embargo, el diagnóstico diferencial entre estos dos grupos no está siempre correctamente explicado en los textos clásicos, principalmente porque a muchas malformaciones vasculares se las conoce todavía con nombres antiguos que sugieren tumores vasculares. Asimismo, el conocimiento genético y patogénico de estas entidades se ha incrementado notablemente en las décadas recientes, de tal manera que investigadores y clínicos tienen ahora una mejor comprensión de las malformaciones vasculares. En este artículo, presentamos las principales claves histopatológicas para reconocer las malformaciones vasculares e identificarlas como tal. También contextualizamos tal información en el conocimiento clínico y patogénico para mejor comprensión de estas entidades.
Vascular anomalies have been long studied and described, using names and concepts coined in 19th Century, many of them by Virchow himself. Many of those names suggested tumors: cavernous hemangioma, lymphangioma, cystic hygroma, lymphangiomyomatosis, and cyrsoid hemangioma. In spite of their inadequacy, these names are many times found in classical texts and even the recent literature.
In the 1990s, the classification of vascular anomalies proposed by the International Society for the Study of Vascular Anomalies (ISSVA) made a crucial contribution: the division into two main groups of vascular anomalies: vascular tumors and vascular malformations (VM).1 At that time, differences between these two groups seemed solid and clear: while tumors were considered proliferating acquired growths made of newly formed vessels, VM were congenital anomalies made of non-proliferating dysmorphic vessels. This new classification allowed for the successful clinical and therapeutic approach of many vascular anomalies. The principles of ISSVA classification have been recently adopted by the WHO in the recently launched classification of Pediatric Tumors.2
As time passed, the international community realized that such dual approach was not as sharp as expected. For instance, VM do in fact proliferate,3 although usually not as much as vascular tumors. Among the VM, lymphatic malformations are the ones that show the highest proliferation rate,4 although even so, much lower than the one identified in vascular tumors. Additionally, since most VM are due to postzygotic somatic mutations, they are made of clonal cells,5 therefore blurring the conceptual difference with tumors as unique clonal proliferations.
There is no universal immunohistochemical marker that allows one to reliably distinguish between VM and vascular tumors. Although certain markers such as WT1 were in the past considered to be related to vascular tumors, it was later demonstrated that they are in fact seen as well in vascular proliferative areas, and therefore, sometimes may be expressed in VM.6 The marker GLUT1 should not be considered a specific marker for tumors, since it is only expressed by endothelial cells in infantile hemangioma7 and also in a subtype of VM, the verrucous venous malformation.8
In spite of this, the dual approach suggested by the ISSVA is found to be useful and practical by many clinicians, surgeons, and researchers, so it is the one we will mainly follow in this review. Our goal is to describe the main histopathological features that can help the dermatopathologist and the general pathologist to identify a VM as such, as well as to classify it depending on the type of vessels identified in them.
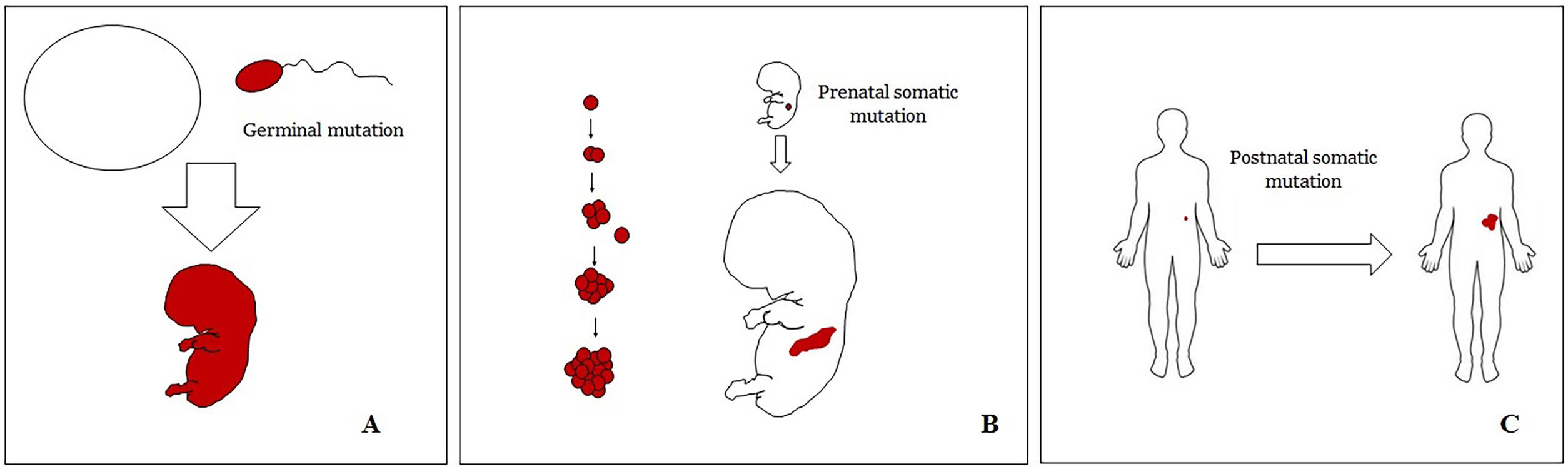
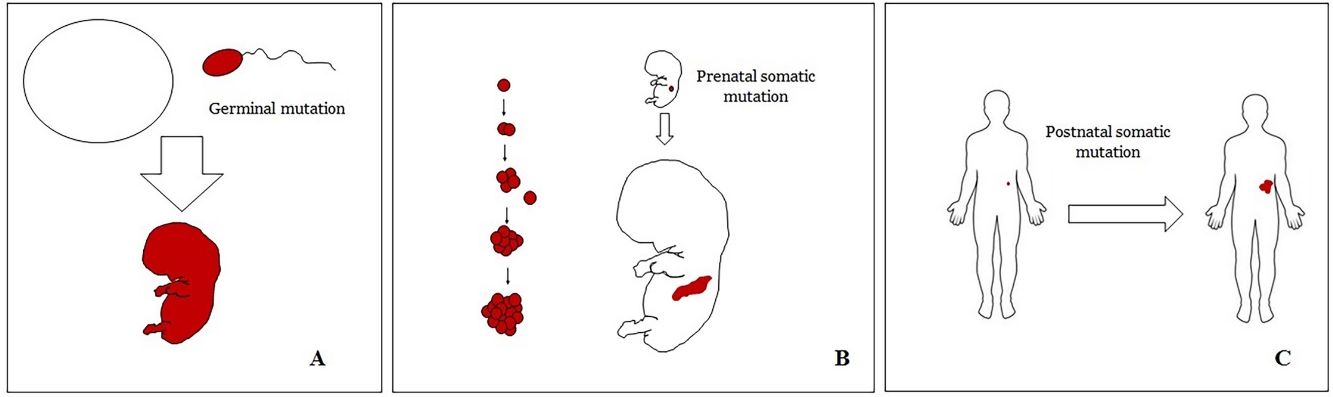
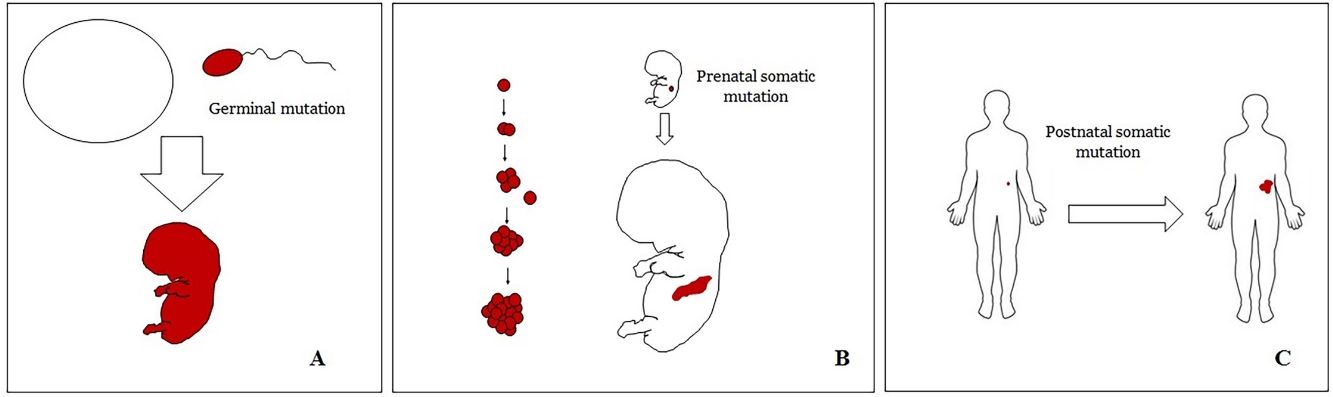
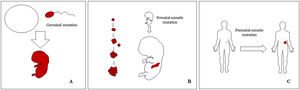
Basic Concepts on VMHuman malformations during the embryologic or fetal development, can happen due to prenatal mutations. Prenatal mutations can be germinal or somatic (Fig. 1). In a prenatal germinal mutation, the mutation is already present in the germ cell (either spermatozoid or ovule). Therefore, all the cells in the individual will carry the mutation (Fig. 1A). In contrast, when a prenatal somatic mutation happens, only the cells that develop from the one that has suffered the mutation will carry the genetic defect, developing a mosaicism. Since such mutated cells follow the normal growth patterns of embryologic development, the distribution of the mutated cells many times adopts a segmental or linear pattern, following the Blaschko lines (Fig. 1B). In contrast, in a postnatal mutation, the cells growing from the mutated cells develop into a tumor (Fig. 1C).
Scheme of prenatal and postnatal mutations and the development of vascular malformations and tumors. Prenatal mutations can be either germinal (A) or somatic (B). In the first case, all the cells of the individual will carry the mutation. In somatic prenatal mutations, in contrast, only the cells developed after the mutation will carry the genetic defect. That is why they are commonly distributed in a linear or segmental way, many times following Blaschko lines (B). Postnatal somatic mutations (C) mainly produce tumors, which do not usually show such linear distribution.
VM are vascular anomalies made of mature dysmorphic vessels. Such dysmorphic features are the result of prenatal mutations (mainly somatic but also germline), therefore, almost virtually all VM are congenital. When VM are caused by prenatal somatic mutations they should be considered a mosaicism9 in which all the cells from the VM carry the same mutation(s). It is well known that some VM are the result of a double hit mechanism, in which a somatic postnatal mutation occurs on cells already carrying a prenatal mutation.10,11 The most classical examples of vascular malformations related to a double hit mechanism are glomulovenous malformations12 and combined capillary-arteriovenous malformations.13 In contrast, vascular tumors are the result of postnatal somatic mutations. Their global morphology is the one of a tumor, without a linear or segmental distribution.
Most VM are clinically evident in childhood. However, that is not always the case. Sometimes a VM can become clinically evident when secondary changes (such as thrombosis or hemorrhage) happen.14
While vascular tumors are made of proliferating vessels, VM either do not proliferate or proliferate at a very low rate. This does not mean that VM do not enlarge with the passage of time. Such enlargement is sometimes due to dilatation of the malformed vessels. Other times, thrombotic phenomena happen in the VM vessels,15 accompanied or not by hemorrhagic phenomena.16 Lastly, in some cases, VM can enlarge due to a slow but constant rate of proliferation.
One of the most important concepts in VM is the one of blood flow.17 This refers to the speed of the blood flow through the dysmorphic vessels of the VM, and therefore, to the blood pressure in such vessels. High-flow VM are the arteriovenous malformations and the arteriovenous fistula. Also, those combined VM that show an arteriovenous component usually have areas of high-flow. Distinguishing low-flow from high-flow VM can be done before surgery by using ultrasound or dynamic magnetic resonance imaging.18 The clinical and therapeutic approach to a VM highly depends on the evidence of high-flow areas or not. When the latter carries a high risk for hemorrhage, invasive therapy (endovascular, surgical, and/or radiation therapy) versus medical management alone, is indicated. Otherwise, medical management can be an option. In contrast, sclerotherapy or surgery is the common indicated treatment for low-flow VM, although some can be managed with drugs.19 In this respect, the identification of certain mutations in VM is crucial for the successful treatment with targeted therapy.20,21
Classification of Vascular MalformationsThere have been several attempts at classifying vascular anomalies. One of the most complete classifications is the one of the International Society for the Study of Vascular Anomalies (ISSVA).22 Such classification is freely available in the ISSVA website (issva.org/classification).
