The arrival of immunotherapy has revolutioned the management of patients with metastatic Merkel cell carcinoma (MCC). We conducted an observational, retrospective study of 14 cases treated with avelumab. The response rate was 57%: complete response was reached in 29% of patients, and partial responses in 29%. The drug proved effective in 83% (5/6) of the patients with a single metastatic site. However, the disease progressed in 75% (3/4) of the patients with bone metastases. PD1-L expression, MCC polyomavirus (MCPyV) positivity, and an impaired neutrophil-to-lypmhocyte ratio (NLR) could not be associated with responses to the therapy. Avelumab is an effective and safe drug for the management of advanced MCC, and its effectiveness appears to be impacted by the number and location of metastases.
La introducción de la inmunoterapia ha supuesto un gran avance en el manejo de los pacientes con carcinoma de células de Merkel (CCM) metastásico. Realizamos un estudio observacional retrospectivo de 14 casos de CCM metastásico tratados con avelumab. La tasa de respuesta fue de 57%, con una completa de 29% y otra parcial de 29%. El fármaco fue efectivo en 83% (5/6) de los sujetos que presentaron una única localización metastásica, pero la enfermedad progresó en 75% (3/4) de aquellos con metástasis óseas. No se detectaron efectos adversos graves en ninguno. No se relacionaron la expresión de PD1-L, la presencia de poliomavirus del CCM (MCPyV), ni la alteración del índice neutrófilo-linfocito (INL) con la respuesta al tratamiento. El avelumab es efectivo y seguro en el CCM en estadios avanzados, con una posible influencia del número y la localización de las metástasis en su efectividad.
Merkel cell carcinoma (MCC) is an aggressive and rare neoplasm primarily affecting elderly and/or immunosuppressed patients.1 Despite accounting for <1% of malignant skin tumors, MCC is the third leading cause of death from skin cancer only after melanoma and squamous cell carcinoma.2,3 Clinical trials with immune checkpoint inhibitors (ICI) have shown clinical activity and durable responses in patients with advanced MCC.4–6 Avelumab—a human anti-PD-L1 monoclonal antibody—is currently the only immunotherapeutic drug approved by the European Medicines Agency (EMA) for the treatment of metastatic MCC.4,6 Despite promising results from clinical trials, real-world experience is still limited, and many predictive factors of response remain unknown.7 The neutrophil-lymphocyte ratio (NLR)—whose predictive value is known in other tumors—has been identified as an independent prognostic factor in MCC.8 The NLR was associated with response to immunotherapy in the work of Zaragoza et al., and its modification after starting therapy seems to be prognostic in other tumors as well.8–10 We conducted the present study to describe the real-world experience of using avelumab to treat metastatic MCC at an oncology specialty center.
MethodWe conducted an observational and retrospective study, including all patients with metastatic MCC treated with avelumab at Instituto Valenciano de Oncología (IVO), Valencia, Spain from January 1st, 2018 through March 15th, 2023, with a total of 14 patients included. Avelumab was IV administered at a dose of 10mg/kg every 2 weeks, continuing until disease progression or unacceptable toxicity (severe and permanent adverse effects).4 During treatment, clinical and laboratory follow-up was performed every 2 weeks, including blood count, biochemical analysis, and periodic radiological studies—computed tomography [CT] and/or positron emission tomography [PET/CT]) —every 3 to 5 months. Response was defined based on the RECIST 1.1 criteria for solid tumors (Annex 1).5 Other evaluated parameters included overall survival (OS), adverse effects, and biomarker analysis (Merkel cell polyomavirus [MCPyV], PD-L1, NLR). NLR was calculated before starting immunotherapy and 6 weeks later according to the work of Suh et al.10 and using reference values from former studies (NLR ≥ 4).8 We performed the descriptive statistical analysis of data using the IBM Statistical Package for the Social Sciences (SPSS) 25.0, and a univariate inferential analysis using Fisher's f (Fig. 1).
