Psoriasis often precedes the onset of psoriatic arthritis (PsA), so dermatologists often face the challenge of early identifying signs of PsA in patients with psoriasis. Our aim was to validate the Spanish version of the PURE-4 questionnaire as a screening tool for PsA, evaluate its performance in terms of sensitivity, specificity, feasibility, reliability, and build validity.
MethodsThis was a cross-sectional, observational, multicenter trial of adult patients with psoriasis. Initially, patients were assessed by a dermatologist and completed 2 self-administered versions (in print and online) of the PURE-4 questionnaire. Afterwards, the rheumatologist, blinded to the PURE-4 results, assessed the presence/absence of PsA, being the reference to determine the performance of the PURE-4 questionnaire.
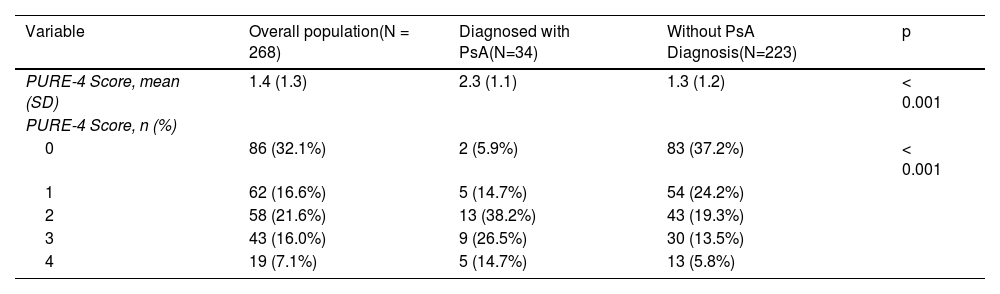
ResultsA total of 268 patients were included (115 [42.9%] women; mean age, 47.1±12.6). The prevalence of PsA according to rheumatologist diagnosis was 12.7% (34 patients). The mean PURE-4 score for patients with psoriasis diagnosed with PsA was 2.3±1.1, and 1.3±1.3 for patients without PsA (P<.001). The cutoff value ≥2 demonstrated the best performance for detecting PsA, with a negative predictive value of 95.1% (95% confidence interval, 90.3-97.6).
ConclusionsThe PURE-4 questionnaire demonstrated good performance in detecting PsA, with an optimal cutoff point ≥2. This simple tool could facilitate early referral of patients to the rheumatology unit.
La psoriasis suele preceder a la aparición de la artritis psoriásica (APs), por lo que los dermatólogos suelen enfrentarse al reto de identificar precozmente los signos de APs en pacientes con psoriasis. El objetivo fue validar la versión española del cuestionario PURE-4 como herramienta de cribado para la APs, evaluando su rendimiento en términos de sensibilidad, especificidad, viabilidad, fiabilidad y validez de constructo.
MétodosSe realizó un estudio transversal, observacional y multicéntrico en pacientes adultos con psoriasis. Inicialmente, los pacientes fueron evaluados por un dermatólogo y completaron dos versiones autoadministradas (en papel y electrónica) del cuestionario PURE-4. Después, el reumatólogo, ciego a los resultados del PURE-4, evaluó la presencia/ausencia de APs, siendo la referencia para determinar el rendimiento del cuestionario PURE-4.
ResultadosSe incluyeron 268 pacientes (115 [42,9%] mujeres; edad media, 47,1±12,6 años). Se diagnosticó de APs a 34 pacientes (12,7%) en una media (DE) de 1,4±1,6 semanas. La puntuación PURE-4 media para los pacientes con psoriasis diagnosticados de APs fue de 2,3±1,1, y de 1,3±1,3 para los pacientes sin APs (p<0,001). El punto de corte óptimo con mejor rendimiento para detectar APs fue ≥2 respuestas positivas, con valor predictivo negativo del 95,1% (intervalo de confianza 95%: 90,3-97,6).
ConclusionesEl cuestionario PURE-4 demostró un buen rendimiento para el cribado de APs, con un punto de corte óptimo ≥2. Esta sencilla herramienta podría facilitar la derivación temprana de los pacientes al servicio de reumatología.
Psoriasis is an inflammatory and immunomediated dermatological disease that affects approximately 1% to 3% of adults in Western Europe.1 Psoriasis can be associated with various comorbidities, including psoriatic arthritis (PsA).2 The estimated prevalence of PsA ranges from 0.05% up to 0.25% in the general population and from 6% up to 41% in psoriatic patients.3 Overall, psoriasis appears before the clinical signs of PsA,4,5 which explains why dermatologists are often the first specialists to face the challenge of identifying patients at risk of developing PsA.