From a histopathological point of view, vascular malformations are classified according to the type of vessels that appear to be malformed in the vascular lesions. Therefore, there are capillary, lymphatic, venous and arteriovenous malformations, but also combined malformations. Additionally, the glomus apparatus can also be malformed. Although morphologically distinctive, these glomuvenous malformations are currently included under the venous malformations group. Glomuvenous malformations should not be confused with glomus tumors. The latter are truly tumors that appear in adult life. Although they show veins, the latter are not malformed.
The current classification of vascular malformations accepted by the ISSVA is shown in Table 1.
Main Types of Simple Vascular Malformations.
| Capillary | Lymphatic | Venous | Arteriovenous |
|---|---|---|---|
| Nevus simplex/salmon patch, “angel kiss”, “stork bite” | Cystic:MacrocysticMicrocysticMixed cystic | Common | Sporadic |
| Cutaneous and/or mucosal (also known as “port wine” stain):NonsyndromicSturge-Weber syndromeWith bone and/or soft tissue overgrowthDiffuse presentation with overgrowth | Generalized lymphatic anomaly including kaposiform lymphangiomiomatosis | Familial cutaneo-mucosal | In hereditary hemorrhagic telangiectasia |
| Reticulate:With microcephalyWith megalencephaly | Gorham-Stout disease | Blue rubber bleb nevus (Bean) syndrome | In capillary malformation |
| Cutis marmorata telangiectatica congenita | Channel type | Glomuvenous malformation* | Congenital arteriovenous fistula |
| Hereditary hemorrhagic telangiectasia | “Acquired” progressive (formerly known as progressive lymphangioma) | Cerebral cavernous | |
| Primary lymphedema | Familial intraosseus | ||
| Verrucous (formerly verrucous hemangioma) |
There are also several combined forms of malformations, defined as two or more types of vascular malformation simultaneously evidenced in the same lesion (capillary+venous; capillary+lymphatic; capillary+lymphatic+venous …).
It should be mentioned that some vascular anomalies have not yet been clearly identified as tumors or malformations, so, perhaps, some of them might be included in the future under the designation of vascular malformation. These are: intramuscular capillary-type hemangioma, also called intramuscular fast flow vascular anomaly; angiokeratoma; sinusoidal hemangioma; acral arteriovenous tumor; multifocal lymphangioendotheliomatosis with thrombocytopenia/cutaneovisceral angiomatosis with thrombocytopenia; PTEN (type) hamartoma of soft tissue/“angiomatosis” of soft tissue; fibroadipose vascular anomaly (FAVA).
Pathogenesis of Vascular MalformationsGenetic alterations happening during embryologic life induce dysregulation in vasculogenesis with the result of dysmorphic vessels. Causal genes have been identified in many VM. (Table 1 shows a list of the known genetic alterations known so far).23 The high flow VMs show anomalies of the Ras/MAPK cell signaling pathway. In contrast, the most common low flow anomalies (venous and lymphatic malformations) are caused by somatic mutation involving genes of the PI3K/AKT/mTOR pathway.
De novo VM also occur, and their pathogenesis still remains unknown and highly speculative. The most accepted hypothesis is that they might need cumulative mutations, that is, somatic postnatal mutations happening in cells with a prenatal mutation, in a double-hit mechanism.10,11
Formerly Known As Vascular Tumors Which Are Presently Considered Vascular MalformationsSome texts still include in their chapters of vascular tumors entities such as cavernous hemangioma, lymphangioma circumscriptum, cystic hygroma, or cyrsoid hemangioma. These entities were recognized as vascular malformations by the ISSVA a long time ago.
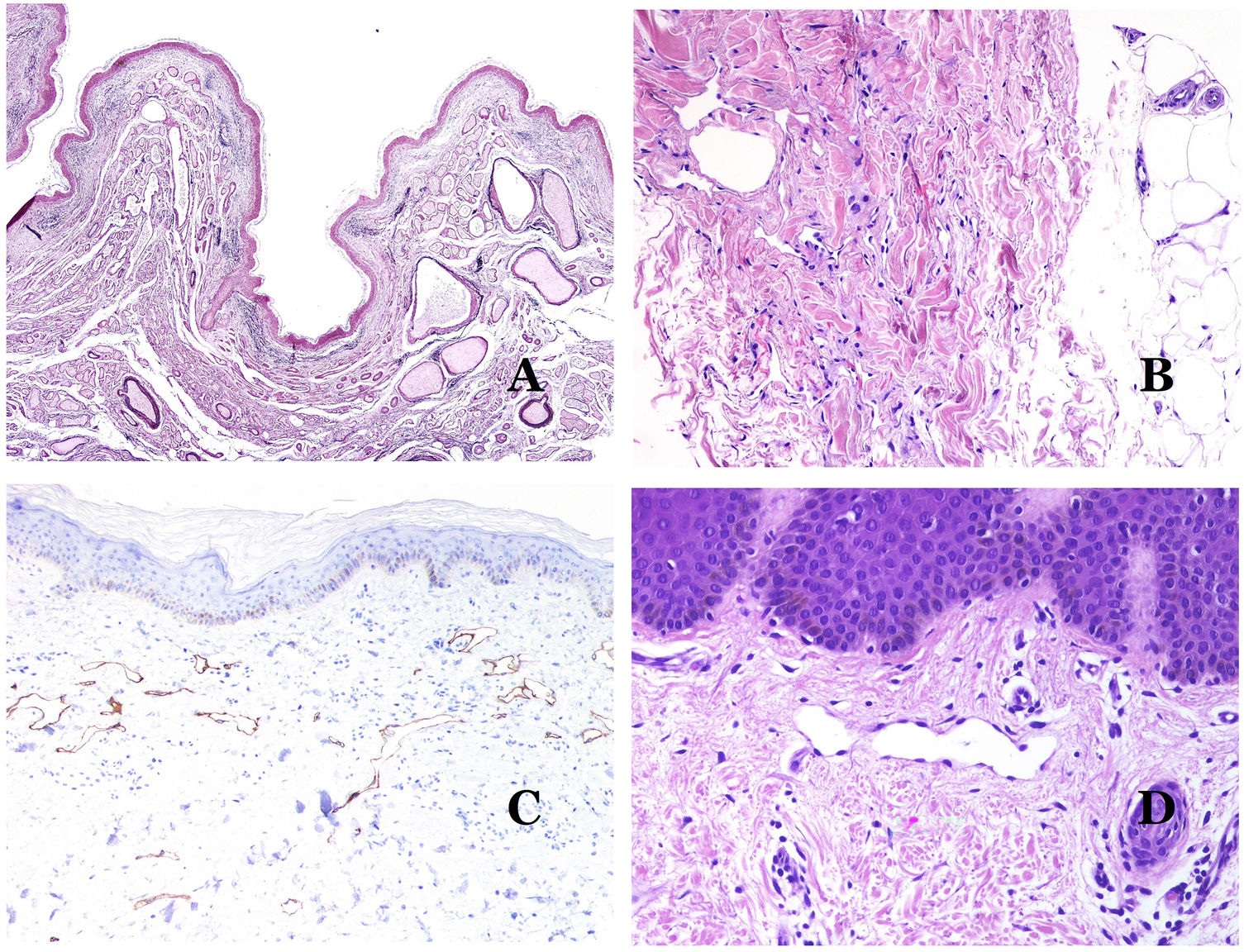
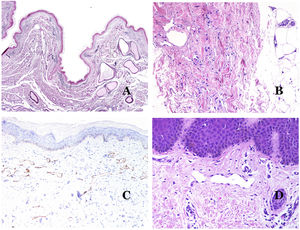
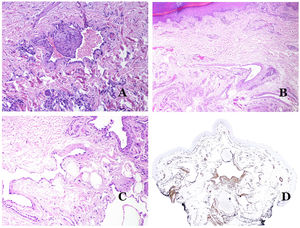
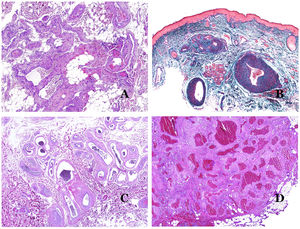
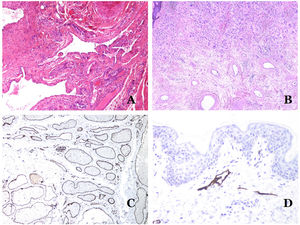
In contrast, there are some entities that have only been recently recognized as malformations. One of the best examples in this group is verrucous hemangioma. The latter term can be found as such in the recent literature, albeit less often than in the past. Most texts correctly refer to it as verrucous venous malformation. This latter malformation carries a somatic mutation in MAP3K3.24,25 Clinically, it presents as erythematous patches and plaques, many of which show a verrucous appearance. Such appearances are due to the verrucous hyperplastic features of the epidermis, which appears hyperkeratotic and hypergranulotic, overlying the vascular malformation which is made of malformed venules occupying the papillary dermis (Fig. 2A), and in most cases extending through the whole dermis, even involving the subcutis.
(A) Verrucous venous malformation. The malformed venules are mainly occupying the superficial dermis, although in most cases they extend deeply in the dermis and hypodermis, where they form nodular aggregates. The overlying epidermis appears verrucous and many times hypergranulotic and hyperkeratotic (orcein ×20). (B) Acquired progressive lymphatic anomaly. The image shows flattened malformed lymphatic vessels that permeate the whole dermis (hematoxylin–eosin ×100). (C) Acquired progressive lymphatic anomaly immunostained with D2-40. The endothelia are positive, showing the angulated and flattened shape of the vessels (D2-40 ×100). (D) Lymphatic vessel in the papillary dermis (hematoxylin–eosin ×200).
Another good example of a vascular anomaly in this group is the acquired progressive lymphatic anomaly.26 The term lymphangioendothelioma was presented long ago in the literature to refer to lymphatic anomalies with a tendency to recur.27 However “benign” lymphangioendothelioma was described as a vascular lesion presenting as slowly growing erythematous macules and plaques, mainly on the extremities or shoulders in young people.28 Histopathologically, it shows flattened malformed lymphatic vessels that permeate the whole dermis and sometimes even the hypodermis29 (Fig. 2B). The vessels are easily highlighted with D2-40 (Fig. 2C). To emphasize its progressive clinical enlargement, this entity was called acquired progressive lymphangioma until not so long ago, but the current classification of the ISSVA includes it as a malformation.
Vascular Malformations Associated With Other AnomaliesSome vascular malformations can present in the context of certain syndromes in which the vascular anomalies are associated with other anomalies, either of the soft tissue, bone, or partial general overgrowths of certain tissues (Table 2).30Table 3 shows the main syndromes in which such situations have been identified.