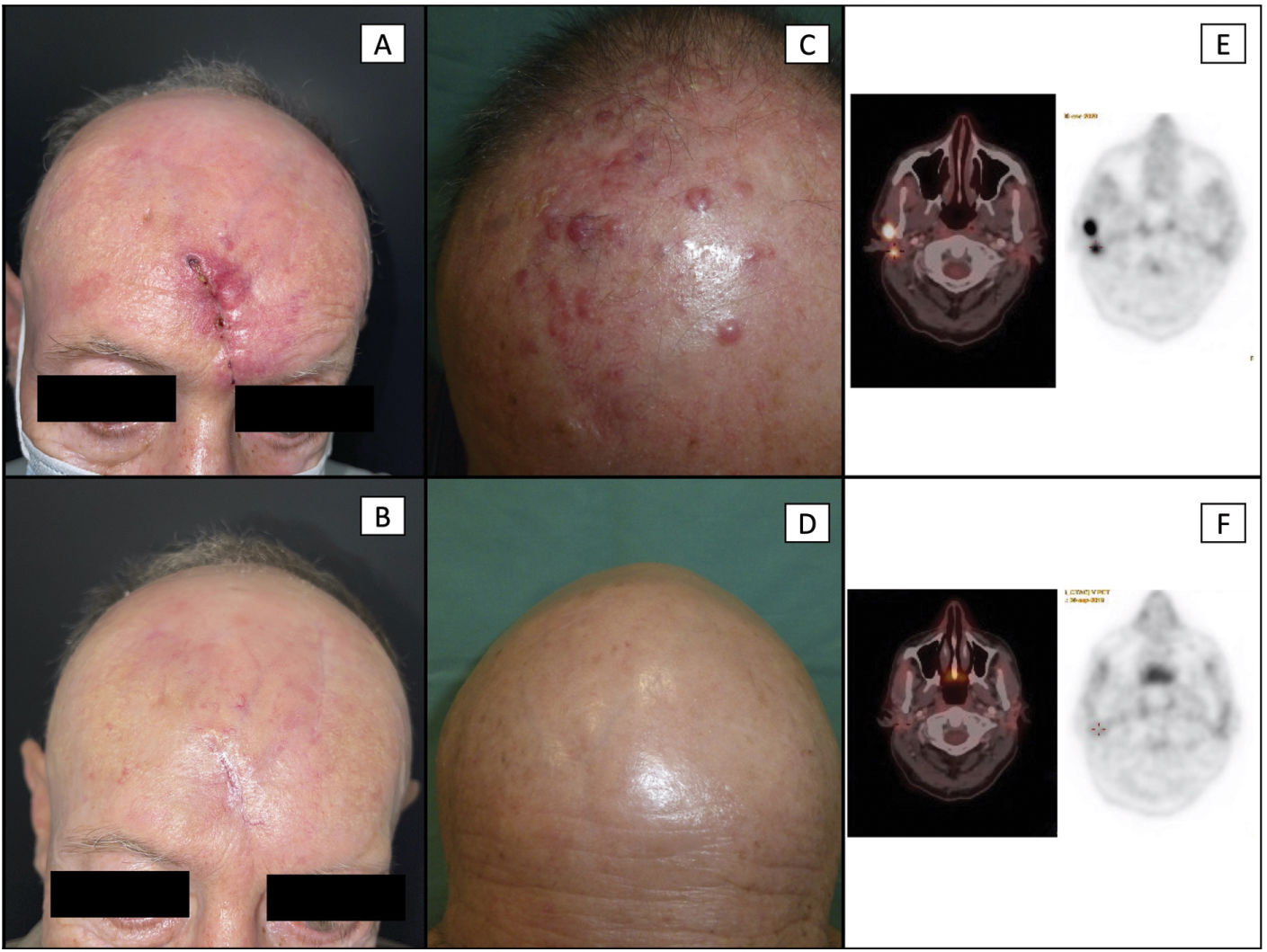
Patient #6 (a-b): local recurrence in the region of the scar from as previously surgically treated MCC. Image A shows an infiltrated and erythematous skin around the scar (the patient also had loco-regional lymph node involvement). Recurrence was histologically confirmed. Image B shows the complete disappearance of previous lesions, with full closure of the scar and absence of MCC lesions after 5 cycles of avelumab.