The estimated prevalence of undiagnosed PsA among psoriatic patients is between 15% up to 40%.4,6,7 Diagnosis is complex and is usually confirmed after several years, highlighting gaps in detection and intervention, when early treatment is known to improve clinical and health outcomes.8 Therefore, the identification of PsA among psoriatic patients is crucial, as it would facilitate rapid referral to the rheumatologist after the onset of the first symptoms. Dermatologists have been recommended to closely monitor signs of PsA in psoriatic patients, at least, once a year and, ideally, every 6 months, conducting a complete physical examination to check for peripheral inflammation and dactylitis, enthesitis, and inflammatory axial pain.9
With the aim of facilitating the early detection of PsA in dermatology clinics, a simple tool, the Psoriatic Arthritis UnclutteRed Screening Evaluation (PURE-4) questionnaire, was developed and validated in a French population with psoriasis.10 The strengths of PURE-4 include its feasibility in clinical practice, good discriminatory ability, and high sensitivity and specificity rates (85.7% and 83.6%, respectively).10 The questionnaire has been culturally adapted into Spanish language following standard methodology,11 and experts recommend its use due to the reduced number of items.9 The objective of this study is to validate the Spanish version of PURE-4 in terms of sensitivity, specificity, feasibility, reliability, and construct validity. Additionally, its electronic version with a virtual assistant (chatbot) was evaluated.
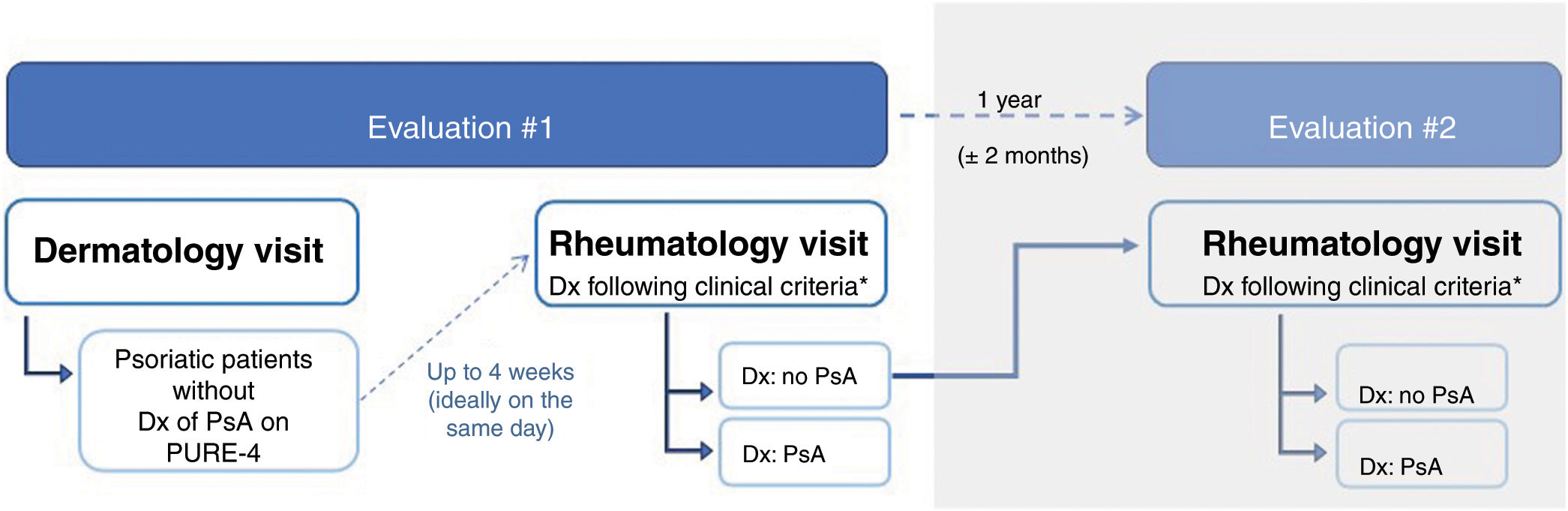
MethodsStudy design and participantsWe conducted an observational and multicenter study among 19 Spanish hospitals, with 2 cross-sectional evaluations. Evaluation #1 included psoriatic patients, without a diagnosis of PsA, and was performed by dermatologists and rheumatologists during routine visits. Evaluation #2 included patients without a diagnosis of PsA (in evaluation #1) and was performed by the same rheumatologists 1 year later (± 2 months) to determine the presence of PsA (Fig. 1). The results of evaluation #2 are not included in this article.