Syndromes With Vascular Malformations (VM).
| Syndrome | Anomalies | Types of VM found |
|---|---|---|
| Kippel-Trenaunay | Low-flow VM with limb overgrowth | Capillary and venous with or without lymphatic |
| Parkes Weber | High-flow VM with limb overgrowth | Capillary and arteriovenous |
| Servelle-Martorell | VM with bone undergrowth | Venous |
| Sturge-Weber | Facial and lepromeningeal VM with eyes anomalies with or without bone and/or soft tissue overgrowth | Capillary |
| Limb capillary malformation with congenital non-progressive limb overgrowth | Limb capillary malformation with congenital non-progressive limb overgrowth | Capillary |
| Maffucci | VM with or without spindle cell hemangioma with enchondroma | Venous |
| Macrocephaly with capillary malformation | Macrocephaly with capillary malformation | Capillary |
| Microcephaly with capillary malformation | Microcephaly with capillary malformation | Capillary |
| CLOVES | VM with lipomatous overgrowth | Lymphatic, venous, and capillary with or without arteriovenous |
| Proteus | VM with asymmetrical somatic overgrowth | Capillary, venous and/or lymphatic |
| Bannayan-Riley-Ruvalcaba | VM with macrocephaly and lipomatous overgrowth | Arteriovenous and venous |
| CLAPO | VM of the lower lip, face, and neck with asymmetry and partial or generalized overgrowth | Capillary and lymphatic |
Main Vascular Malformations Which Have an Identified Gene Involved in Their Pathogenesis.
| Capillary malformations (CM) |
| Cutaneous and/or mucosal CM (also known as “port-wine” stain): |
| Non-syndromic CM (GNAQ) |
| CM with central nervous system and/or ocular anomalies (Sturge-Weber syndrome) (GNAQ) |
| CM with bone and/or soft tissues overgrowth (GNA11) |
| Diffuse CM with overgrowth (DCMO) (GNA11) |
| Reticulate CM |
| CM of microcephaly-capillary malformation (STAMBP) |
| CM of megalocephaly-capillary malformation-polymicrogyria (PIK3CA) |
| CM of CM-AVM (RASA1/EPHB4) |
| Hereditary hemorrhagic telangiectasia (HHT) (HHT1: ENG, HHT2: ACVRL1, HHT3, JPHT: SMAD4) |
| Lymphatic malformations (LM) |
| Common (cystic) LM (PIK3CA) |
| Primary lymphedema |
| Nonne-Milroy syndrome (FLT4/VEGFR3) |
| Primary hereditary lymphedema (VEGFC) |
| Primary hereditary lymphedema (GJC2)/Connexin 47 Gen |
| Lymphedema-distichiasis (FOXC2) |
| Hypotrichosis-lymphedema-telangiectasia (SOX18) |
| Primary lymphedema with myelodysplasia (GATA2) |
| Primary generalized lymphatic anomaly (Hennekam lymphangiectasia-lymphedema syndrome) (CCBE1) |
| Microcephaly with or without chorioretinopathy, lymphedema, or mental retardation syndrome (KIF11) |
| Lymphedema-choanal atresia (PTPN14) |
| Venous malformations (VeM) |
| Common VeM TEK (TIE2)/PIK3CA |
| Familial VeM cutaneo-mucosal: TEK (TIE2) |
| Multifocal sporadic VeM: TEK (TIE2) |
| Blue rubber bleb nevus (Bean) syndrome VM: TEK (TIE2) |
| Glomuvenous malformation (Glomulin gen) |
| Cerebral cavernous malformation (CCM) (CCM1: KRIT1, CCM2: Malcavernin gen, CCM3: PDCD10) |
| Familial intraosseous vascular malformation (ELMO2) |
| Verrucous venous malformation (formerly verrucous hemangioma) (MAP3K3) |
| Arteriovenous malformations (AVM) and fistulas |
| Sporadic (MAP2K1) |
| In HHT (HHT1: ENG, HHT2: ACVRL1, HHT3: JPHT SMAD4) |
| In CM-AVM: RASA1/EPHB4 |
Abbreviations: CM: capillary malformation; LM: lymphatic malformation; VeM: venous malformation; AVM: arteriovenous malformation; HHT: hereditary hemorrhagic telangiectasia; CCM: cerebral cavernous malformation.
Two main types of vascular networks are described in humans: the lymphatic system and the blood system. The first one is an open system through which lymph circulates and from where lymph passes to the connective tissue. In contrast, the blood vessels system is a close circuit in which selected cells and products cross the blood vessel walls.
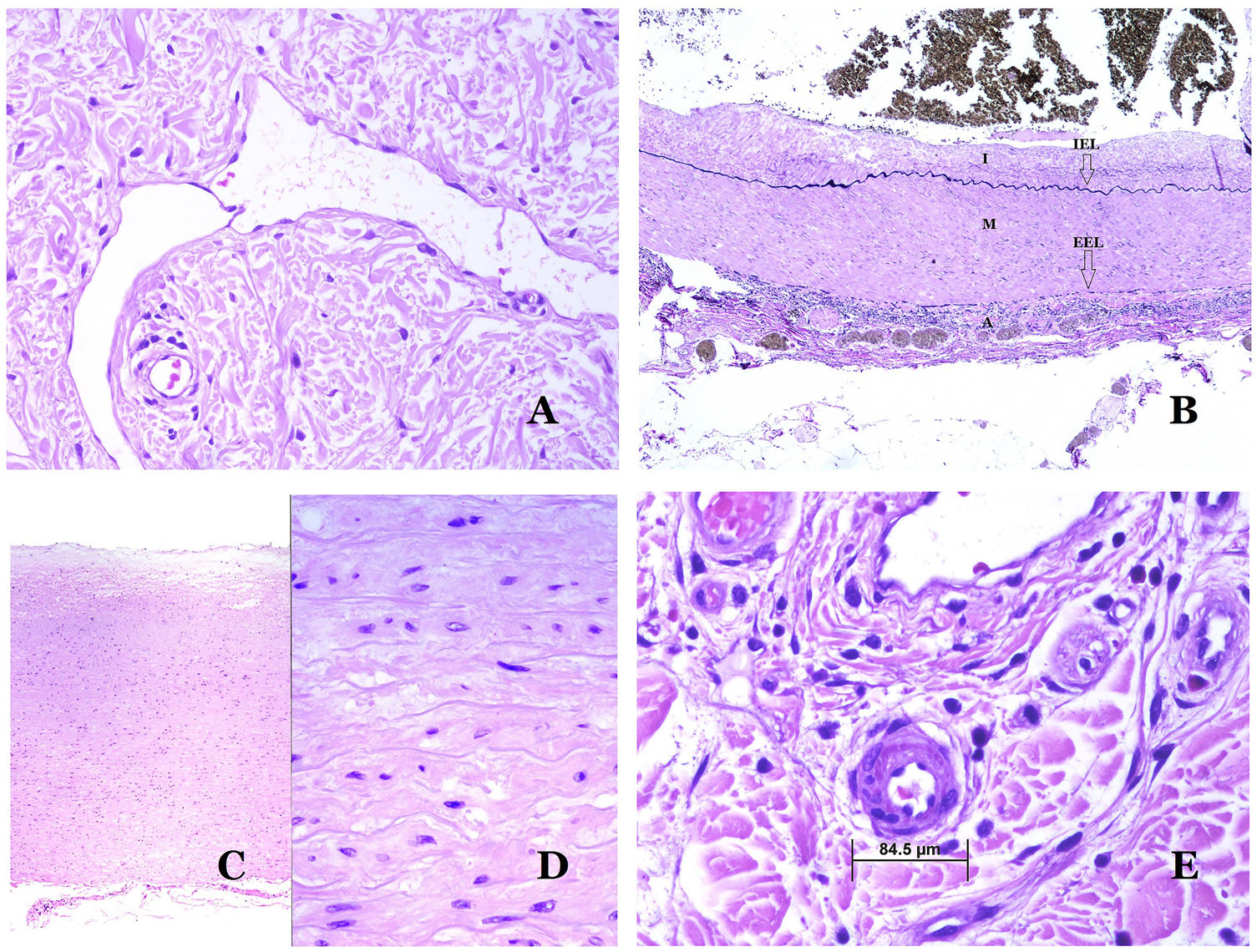
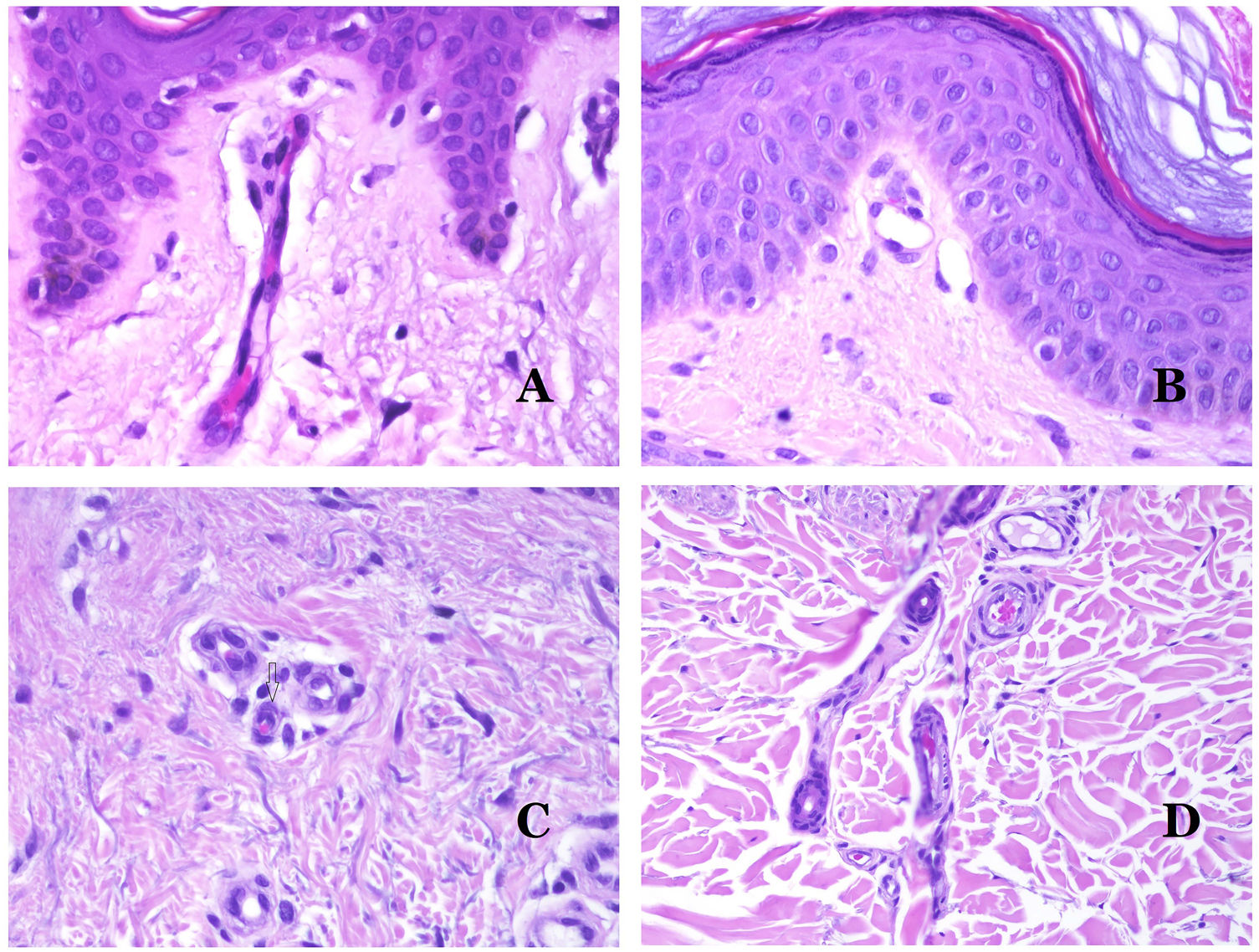
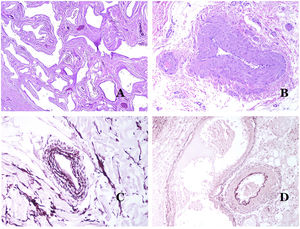
Lymphatic vessels are made of a discontinuous endothelium underneath which there is a basement membrane (Fig. 2D). Lymphatic vessels are devoid of a prominent net of elastic fibers (as veins have) and therefore, circulation of lymph cannot rely on the elastic properties of their wall. In contrast, lymph circulates along these vessels mainly helped by the action of the surrounding musculature which compress the lymphatic walls while we move. Lymphatic valves successfully avoid the return of the lymph. Such valves are sometimes found in the sections of lymphatic vessels (Fig. 3A), which is an important clue in identifying them as such. While the smallest lymphatic vessels show a very thin wall (only made of endothelium and basement membrane) some larger vessels can show foci of muscular layer. Additionally, foci of lymphocytic infiltrates close to the vessel walls are a common feature of lymphatics.