Patient #2 (c-d): loco-regional recurrence in the form of multiple satellitosis in a patient with a past medical history of primary MCC on the scalp. Image C shows multiple erythematous-violet papules and nodules corresponding to loco-regional cutaneous metastases (the patient also exhibited loco-regional lymph node involvement). Image D shows the complete disappearance of all cutaneous lesions after 7 cycles of avelumab plus loco-regional adjuvant radiotherapy.
Patient #2 (e-f): comparative axial views of PET/CT images at cervical level. Image E shows 2 hypermetabolic nodular lesions on the PET scan, corresponding to metastatic lymphadenopathy. Image F shows the disappearance of these lesions after 7 cycles of avelumab.
MCC: Merkel cell carcinoma; PET/CT: positron emission tomography/computed tomography.
We included a total of 14 subjects (13 men and 1 woman) with a median age of 70 years. Only the woman had a history of immunosuppression (chronic lymphocytic leukemia). The main clinical characteristics and treatment data are shown in Table 1. The most common location of the primary tumor was the head (6) and lower limbs (6), with a median tumor size of 3.75cm. Most patients presented with stage IIIB cancer (macroscopic nodal involvement; 8), and all developed metastases within 2 years of diagnosis. The most common site of metastases was nodal (13), followed by cutaneous (5).
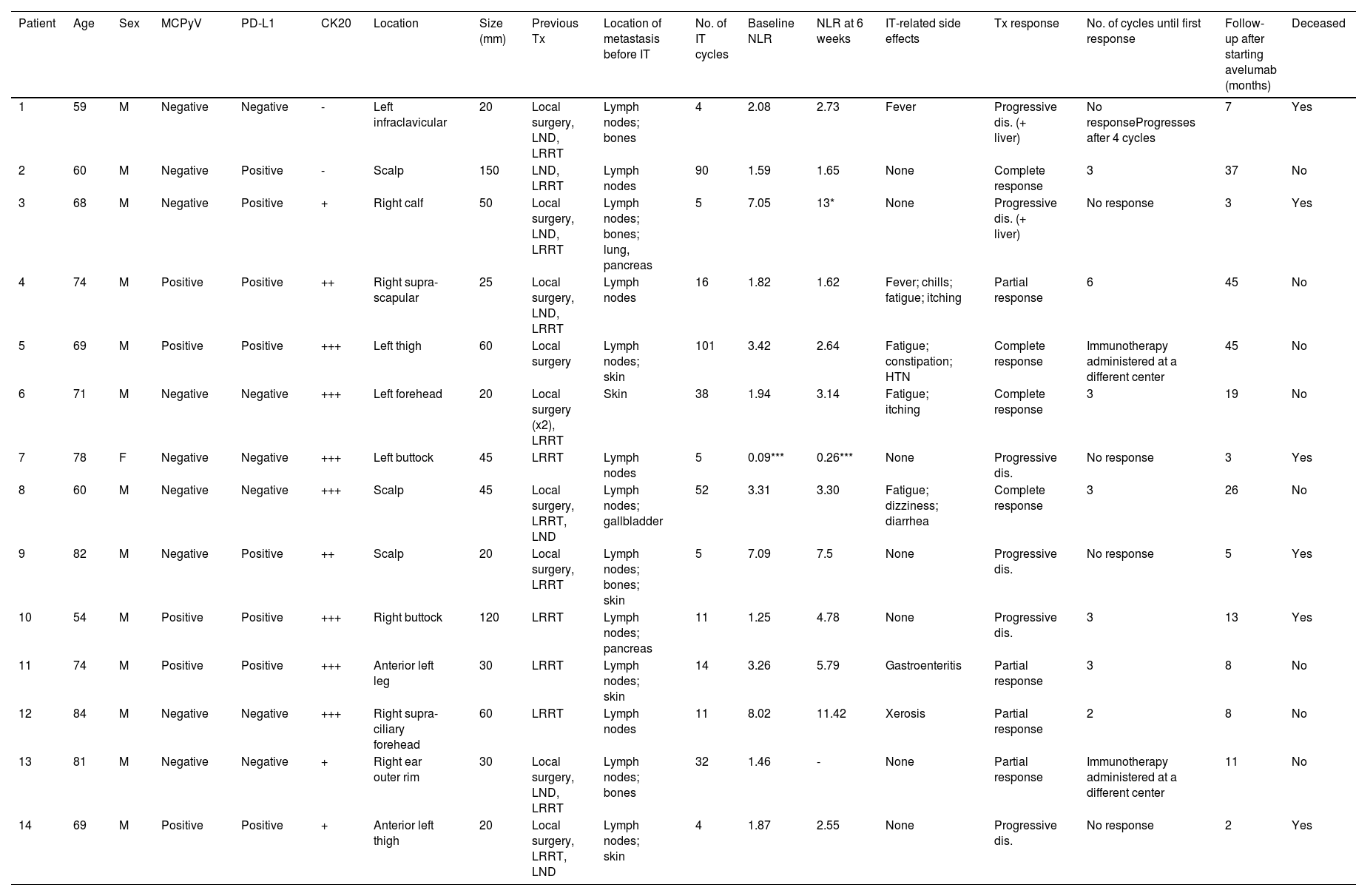
Main clinical and treatment characteristics.
| Patient | Age | Sex | MCPyV | PD-L1 | CK20 | Location | Size (mm) | Previous Tx | Location of metastasis before IT | No. of IT cycles | Baseline NLR | NLR at 6 weeks | IT-related side effects | Tx response | No. of cycles until first response | Follow-up after starting avelumab (months) | Deceased |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 59 | M | Negative | Negative | - | Left infraclavicular | 20 | Local surgery, LND, LRRT | Lymph nodes; bones | 4 | 2.08 | 2.73 | Fever | Progressive dis. (+ liver) | No responseProgresses after 4 cycles | 7 | Yes |
| 2 | 60 | M | Negative | Positive | - | Scalp | 150 | LND, LRRT | Lymph nodes | 90 | 1.59 | 1.65 | None | Complete response | 3 | 37 | No |