From November 15 through December 30, 2020, adult patients with psoriasis without a previous diagnosis of PsA who could respond to the PURE-4 questionnaire in paper and electronic formats were recruited. Considering that the probability of finding PsA in patients with moderate-to-severe psoriasis increases, and to ensure a PsA prevalence of 15%, at least, 60% of included patients should have moderate-to-severe psoriasis (Psoriasis Area and Severity Index [PASI] ≥7).12,13 All participants gave their prior written informed consent before recruitment. The study was approved by Hospital de Bellvitge Ethics Committee (reference PR384/20 [CSI 20/89]) and was conducted following the principles described in the Declaration of Helsinki.
ProceduresThe first visit to the dermatologist was followed by an independent visit to the rheumatologist (ideally, on the same day, or within 4 weeks following the dermatologist's visit). At the time of inclusion, demographic and clinical data were recorded (see Appendix A, “Supplementary Methods,” in the supplementary data), and patients completed the paper version of the PURE-4. Before concluding the visit, the dermatologist provided the patient with the electronic version of the PURE-4 and collected the patient's opinions and preferences regarding the paper and electronic versions of the questionnaire. Subsequently, the rheumatologist, blinded to the PURE-4 results, assessed the presence/absence of PsA based on the patient's health history and examination. When additional tests were necessary for the diagnosis of PsA according to clinical practice, an additional 2 months were allowed for confirmation purposes.
PURE-4 QuestionnaireThe PURE-4 questionnaire consists of 4 questions with affirmative or negative answers about signs of dactylitis, inflammatory heel pain, bilateral buttock pain, and peripheral joint pain with inflammation before the age of 50 (see Appendix A, Figure S1 of the supplementary data). Each positive response scores 1 point, with the maximum score being the sum of “Yes” responses (0 to 4 points).10 An electronic version of the PURE-4 questionnaire in Spanish was also evaluated, supported by a chatbot or virtual assistant, with information and images explaining the meaning of the items.
Study outcomesThe performance of the Spanish paper version of the PURE-4 questionnaire was examined, with the clinical diagnosis being confirmed by the rheumatologist as the reference. Patient perceptions (simple, quick, comfortable, and useful) of the paper and electronic versions were compared using a visual analog scale from 0 up to 10, as well as patient preferences between both versions.