(A) Lymphatic vessel. The endothelium lies directly on the basement membrane. Two valves are easily seen in the vessel (hematoxylin–eosin ×200). (B) Artery. This histochemical stain for elastic tissue shows the three layers of a normal artery: the intimal (I), medial (M), and adventitial (A). Two thick elastic bands divide these three layers: the internal elastic lamella (IEL) and the external elastic lamella (EEL) (Van Gieson elastic staining ×20). (C) Aorta. The medial layer is rich in elastic fibers (hematoxylin–eosin ×20). Such feature is better appreciated at a high magnification (hematoxylin–eosin ×200). (D) Arteriole (hematoxylin–eosin ×400).
The blood system has to carry the blood through a closed system that guarantees the distribution from the heart to the lungs, from the lungs to all the organs, and from them, back to the heart. Although there is a selective interchange of cells and products across the walls of blood vessels all along this circuit, scape of large quantities of blood (that is hemorrhage) is not a physiological phenomenon. To accomplish all of these requirements, the blood system is made of five main types of blood vessels: arteries, arterioles, capillaries, venules and veins.
Arteries are the largest out of all of them. They have three layers: intimal, medial and adventitial31 (Fig. 3A). An elastic band is found between the intimal and medial layers, as well as between the medial and the adventitial.31 These two bands are known as internal and external elastic lamellas, respectively (Fig. 3B). Arteries can be elastic or muscular, depending on the abundance or not of elastic fibers in the medial layer. While arteries which are far from the heart are muscular, the elastic component of the medial layer progressively increases with the proximity of the vessels to the heart (Fig. 3C). Large arteries directly in contact to the heart (such as the aorta) have a medial layer with prominence of elastic fibers. Although the intima is a virtual space at birth, this layer slowly thickens with age, mainly by an increase in the amount of collagen in it. Such increase can show a disorganized appearance with aging, and the intimal layer appears therefore fibrotic.
Although the term “arteriole” is widely accepted in the literature to refer to vessels with the structure of an artery but with a diameter less than 100μm, there is no histological sign to distinguish small arteries from large arterioles.32 Their media is formed by 1 or 2 circular layers of smooth muscle, which can act as a precapillary sphincter (Fig. 3D). When arterioles are very small, they might lack the internal elastic lamella.32
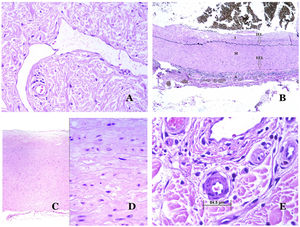
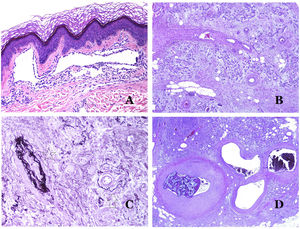
Capillaries are small blood vessels (5–15μm) which do not have a medial layer or elastic fibers (Fig. 4A). Their wall is made of endothelial layer directly positioned on the basement membrane33 (Fig. 4B). Surrounding the basement membrane, pericytes can be found in some capillaries (Fig. 4C).
(A) Capillary blood vessel. These vessels have a narrow diameter and their wall is made of endothelial cells plus the basement membrane (hematoxylin–eosin ×400). (B) Capillary. The wall is composed only of endothelia positioned directly on the basement membrane (hematoxylin–eosin ×400). (C) Pericytes (arrow) are sometimes clearly evidenced in some capillaries with the hematoxylin–eosin stain (hematoxylin–eosin ×400). (D) Venule. A thin layer of connective tissue is seen underneath the basement membrane (hematoxylin–eosin ×200).
The small veins immediately seen after the capillaries are called venules. In contrast to capillaries, they show a connective tissue underneath the basement membrane (Fig. 4D). Additionally, a media made of smooth muscle cells, irregularly distributed, is evidenced. Some of them can also show pericytes. In some organs, certain venules appear dilated and large, and they are specialized in collecting blood (Fig. 5A). These venules are called sinusoids.
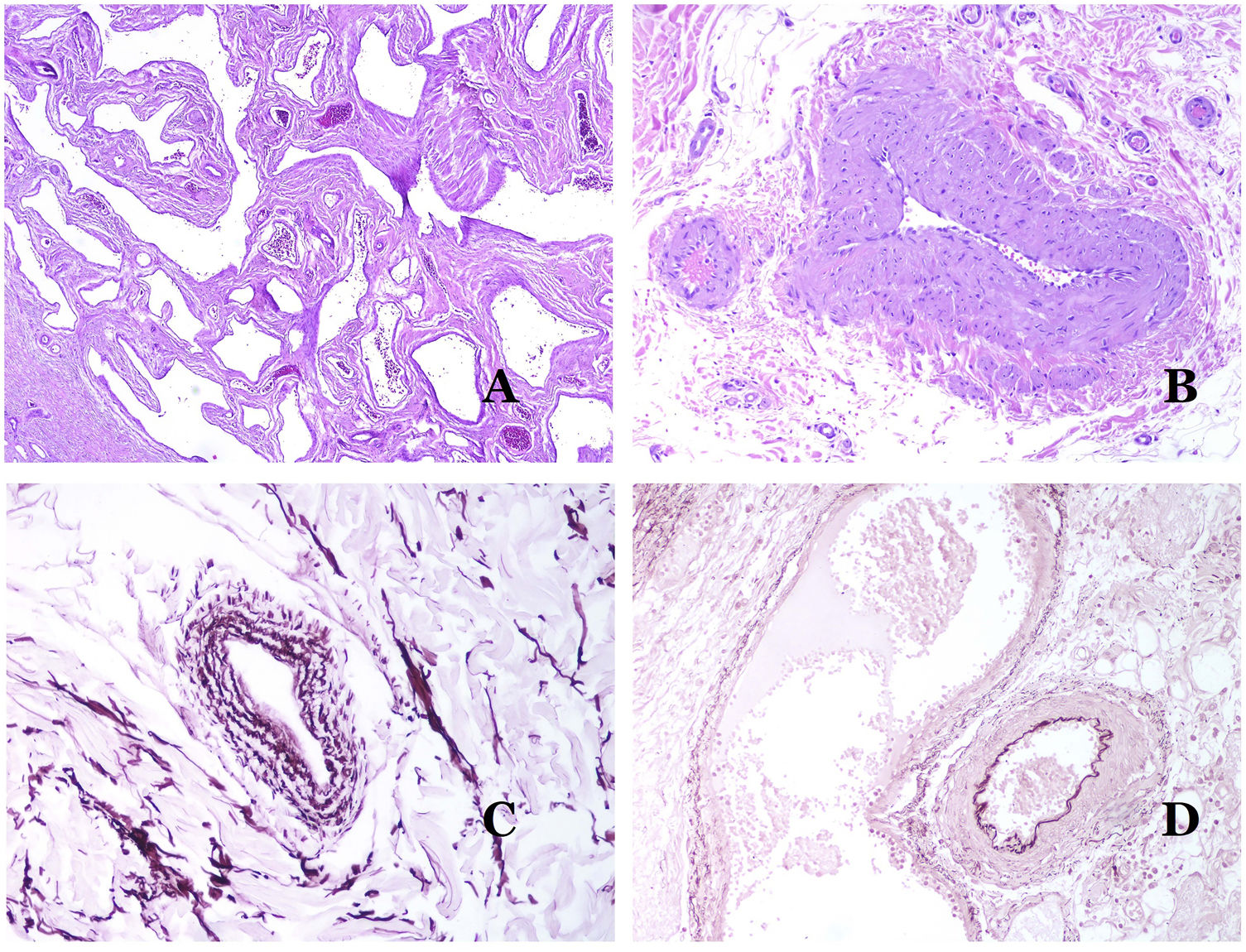
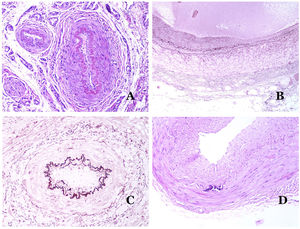
(A) Veins from the glans. At this location, the veins are specialized in collecting blood and appear dilated and organized in the corpus spongiosum. This should not be mistaken for a venous malformation (hematoxylin–eosin ×20). (B) Small vein with a continuous muscular layer (hematoxylin–eosin ×100). (C) Histochemical staining for elastic tissue showing the multiple concentrical discontinuous elastic bands evidenced in the muscular layer of a vein (orcein ×200). (D) Comparison between an artery (right) and a vein (left). The artery shows a thick wall when compared to the diameter of the vessel. In contrast, the vein (right) has a thinner wall. While a prominent internal elastic lamella is identified in the artery, it is not evident in veins. In contrast, veins have many discontinuous elastic fibers all over their wall (orcein ×20).
The small peripheral veins already show a continuous and regular spiral smooth muscle layer (Fig. 5B) with many discontinuous concentric bands of elastic fibers (Fig. 5C). Additionally, valves can be seen in them. Since veins are more elastic than contractile, these valves are important to guarantee the blood flow, preventing the blood return. Valves are made of paired infoldings of the intimal layer. Nevertheless, veins in some organs do not have valves. Medium and large veins have a wall which is well structured in three layers: intima, media and adventitial.
How to Distinguish an Artery From a Vein?In a transversal section of the blood vessel, arteries are round and symmetrical while veins might sometimes show an oval section. In comparison, arteries usually have a thicker wall when compared to the diameter of the vessel (Fig. 5D). In contrast, veins have a thinner wall when compared to the diameter of the vessel.34,35
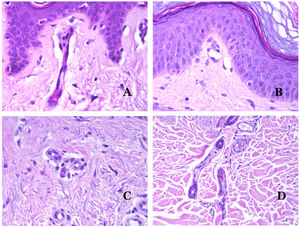
Arteries commonly have a prominent internal elastic lamella which is not evident in veins (Fig. 6A). In contrast, veins have many discontinuous elastic fibers all over their wall.34,35 Last, in the muscular layer of veins, the muscle fascicles are interrupted by bundles of collagen tissue, something that does not happen in arteries.