| 3 | 68 | M | Negative | Positive | + | Right calf | 50 | Local surgery, LND, LRRT | Lymph nodes; bones; lung, pancreas | 5 | 7.05 | 13* | None | Progressive dis. (+ liver) | No response | 3 | Yes |
| 4 | 74 | M | Positive | Positive | ++ | Right supra-scapular | 25 | Local surgery, LND, LRRT | Lymph nodes | 16 | 1.82 | 1.62 | Fever; chills; fatigue; itching | Partial response | 6 | 45 | No |
| 5 | 69 | M | Positive | Positive | +++ | Left thigh | 60 | Local surgery | Lymph nodes; skin | 101 | 3.42 | 2.64 | Fatigue; constipation; HTN | Complete response | Immunotherapy administered at a different center | 45 | No |
| 6 | 71 | M | Negative | Negative | +++ | Left forehead | 20 | Local surgery (x2), LRRT | Skin | 38 | 1.94 | 3.14 | Fatigue; itching | Complete response | 3 | 19 | No |
| 7 | 78 | F | Negative | Negative | +++ | Left buttock | 45 | LRRT | Lymph nodes | 5 | 0.09*** | 0.26*** | None | Progressive dis. | No response | 3 | Yes |
| 8 | 60 | M | Negative | Negative | +++ | Scalp | 45 | Local surgery, LRRT, LND | Lymph nodes; gallbladder | 52 | 3.31 | 3.30 | Fatigue; dizziness; diarrhea | Complete response | 3 | 26 | No |
| 9 | 82 | M | Negative | Positive | ++ | Scalp | 20 | Local surgery, LRRT | Lymph nodes; bones; skin | 5 | 7.09 | 7.5 | None | Progressive dis. | No response | 5 | Yes |
| 10 | 54 | M | Positive | Positive | +++ | Right buttock | 120 | LRRT | Lymph nodes; pancreas | 11 | 1.25 | 4.78 | None | Progressive dis. | 3 | 13 | Yes |
| 11 | 74 | M | Positive | Positive | +++ | Anterior left leg | 30 | LRRT | Lymph nodes; skin | 14 | 3.26 | 5.79 | Gastroenteritis | Partial response | 3 | 8 | No |
| 12 | 84 | M | Negative | Negative | +++ | Right supra-ciliary forehead | 60 | LRRT | Lymph nodes | 11 | 8.02 | 11.42 | Xerosis | Partial response | 2 | 8 | No |
| 13 | 81 | M | Negative | Negative | + | Right ear outer rim | 30 | Local surgery, LND, LRRT | Lymph nodes; bones | 32 | 1.46 | - | None | Partial response | Immunotherapy administered at a different center | 11 | No |
| 14 | 69 | M | Positive | Positive | + | Anterior left thigh | 20 | Local surgery, LRRT, LND | Lymph nodes; skin | 4 | 1.87 | 2.55 | None | Progressive dis. | No response | 2 | Yes |