Data analysisData are expressed as mean ± standard deviation (or median and interquartile range) for quantitative variables and as frequencies and percentages for categorical variables. Comparisons between participants with and without PsA were made using the Student's t-test, Fisher's exact test, and the Mantel-Haenszel test, as appropriate. The receiver operating characteristic (ROC) curve and the area under the curve (AUC) were used to evaluate the performance of PURE-4 in detecting PsA, and validity, sensitivity, specificity, positive predictive value, negative predictive value, and Youden's index were analyzed to find the optimal cutoff value for patient classification. Additional validation tests are described in Appendix A, “Supplementary Methods” of the supplementary data. The intraclass correlation coefficient (ICC) was used to assess internal consistency between paper and electronic versions of the questionnaire. Statistical analyses were performed using SAS software version 7.15.
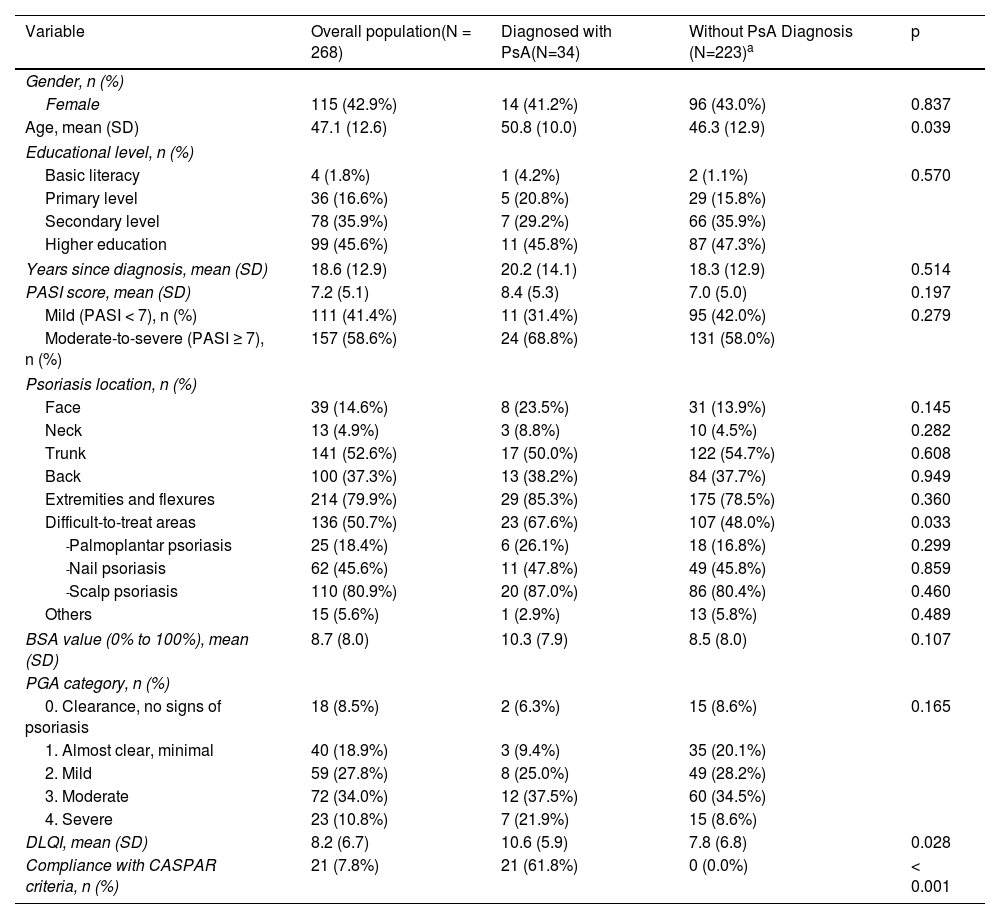
ResultsStudy participants and clinical evaluationsA total of 283 psoriatic patients were recruited; 3 were excluded for having PsA or another rheumatologic condition, and 12 because they did not attend their rheumatology appointment. The final analysis included a total of 268 patients (115 [42.9%] women; mean age, 47.1±12.6). Table 1 shows the demographic and clinical data of patients with and without PsA. The mean PASI score was 7.2±5.1. A total of 238 (88.8%) patients were on active treatment for psoriasis. The mean time between dermatology and rheumatology visits was 1.4±1.6 weeks. In evaluation #1, the prevalence of PsA based on the rheumatologist's diagnosis was 12.7% (n=34); it was not possible to confirm or rule out the diagnosis of PsA in 11 patients, even after obtaining additional test results within this period. Overall, the most common psoriasis locations were the extremities and flexural areas (PsA, 85.3% vs non-PsA, 78.5%; p=0.360), trunk (PsA, 50.0% vs non-PsA, 54.7%; p=0.608), and difficult-to-treat locations (PsA, 67.6% vs non-PsA, 48.0%; p=0.033). Information on inflammatory signs, imaging findings, and additional tests is shown in Appendix A, Table S1 of the supplementary data.
Demographic and clinical characteristics of all patients with psoriasis and those with and without PsA.
| Variable | Overall population(N = 268) | Diagnosed with PsA(N=34) | Without PsA Diagnosis (N=223)a | p |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Female | 115 (42.9%) | 14 (41.2%) | 96 (43.0%) | 0.837 |