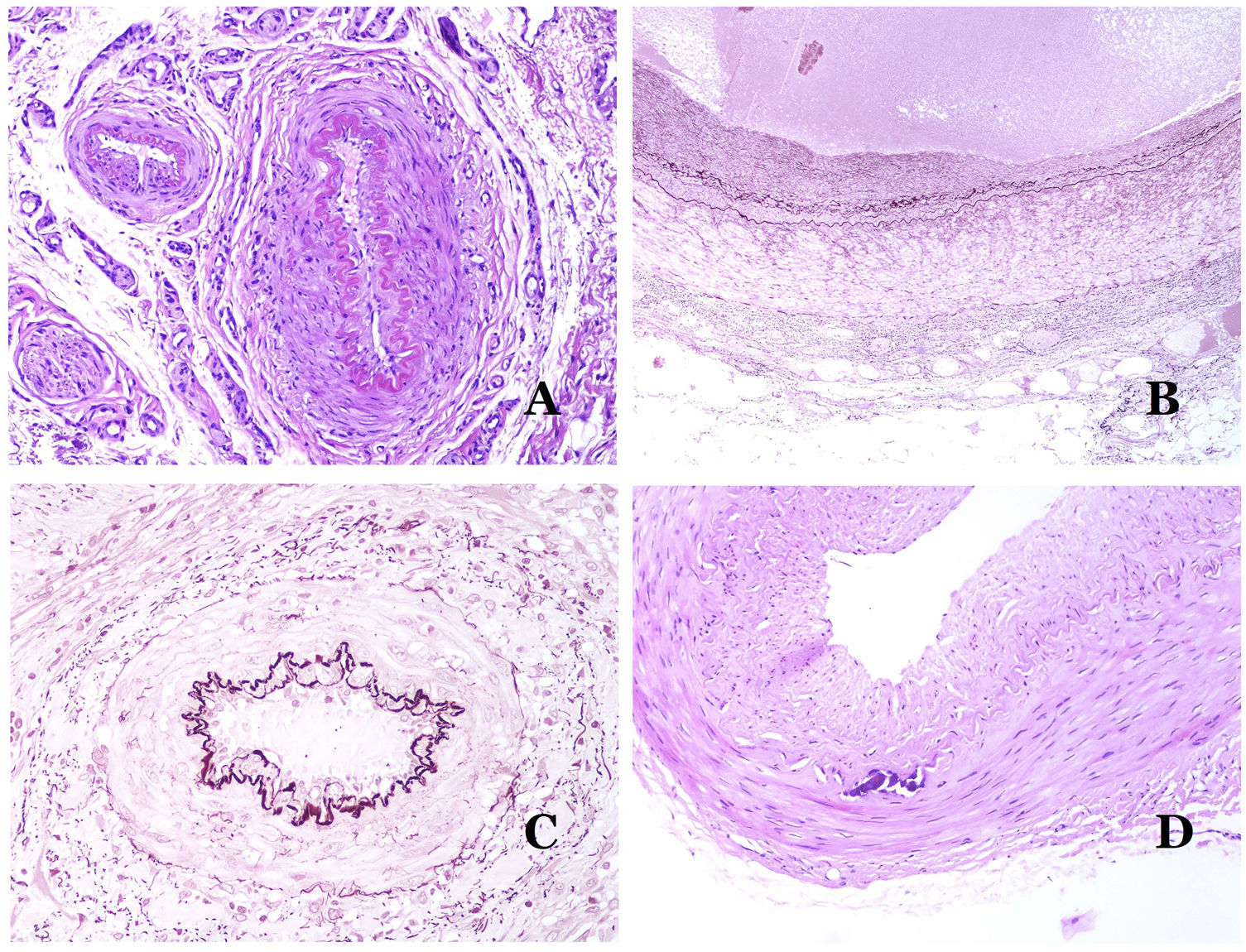
(A) Small artery in a skin biopsy. A prominent internal elastic lamella is evident even without special ancillary techniques (hematoxylin–eosin ×100). (B) This artery shows clear changes due to aging: the internal layer shows zonal thickening. The internal elastic lamella shows reduplication (orcein ×40). (C) Reduplication of the elastic internal lamella is a common phenomenon in aged arteries (orcein ×40). (D) Temporal artery showing a focus of calcification. Such foci are common with aging (hematoxylin–eosin ×100).
All blood vessels suffer morphologic changes with age, so much so, that it is debated if they should be considered as variants of normality.
In arteries, the intima thickens with age, mainly due to fibrosis31 (Fig. 6B). Additionally, fragmentation as well as reduplication of the internal elastic lamella is common with age32 (Fig. 6C). These acquired changes can be the source of weak points in the wall of the artery, inducing zonal dilatation and tortuosity, which should not be mistaken with the vascular dysmorphia evidenced in VM. Calcification is another common change with age (Fig. 6D). At the beginning, small calcium deposits are seen in the internal elastic lamella but, with progression, thicker deposits in the whole wall are evidenced. The amount of collagen and elastin also increases with age, in the muscular layer of arteries. All these aging in arteries can cause dilatation and tortuosity of the vessel.
Additional to intimal fibrosis and reduplication of elastic tissue, hyalinization of the wall is a common finding in aged arterioles (Fig. 7A), especially in the context of hypertension or diabetes.
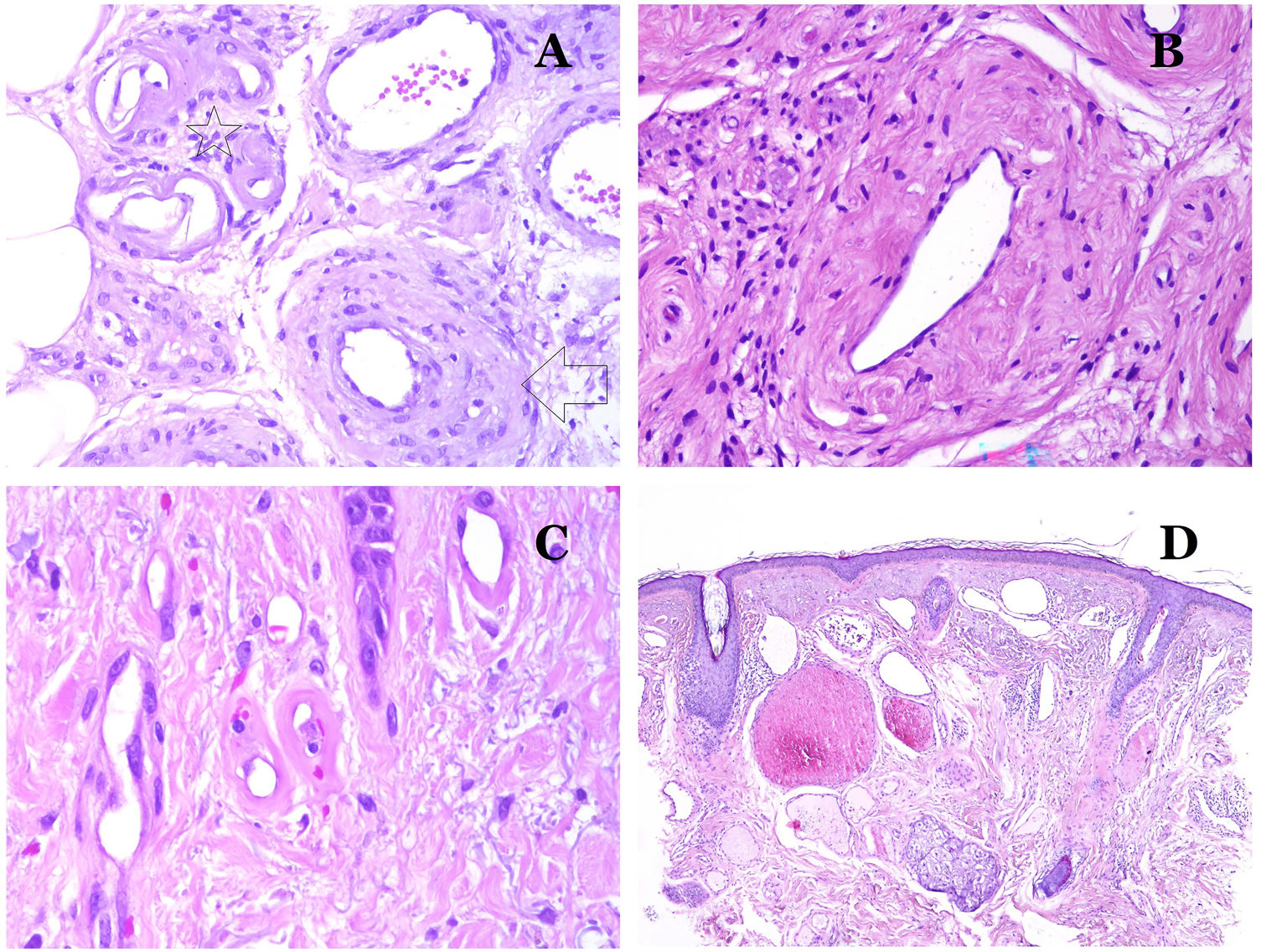
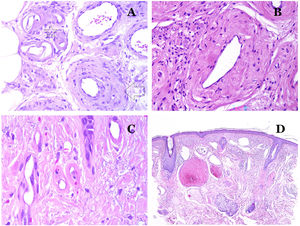
(A) This image shows changes in blood vessels due to aging. The arterioles show hyalinization of their wall (star) and a vein shows thickening of the muscular layer (arrow) (hematoxylin–eosin ×200). (B) Intimal fibrosis in a vein. This change is common with age (hematoxylin–eosin ×200). (C) Thickening of the basement membrane of capillaries, evidenced with aging (hematoxylin–eosin ×400). (D) Capillary-venous malformation. The dysmorphic capillaries appear dilated and in a haphazard distribution (hematoxylin–eosin ×20).
Changes seen in veins with aging include intimal fibrosis (Fig. 7B) and muscular hyperthrophy.32,33 Additionally, some veins are under the influence of high orthostatic pressure (such as the ones in legs) and, as a consequence, they can show “arterialization” of their wall, that is, anatomical changes that make them look like arteries, with prominence of an internal elastic lamella and a thick muscular layer.35
In capillaries, the basement membrane increases its thickness with age32,33 (Fig. 7C).
Main Morphologic Signs of Dysmorphic Vessel of VMDysmorphic capillaries (in capillary malformations) appear many times dilated and ectatic, with a haphazard distribution (Fig. 7D). Their walls might be thickened, with small amounts of muscle and fibrous tissue. These vascular changes may be accompanied by epidermal and mesenchymal changes, such as epidermal hyperplasia, focal dermal mucin deposition, increased collagen and elastic tissue, follicular hamartomatous changes, infundibular cysts, trichofolliculoma-like changes, and neural hamartomatous changes. Changes are very subtle in biopsies from younger children and become more prominent with age.