CK20: cytokeratin 20; dis.: disease; F: female; IT: immunotherapy; LND: lymph node dissection; M: male; MCPyV: Merkel cell polyomavirus; Tx: therapy; LRRT: local/regional radiotherapy; -: negative; +: weak; ++: moderate; +++: intense.
All patients received avelumab as first-line therapy. A median of 12.5 cycles (range, 4 up to 101) was administered. Eight patients (57%) responded to avelumab, 4 of them (29%) achieved complete lesion response and 4 (29%) partial lesion response. Responders received a median of 33 cycles. Currently, all responders are still on avelumab, except for 1 patient who stop using the drug after 16 cycles due to disease progression. This patient later received 6 cycles of cisplatin-etoposide chemotherapy with good tolerance, achieving a durable complete response with the cytotoxic regimen, remaining disease-free for 4 years and 6 months after the primary tumor diagnosis. The drug was discontinued in the other 6 patients due to tumor progression during treatment; all died from the disease. The median OS was 9.5 months (range, 2-46) (19 months in responders).
A total of 50% of the patients on avelumab experienced no adverse reactions, and most adverse events were mild (G1) (Table 1). The most common side effects were asthenia, pruritus, and diarrhea. Only 1 patient had to discontinue therapy due to immune-mediated colitis after 16 cycles (G2), which resolved with oral corticosteroid therapy, with subsequent reintroduction and tolerance of the drug.
Immunohistochemical expression of MCPyV was positive in 5 cases, and PD-L1 in 8. Neither parameter appeared to be related to treatment response. Finally, 3 patients had an elevated NLR: 2 of them exhibited rapidly progressive disease unresponsive to avelumab. The third patient has shown a partial response to the drug so far. None of the patients with a complete response had an altered NLR. No statistically significant relationship between an altered NLR and the lack of treatment response could be established. No significant changes were found either between the baseline NLR and 6 weeks after starting therapy (Table 1).