| Age, mean (SD) | 47.1 (12.6) | 50.8 (10.0) | 46.3 (12.9) | 0.039 |
| Educational level, n (%) | ||||
| Basic literacy | 4 (1.8%) | 1 (4.2%) | 2 (1.1%) | 0.570 |
| Primary level | 36 (16.6%) | 5 (20.8%) | 29 (15.8%) | |
| Secondary level | 78 (35.9%) | 7 (29.2%) | 66 (35.9%) | |
| Higher education | 99 (45.6%) | 11 (45.8%) | 87 (47.3%) | |
| Years since diagnosis, mean (SD) | 18.6 (12.9) | 20.2 (14.1) | 18.3 (12.9) | 0.514 |
| PASI score, mean (SD) | 7.2 (5.1) | 8.4 (5.3) | 7.0 (5.0) | 0.197 |
| Mild (PASI < 7), n (%) | 111 (41.4%) | 11 (31.4%) | 95 (42.0%) | 0.279 |
| Moderate-to-severe (PASI ≥ 7), n (%) | 157 (58.6%) | 24 (68.8%) | 131 (58.0%) | |
| Psoriasis location, n (%) | ||||
| Face | 39 (14.6%) | 8 (23.5%) | 31 (13.9%) | 0.145 |
| Neck | 13 (4.9%) | 3 (8.8%) | 10 (4.5%) | 0.282 |
| Trunk | 141 (52.6%) | 17 (50.0%) | 122 (54.7%) | 0.608 |
| Back | 100 (37.3%) | 13 (38.2%) | 84 (37.7%) | 0.949 |
| Extremities and flexures | 214 (79.9%) | 29 (85.3%) | 175 (78.5%) | 0.360 |
| Difficult-to-treat areas | 136 (50.7%) | 23 (67.6%) | 107 (48.0%) | 0.033 |
| -Palmoplantar psoriasis | 25 (18.4%) | 6 (26.1%) | 18 (16.8%) | 0.299 |
| -Nail psoriasis | 62 (45.6%) | 11 (47.8%) | 49 (45.8%) | 0.859 |
| -Scalp psoriasis | 110 (80.9%) | 20 (87.0%) | 86 (80.4%) | 0.460 |
| Others | 15 (5.6%) | 1 (2.9%) | 13 (5.8%) | 0.489 |
| BSA value (0% to 100%), mean (SD) | 8.7 (8.0) | 10.3 (7.9) | 8.5 (8.0) | 0.107 |
| PGA category, n (%) | ||||
| 0. Clearance, no signs of psoriasis | 18 (8.5%) | 2 (6.3%) | 15 (8.6%) | 0.165 |
| 1. Almost clear, minimal | 40 (18.9%) | 3 (9.4%) | 35 (20.1%) | |
| 2. Mild | 59 (27.8%) | 8 (25.0%) | 49 (28.2%) | |
| 3. Moderate | 72 (34.0%) | 12 (37.5%) | 60 (34.5%) | |
| 4. Severe | 23 (10.8%) | 7 (21.9%) | 15 (8.6%) | |
| DLQI, mean (SD) | 8.2 (6.7) | 10.6 (5.9) | 7.8 (6.8) | 0.028 |
| Compliance with CASPAR criteria, n (%) | 21 (7.8%) | 21 (61.8%) | 0 (0.0%) | < 0.001 |
BSA, body surface area; CASPAR, Classification Criteria for Psoriatic Arthritis; DLQI, Dermatology Life Quality Index; PASI, Psoriasis Area Severity Index; PGA, Physician's Global Assessment; PsA, psoriatic arthritis; SD, standard deviation.
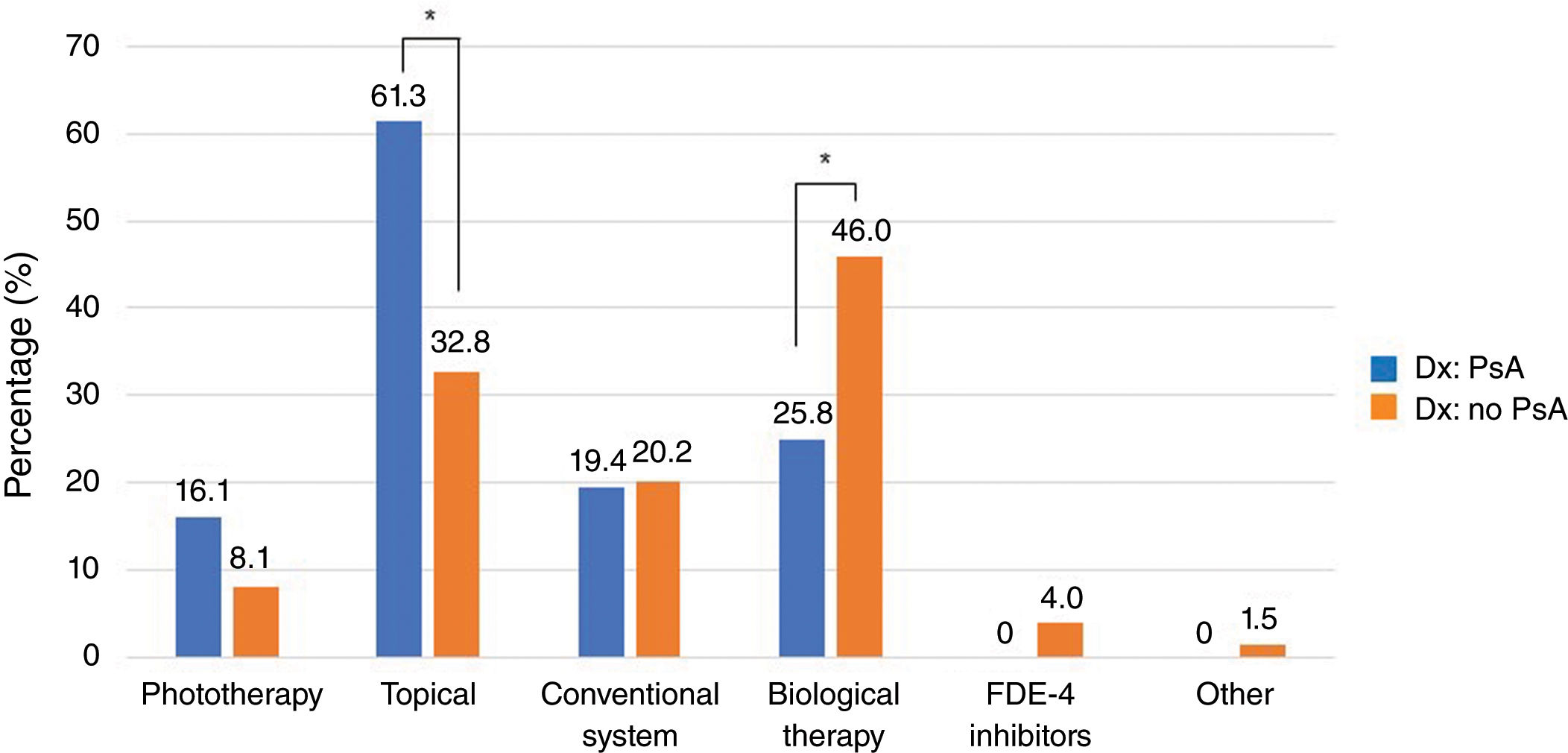
Figure 2 illustrates the distribution of current psoriasis treatment among patients with and without PsA. Biological therapy was received by 25.8% of patients with PsA vs 46.0% of patients without PsA (p=0.035), while topical treatments were used by 61.3% vs 32.8%, respectively (p=0.002).