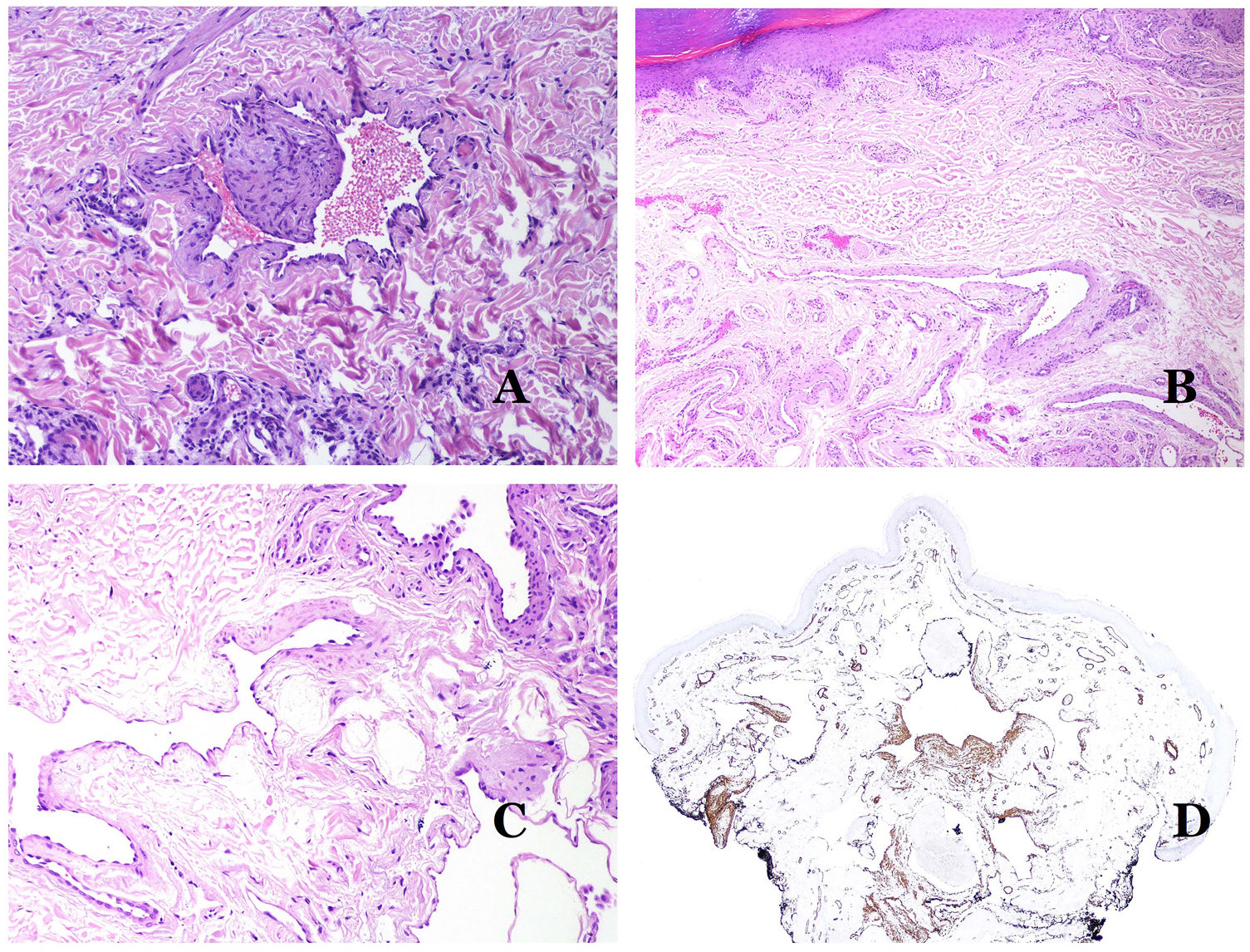
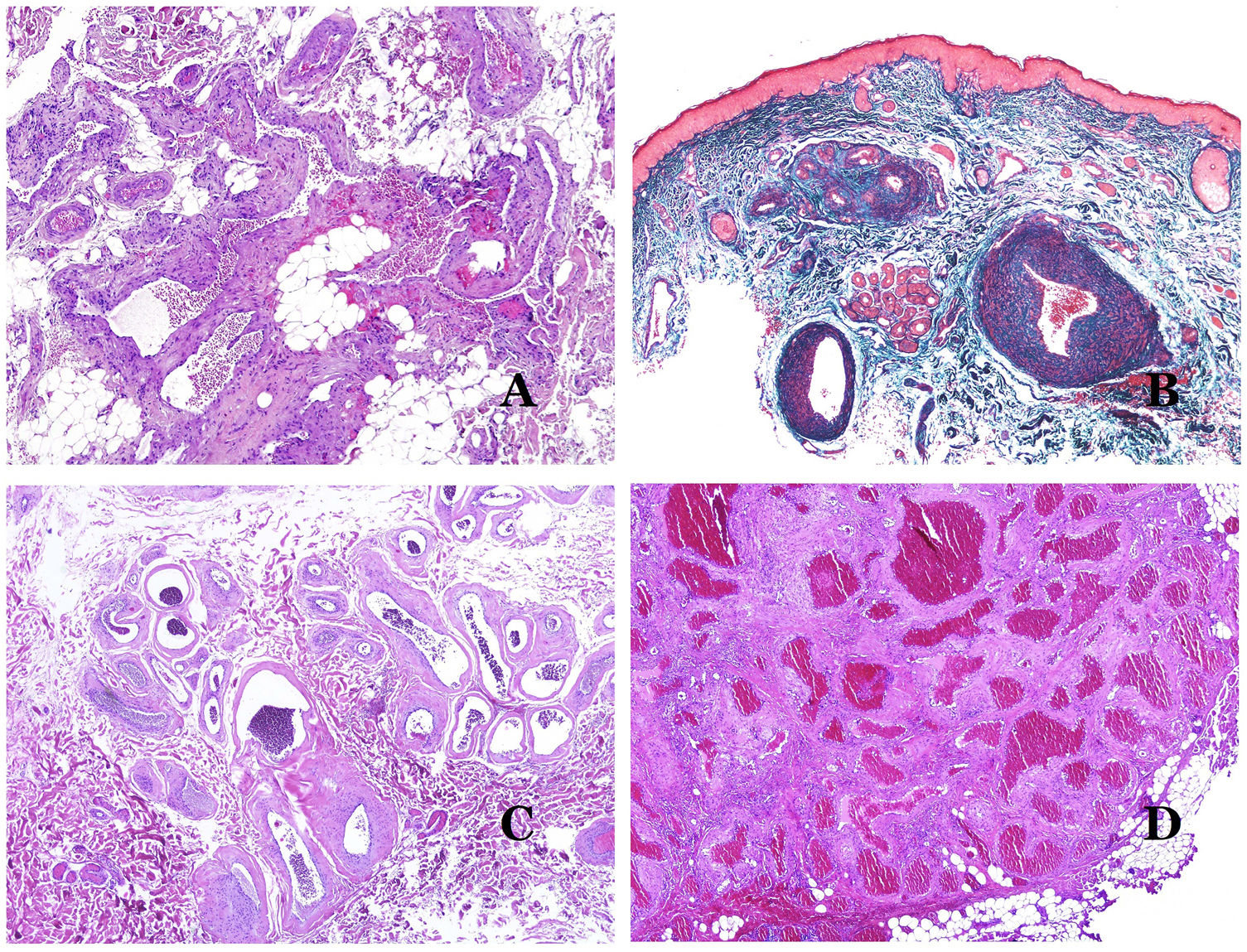
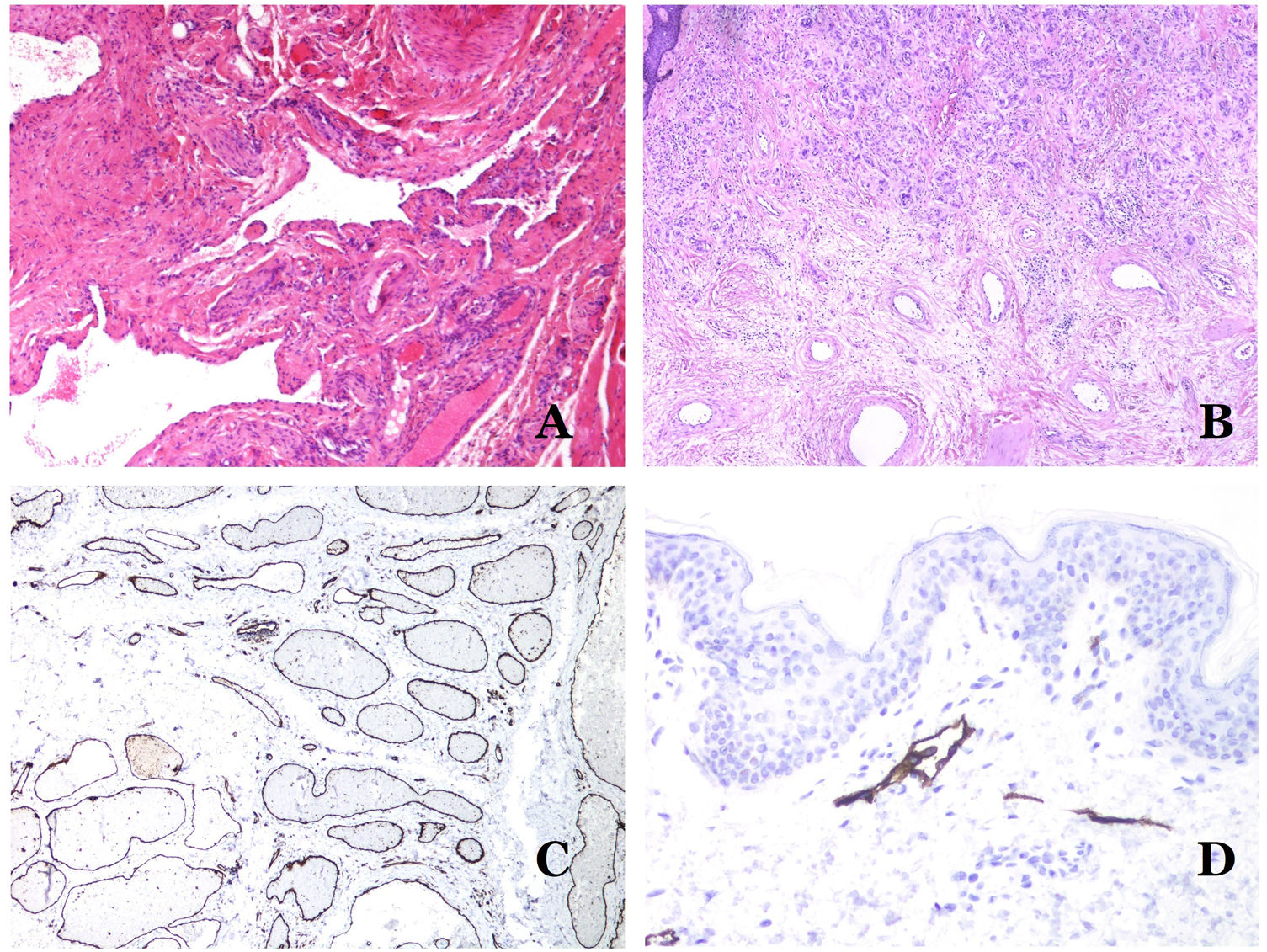
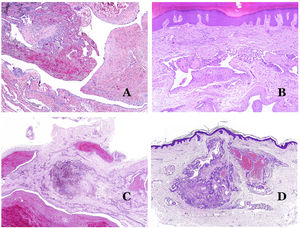
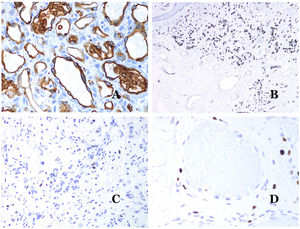
Dysmorphic veins (in venous malformations) are recognized by irregular shapes, sometimes angulated, with also irregularities in their walls (Fig. 8A). This is mainly due to variations in the muscular layer thickness25 (Fig. 8B), which in areas can be very thin or even absent, while in others, is enlarged and pronounced (Fig. 8C). This latter feature can be better demonstrated with an immunostain for smooth muscle actin (Fig. 8D). The dysmorphic vessels can extend through all the layers of the skin, many times involving the hypodermis (Fig. 9A). The venous wall, however, retains the normal structure of muscle fascicles separated by collagen, which is easily evidenced with the appropriate histochemical techniques (Fig. 9B). These malformed veins many times show a haphazard distribution, even forming clusters (Fig. 9C) or back-to-back arrangements (Fig. 9D). Thrombotic phenomena are not rare (Fig. 10A), and sometimes they are followed by partial recanalization and intravascullary papillary endothelial hyperplasia (Fig. 10B) and areas of hemorrhage, sometimes with hemosiderin deposits (Fig. 10C). When thrombi are incorporated into the vein wall, they can form fibromyxoid nodules. Thrombi may calcify forming phleboliths. Glomuvenous malformations are included among the venous malformations in the ISSVA classification. However, they show very distinctive features: malformed veins are surrounded by one or more layers of glomus cells (Fig. 10D).
(A) Dysmorphic veins of a venous malformation. Veins appear irregular and angulated. Their walls show an irregular thickness with some areas in which the muscular layer is absent while it is thickened in others (hematoxylin–eosin ×100). (B) Venous malformation. The dysmorphic veins show great variation in their wall thickness. This is mainly due to the irregularities of the muscular layer thickness (hematoxylin–eosin ×40). (C) Venous malformation. The dysmorphic veins show an irregular wall with irregularities in their muscular layer: while muscle is thin or even lacking in some areas, it is hypertrophied in others. (D) Venous malformation. An immunostain for smooth muscle actin marks the irregular muscular layer of the dysmorphic veins (smooth muscle actin ×20).
(A) Venous malformation. Dysmorphic veins are identified deep in the subcutaneous tissue (hematoxylin–eosin ×40). (B) Venous malformation. The dysmorphic veins show a muscular layer in which the muscles are divided in fascicles by collagen bundles, similar to what happens in normal veins (Masson trichrome ×20). (C) Venous malformation with a cluster arrangement of the dysmorphic veins (hematoxylin–eosin ×20). (D) Venous malformation with a back to back arrangement of the dysmorphic veins (hematoxylin–eosin ×20).