DiscussionMCC is one of the most aggressive skin tumors, with 5-year OS rates ranging from 40% up to 60% in patients with nodal involvement, and 10% up to 20% in those with visceral metastatic disease.11,12 Before the arrival of immunotherapy, the treatment of this disease was based on platinum-based chemotherapy.6,7,13 Of note that the relatively high objective response rates (ORR) to first-line chemotherapy (50% up to 70%) were short in time, with median response durations between 3 and 8 months, without a proven benefit in OS.5,11,13–15
In clinical trials leading to its approval, avelumab demonstrated an ORR of 33% when used as a second-line therapy after chemotherapy,5,14 and a 39.7% ORR when used as first-line therapy.15 After the drug was approved, an expanded access program was initiated in 38 countries, confirming the safety and efficacy data of the JAVELIN 200 trial, with a 47% ORR and no new detectable safety events.16 In addition to the results of this expanded program, the real-world use of avelumab remains limited and consists of cohorts of a few dozen patients. Cowey et al.17 reported a 64% ORR as a first-line therapy. Levy et al.18 reported a 57% ORR in a Dutch cohort, in which most patients received avelumab as first-line therapy. Lastly, Averbuch et al.19 achieved a 59% ORR, also in most treatment-naive patients. Both in real-world and clinical studies, responses occurred quickly, between 7 and 10 weeks.4,19 In our series, responses began to be observed between the 2nd and 6th cycles of treatment (i.e., 4 to 12 weeks after starting therapy).
In our series, the ORR was 57%, which is similar to the ORR of other real-world clinical cohorts, and in all cases, higher than the initial results reported in pivotal studies, with response rates to avelumab being higher when the drug is used as a first-line therapy for metastatic disease. This underscores the importance of using immunotherapy as the first-line therapy.4,19,20 In clinical trials, the median OS ranged from 12 up to 20 months,4,15 while in real-world cohorts, it ranged from 10 up to 25 months.17–19 In our series, the median OS was 8.5 months (22.5 months in responders). These data are significantly higher than those of conventional chemotherapy.13,20
The most frequently reported adverse events in all reports have been grade 1-2, with a low incidence rate of severe adverse events (G3 and G4) that led to treatment discontinuation.4,14,15,17–21 In our series, the most common adverse event reported was asthenia, which is also described as one of the most frequent in the literature.7,21
To date, predictive factors of response to immunotherapy with anti-PD1/PD-L1 drugs are mostly unknown.22–24 Although some studies suggest that PD-L1 expression may be associated with a higher response rate, this has not been validated by other studies.1,4,6,15 Both MCPyV+ and MCPyV− MCCs seem to respond similarly to these drugs.1,6,19,24 Neither one of these 2 factors was associated with a greater response to avelumab in our series, being similarly distributed among responders and non-responders. On the other hand, NLR is an interesting marker that—although associated with a worse prognosis in MCC—has not yet been associated with immunotherapy response.24 However, studies have associated the NLR with immunotherapy response in other solid tumors.8–10 In the present work, although we did not find a correlation between this index and immunotherapy response, we should mention that none of the 4 patients with a complete response and only 1 of those with a partial response, had an altered NLR (Table 1). The small sample size likely limits the possibility of finding a statistical association in this series. Furthermore, we did not find any significant variations that could be associated with response in baseline NLR vs the NLR calculated 6 weeks after starting therapy.
Three of the 4 patients with bone metastases from our series did not respond to the drug, which could link this metastatic site to a poorer treatment response. On the other hand, 5 of the 6 with single metastatic localization responded to the drug (all except for patient #7, an immunosuppressed woman). Although a recent multicenter study identified the absence of immunosuppression and the presence of a single metastasis as predictors of response to avelumab, to date, no study has ever linked bone metastases to a lower response rate, even though they are associated with a worse prognosis in other solid tumors.22 Finally, some studies have associated the presence of adverse effects with a higher probability of response, something we were unable to corroborate in our series.6
The main limitations of our research—beyond those inherent to a retrospective study—are the lack of a control group and the small sample size.
ConclusionsIn this real-world observational study, avelumab proved to be safe and effective for the treatment of MCC in clinical practice and seems to exceed the safety and efficacy data previously presented in clinical trials. Although some studies suggest the absence of immunosuppression, single metastatic localization, and the presence of PD-L1 as predictors of response to immunotherapy, none of them has yielded definite conclusions. In our series, the presence of bone metastases and multiple metastatic sites were associated with a poorer treatment response.
FundingNone declared.
Conflicts of interestBLLC declares having worked as a consultant and received fees from Merck, Amgen, Sanofi, Sunpharma, and Roche.