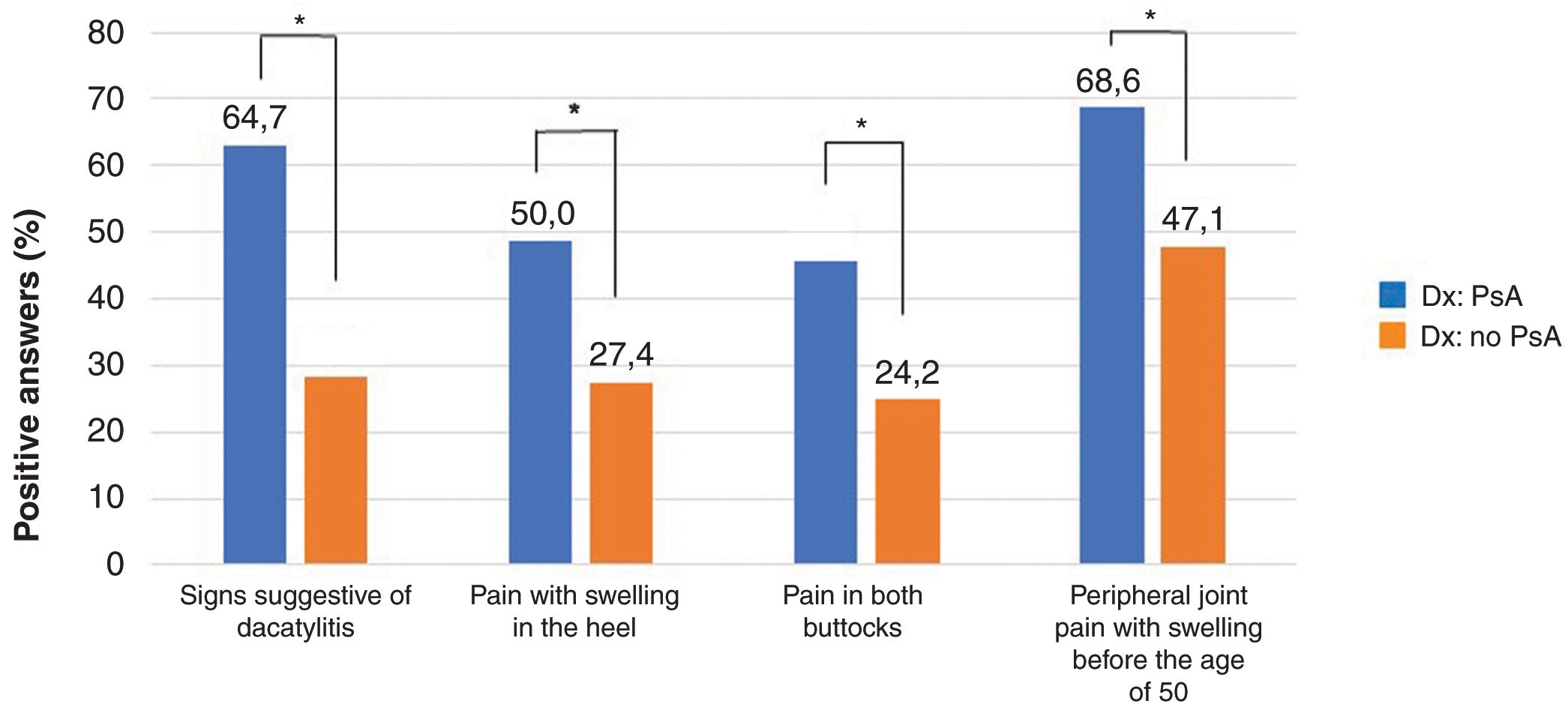
Questionnaire responses and screening resultsAll patients completed the PURE-4 questionnaire on paper. Table 2 shows the mean PURE-4 score among patients with PsA as 2.3±1.1, and 1.3±1.3 among patients without a diagnosis of PsA (p<0.001). Peripheral joint pain with inflammation before the age of 50 was the item most frequently answered affirmatively by 137 (51.1%) patients (Fig. 3).