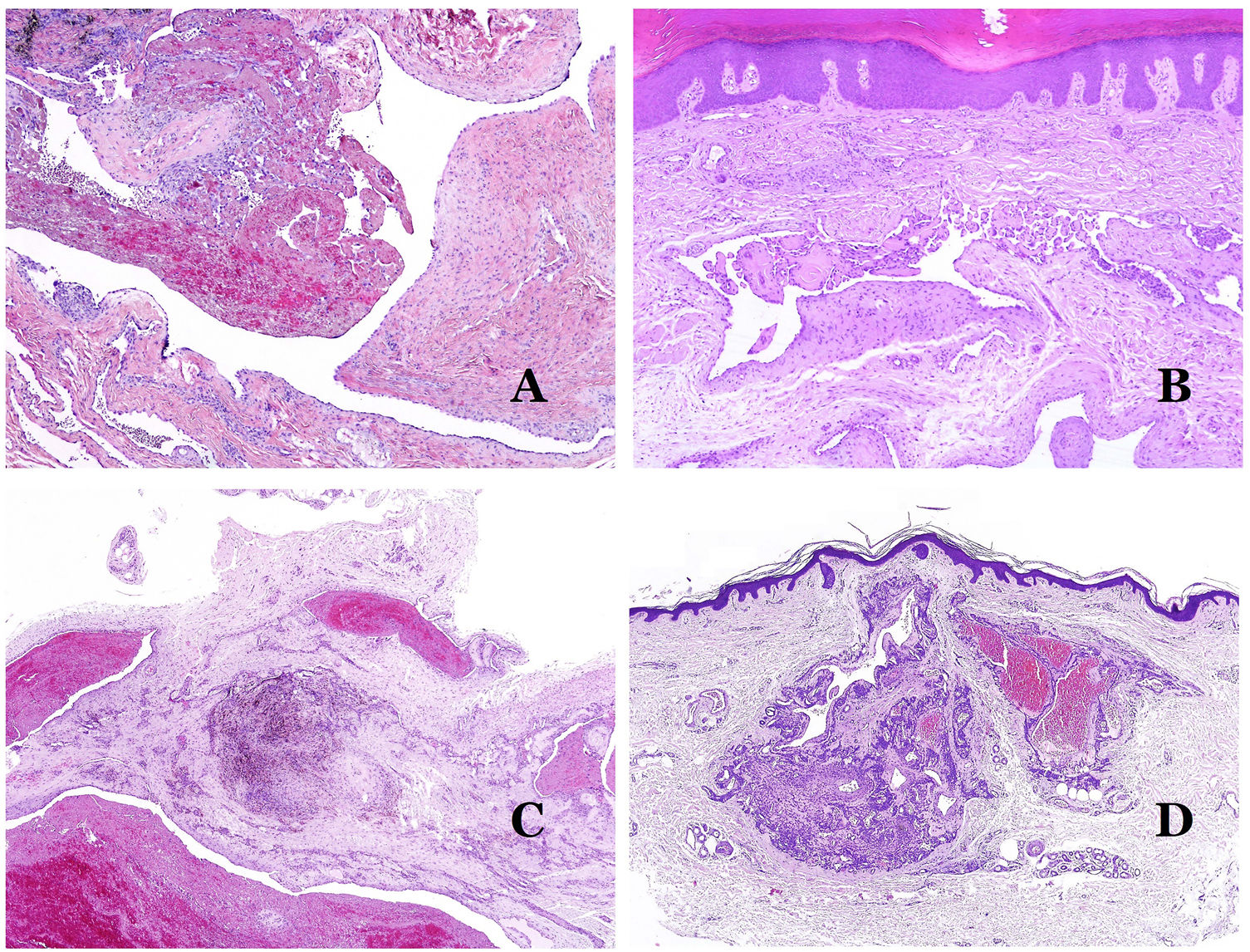
(A) Venous malformation with a partially recanalized thrombus inside (hematoxylin–eosin ×40). (B) Venous malformation with endothelial papillary intravascular hyperplasia (hematoxylin–eosin ×40). (C) Venous malformation with hemosiderin deposits in an area of old hemorrhage (hematoxylin–eosin ×20). (D) In this example of glomuvenous malformation, dysmorphic veins are accompanied by irregularly distributed roundish monomorphous glomus cells (hematoxylin–eosin ×20).
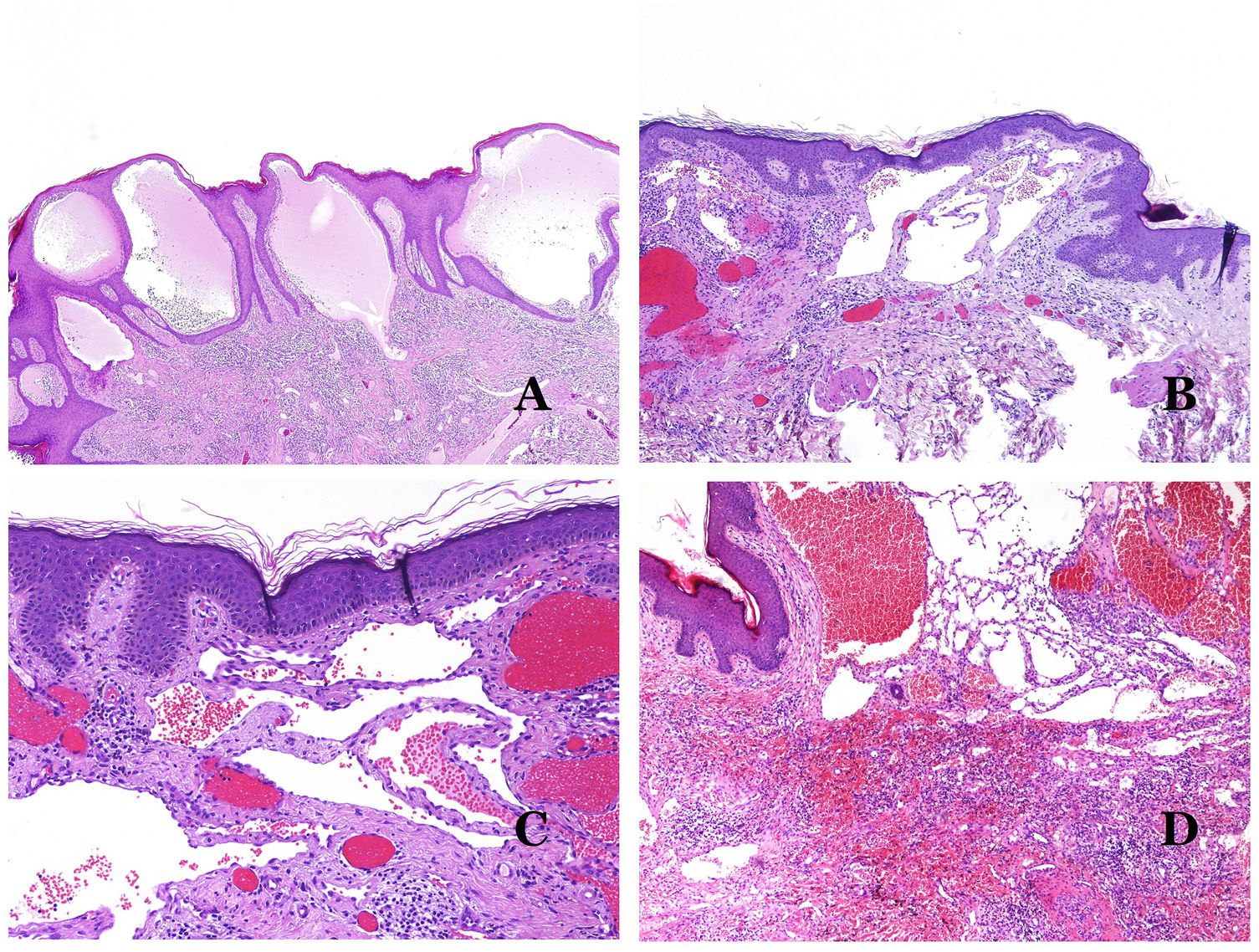
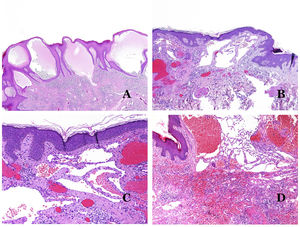
Dysmorphic lymphatics (in lymphatic malformations) appear dilated (Fig. 11A), sometimes extremely. Valves are sometimes seen, helping to identify such vessels as lymphatics (Fig. 11B). Lymphatic walls are commonly very thin but many times they can show foci of thickening, with smooth muscle and myxoid fibrous tissue. Disorganized fascicles of smooth muscle between the malformed channels is not rarely seen. The lymphatic lumens can sometimes show red cells, which is not incompatible with a lymphatic nature of these vessels: blood can accidentally enter these malformed lymphatics (Fig. 11C) but the event many times end up as hemorrhages with hemosiderin stromal deposits (Fig. 11D). Other feasible accompanying changes are hyperkeratosis, epidermal hyperplasia (sometimes even verrucous), ulceration, or inflammatory infiltrates (Fig. 12A). The size of the malformed vessel is variable, ranging from microscopic slit-like channels dissecting between the dermal collagen bands, to very large cysts.
(A) Lymphatic malformation. The dysmorphic lymphatic vessel appears dilated in the papillary dermis. This presentation was named in the past as “circumscribed lymphangioma” (hematoxylin–eosin ×20). (B) Lymphatic malformation. Valves are evident in some of the dysmorphic lymphatic vessels (hematoxylin–eosin ×20). (C) Lymphatic malformation. Dysmorphic lymphatic vessels, some of them containing blood. A valve is also seen in one of the vessels. The stromal lymphocytic infiltrate is also a common feature (hematoxylin–eosin ×40). (D) Hemorrhagic area besides a lymphatic malformation. This is a frequent phenomenon due to the low resistance of the lymphatic wall to keep the blood inside the vessel. The long standing hemorrhages can sometimes lead to hemosiderotic deposits (hematoxylin–eosin ×20).
(A) Lymphatic malformation. An accompanying inflammatory lymphocytic infiltrate is a common finding in lymphatic malformations (hematoxylin–eosin ×100). (B) Arteriovenous malformation. In this type of malformation, some vessels are recognized as dysmorphic arteries, some as dysmorphic veins, and even some as having intermediate features between an artery and a vein (hematoxylin–eosin ×100). (C) Arteriovenous malformation. In this stain for elastic fibers, the dysmorphic artery (arrow) shows disruption of the internal elastic lamina. In comparison, a dysmorphic vein is shown in the same field (star) (orcein ×40). (D) Arteriovenous malformation. The dysmorphic veins appear enlarged with a thick fibrotic wall. One of the veins shows foreign material used for sclerosis in its lumen. Large areas of small proliferative blood vessels are also seen (hematoxylin–eosin ×20).