PURE-4 Results in Evaluation #1.
| Variable | Overall population(N = 268) | Diagnosed with PsA(N=34) | Without PsA Diagnosis(N=223) | p |
|---|---|---|---|---|
| PURE-4 Score, mean (SD) | 1.4 (1.3) | 2.3 (1.1) | 1.3 (1.2) | < 0.001 |
| PURE-4 Score, n (%) | ||||
| 0 | 86 (32.1%) | 2 (5.9%) | 83 (37.2%) | < 0.001 |
| 1 | 62 (16.6%) | 5 (14.7%) | 54 (24.2%) | |
| 2 | 58 (21.6%) | 13 (38.2%) | 43 (19.3%) | |
| 3 | 43 (16.0%) | 9 (26.5%) | 30 (13.5%) | |
| 4 | 19 (7.1%) | 5 (14.7%) | 13 (5.8%) | |
PsA, psoriatic arthritis; PURE-4, Psoriatic Arthritis UnclutteRed Screening Evaluation; SD, standard deviation.
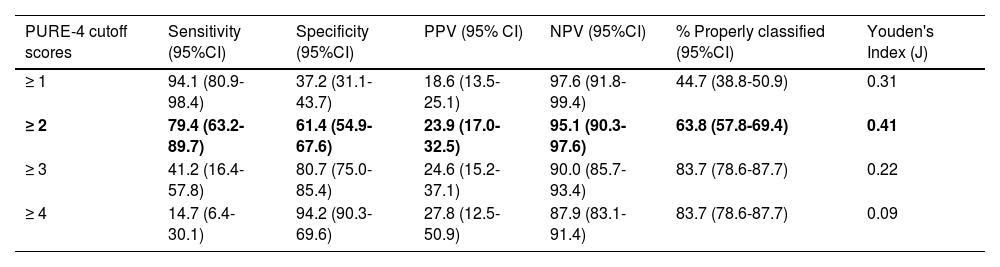
Using the Youden index, the optimal cutoff value was ≥2, with the highest percentage of properly classified patients, showing a sensitivity rate of 79.4% (95% confidence interval [CI], 63.2-89.7) and a specificity rate of 61.4% (95%CI, 54.9-67.6%) for detecting PsA (Table 3). A total of 63.8% of patients were classified the same by the PURE-4 questionnaire and the rheumatologist, with a negative predictive value of 95.1%. The ROC curve showed an AUC of 0.729 (95%CI, 0.649-0.809) (Fig. 4), indicating good performance of PURE-4. Patient distribution based on the PURE-4 results is shown in Appendix A, Figure S2 of the supplementary data.
Sensitivity and specificity of the PURE-4 questionnaire with different cutoff scores.
| PURE-4 cutoff scores | Sensitivity (95%CI) | Specificity (95%CI) | PPV (95% CI) | NPV (95%CI) | % Properly classified (95%CI) | Youden's Index (J) |
|---|---|---|---|---|---|---|
| ≥ 1 | 94.1 (80.9-98.4) | 37.2 (31.1-43.7) | 18.6 (13.5-25.1) | 97.6 (91.8-99.4) | 44.7 (38.8-50.9) | 0.31 |
| ≥ 2 | 79.4 (63.2-89.7) | 61.4 (54.9-67.6) | 23.9 (17.0-32.5) | 95.1 (90.3-97.6) | 63.8 (57.8-69.4) | 0.41 |
| ≥ 3 | 41.2 (16.4-57.8) | 80.7 (75.0-85.4) | 24.6 (15.2-37.1) | 90.0 (85.7-93.4) | 83.7 (78.6-87.7) | 0.22 |
| ≥ 4 | 14.7 (6.4-30.1) | 94.2 (90.3-69.6) | 27.8 (12.5-50.9) | 87.9 (83.1-91.4) | 83.7 (78.6-87.7) | 0.09 |
CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value; PURE-4, Psoriatic Arthritis UnclutteRed Screening Evaluation.
In bold: optimal cut-off point, with the highest percentage of patients correctly classified.