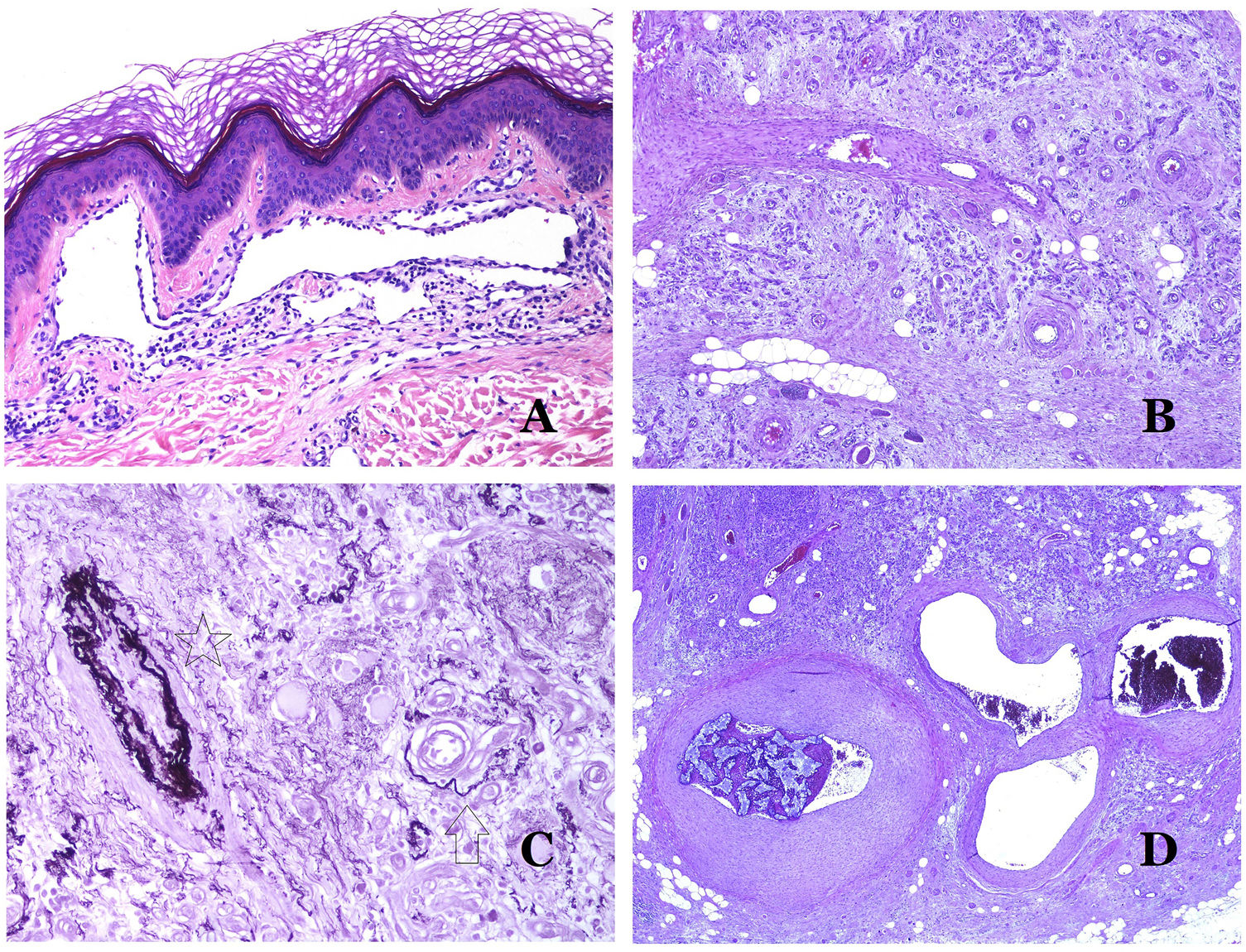
Arteriovenous malformations are made of dysmorphic arteries and veins, as well as vessels with morphologic features intermediate between a vein and an artery (Fig. 12B). The dysmorphic arteries show disruption of the internal elastic (Fig. 12C). Due to the high blood pressure, the venous channels many times develop a fibrotic wall, devoid of elastic fibers (Fig. 12D). Due to the high flow, thrombotic phenomena are not a feature of AVMs (Fig. 13A). Foci of microvascular proliferation are very common36 (Fig. 13B). They are allegedly proliferative expanding reactive foci. Most times they are seen as capillary-like groups but they can also show pyogenic granuloma-like morphology, or even pseudo-kaposiform changes.
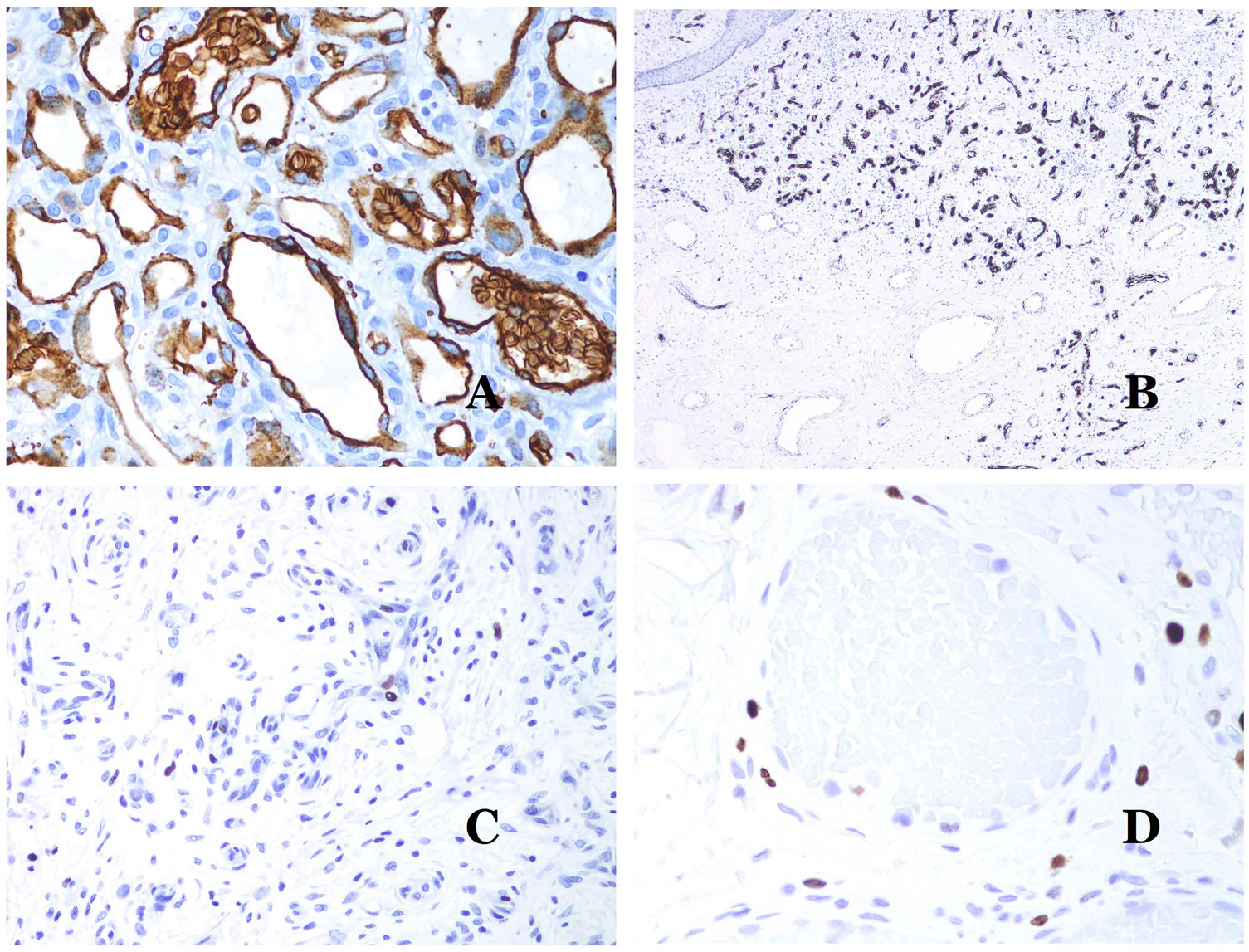
(A) Arteriovenous malformation (hematoxylin–eosin ×40). (B) Focus of vascular proliferative small vessels in an arteriovenous vascular malformation (hematoxylin–eosin ×40). (C) Venous malformation immunostained with CD31. The positive endothelia highlight the abnormal morphology of the dilated vessels (CD31 ×40). (D) Immunostaining for D2-40 showing positive endothelia in the lymphatics of the papillary dermis (D2-40 ×200).
There are several antibodies of help in the diagnosis of VMs.
The irregular or dilated shape of the vessels can be highlighted by using common endothelial markers such as ERG, CD31, Factor VIII or CD34 (Fig. 13C).25 The four of them stain the endothelia of both blood vessels and lymphatic vessels, however, CD34 can be only focally positive or completely negative in some malformed lymphatic vessels, particularly the largest ones.
Lymphatic vessels endothelia are positive for D2-40 (Fig. 13D), Lymphatic vessel endothelial hyaluronan receptor (LYVE-1), PROX1 and vascular endothelial growth factor receptor-3 (VEGFR-3).32 Podoplanin is a transmembrane protein found on lymphatic endothelium. Several commercialized antibodies react to podoplanin, among which, D2-40 is the most commonly used. Podoplanin expression is regulated by the PROX1 gene. Antibodies against the protein transcribed by the gene do exist and the cytoplasmic positivity of endothelia is considered as a marker of lymphatic nature.37 There are antibodies against PROX1 and the immunostaining is nuclear instead of cytoplasmic. LYVE-1 is a hyaluronan receptor and some studies have found it as effective as D2-40 in staining endothelia of lymphatic malformations.38 VEGFR-3 is expressed by lymphatic endothelia and might play an important role in lymphangiogenesis.39 However, VEGFR expression has also been found in blood vessel angiogenesis. Therefore, this marker is much less used than D2-40 or LYVE-1 in the identification of lymphatic vessels.
Glucose transporter isoform 1 (GLUT-1) is a member of the family of glucose transporters across cell membranes. GLUT1 upregulation has been identified in several neoplasms and, therefore, it has been found useful to distinguish them from certain mimicking entities. Immunohistochemistry for GLUT1 is useful in this sense. The differential diagnosis between infantile hemangioma (GLUT1-positive)7 (Fig. 14A) and VM (GLUT1-negative) is a good example. Some vascular malformations, such as verrucous venous malformation, are focally positive for GLUT-1.
(A) Infantile hemangioma positive for GLUT-1. The positive endothelia are clearly evidenced. Positivity of the red cells is useful as a control (GLUT-1 ×400). (B) Immunoexpression of WT1 in an area of small proliferative blood vessels evidenced in an arteriovenous malformation (hematoxylin–eosin ×40). (C) The proliferative areas mainly seen in arteriovenous malformations are positive for Ki67 in a small number of endothelial nuclei (Ki67 ×200). (D) Vascular malformation. Immunostaining with Ki67. Endothelial cells are negative. Caution must be taken not to misread the surrounding positive cells (Ki67 ×400).
Wilms tumor 1 (WT1) antibody recognizes the Wilms tumor protein, encoded by a gene located on chromosome 11p13. The protein is expressed by several tumors with a nuclear or a cytoplasmic pattern. It was for long claimed that vascular tumors are WT1-positive (cytoplasmic expression) while VM are negative. This is not sustained any longer.40,41 The protein is expressed by vascular proliferative areas, whether they happen in a tumor or in a malformation. Areas of microvascular proliferation, which are commonly seen in arteriovenous malformations are WT1-positive as can be the endothelial cells of the larger vessels (Fig. 14B). WT1 could also be positive in verrucous venous malformations in some types of capillary malformations like those seen in the capillary malformation-arteriovenous malformation syndrome. In the evaluation of WT1 immunostaining, another frequent pitfall should be considered: positivity by pericytes and/or smooth muscle cells. Pericytes and smooth muscle cells can be marked with smooth muscle actin (SMA),42 in contrast to the endothelial layer which is SMA-negative. This is also important to correctly identify mitoses and Ki67 expression in endothelial cells (SMA-negative) from the mitoses sometimes seen in pericytes (SMA-positive). While vascular tumors are proliferating lesions and therefore, their endothelial cells stain with Ki67, VM do not stain with Ki67 or they only show proliferation in the newly formed proliferative small blood vessels (Fig. 14C). Out of such foci, endothelial cells are negative in VM, in contrast with the surrounding connective and muscular cells (Fig. 14D).
ConclusionsWhen a dermatopathologist identifies a vascular anomaly in a biopsy, several signs should alert them to the possibility of a VM, such as the evidence of dysmorphic vessels or a low or null proliferation rate. The identification of the type of dysmorphic vessel will be essential to diagnose the type of vascular malformation. The morphology of an arteriovenous malformation equals a high-flow VM, which can be corroborated by doppler sonography or dynamic magnetic resonance imaging. A complete clinical history will help to identify those cases in which the VM is part of a syndrome. Last, when needed, genetic studies may help to specifically identify certain VM or syndromes.
Conflict of InterestsThe authors declare that they have no conflict of interest.