The internal consistency of the PURE-4 questionnaire obtained a Cronbach's α value of 0.610, indicative of good consistency. Supplementary data (Appendix A, Table S2 of the supplementary data) describes the relationship between the PURE-4 score and the clinical variables evaluated by rheumatologists (dactylitis, enthesitis, inflammatory back pain, and peripheral joint pain), all of which were statistically significant (p<0.05). The mean PURE-4 score (2.19±1.29) was significantly higher in patients who met the CASPAR criteria than in those who did not (1.29±1.26; p=0.002). The agreement percentage between both diagnostic criteria (PURE-4 and CASPAR) was 61.4%.
Paper and electronic versions of the PURE-4 questionnaireFor the comparison of both questionnaire versions, a total of 280 patients with available information were included. A total of 98.9% (n=277) of those who completed both versions of the questionnaire, and only 16 respondents (5.8%) needed help from the virtual assistant. Responses to both versions of the PURE-4 questionnaire showed good correlation (ICC=0.965) and both were considered simple, quick, comfortable, and useful tools, although the paper questionnaire received slightly higher ratings (Appendix A, Table S3 of the supplementary data). More patients preferred the paper (39.0%) than the electronic version (27.6%); one third of respondents rated both options equally (33.5%).
DiscussionThis study was motivated by the need to address the challenge of early recognition of PsA by dermatologists treating psoriatic patients. Based on results, the performance of the PURE-4 questionnaire was slightly better than that reported for other PsA screening tools such as the Psoriatic Arthritis Screening Evaluation (PASE), the Psoriasis Epidemiology Screening Tool (PEST), the Toronto Psoriatic Arthritis Screen (ToPAS), or the CONTEST questionnaire,14,15 although diagnostic accuracy shows great heterogeneity across screening tools.16
The ROC curve analysis showed good negative predictive values for detecting PsA, indicating that the PURE-4 questionnaire is suitable for use as an initial screening tool, albeit with some probability of false positive results. Therefore, patients with a score above the cutoff value (>2) should undergo formal rheumatological examination to verify the diagnosis of PsA, which is consistent with the nature of a screening test. There are several benefits associated with the PURE-4 questionnaire: it is a rapid and useful screening method in busy clinics; it streamlines referral to rheumatology, eventual confirmation of PsA,9 and increases the likelihood of patients receiving appropriate treatment to reduce deterioration in their quality of life.17
In our cohort, the prevalence of PsA was 12.7%, which is similar to the one seen in the validation study conducted by Audureau et al.,10 although they used CASPAR criteria as the reference. Although psoriasis severity and duration seem to increase the risk of developing PsA,18,19 in our study, psoriasis severity was similar between those with and without PsA, except for difficult-to-treat areas, which were more common in those with PsA. Additionally, quality of life, assessed by the Dermatology Life Quality Index, was more affected in patients with PsA. Recent data indicate that biological therapies could also have an impact on the incidence of PsA, decreasing or delaying joint involvement.20–22 Our results showed that patients on biological therapy had a lower frequency of PsA. Nonetheless, actively seeking signs of PsA, at least, once a year and, ideally, every 6 months is advisable,9 regardless of the patient's treatment or psoriasis severity.
Digital tools can encourage patient self-care and facilitate more effective communication between patients and doctors, improving long-term clinical management.23 In this context, the use of new technologies represents a valuable facilitator for implementing digital screening for PsA,24 allowing more active monitoring of patients with psoriasis. The electronic version of the PURE-4 questionnaire showed high correlation (ICC=0.963) with the conventional questionnaire. This tool could facilitate the remote detection of joint symptoms, in a simple and quick manner. Since timely treatment can reduce cutaneous symptoms, pain, and subclinical inflammation,25,26 an accurate detection method has the potential to change the clinical course of PsA. Earlier intervention can prevent arthritis progression, reduce flares, and thus improve disease control and quality of life.27
The limitations of this study are as follows: although the two modalities of the Spanish version of the PURE-4 questionnaire could be used to facilitate PsA detection, the results are applicable to the Spanish context and may not be generalizable to other countries. Additionally, to minimize selection bias, all participants completed the PURE-4 questionnaire regardless of the presence or absence of PsA signs, albeit without confirmed clinical diagnosis. While this could be perceived as a limitation of the patient flow in dermatology clinics, the present study was designed for PURE-4 validation for early PsA detection following linguistic adaptation.11 Therefore, it was established that, at least, 60%12 of the sample should have moderate-to-severe psoriasis, defined by a PASI≥7,13 as moderate-to-severe psoriasis presents a higher risk of developing PsA.5 Furthermore, 43% of patients were on biological therapy, which likely reduced PsA-related incidence.20–22,28 Another limitation, affecting only the comparison between paper and electronic versions of PURE-4, is the fact that patients completed both versions in the same visit and order, which may have introduced a recall bias in electronic questionnaire responses. However, this does not have an impact on the main objective of the study, as the paper version was always administered first, eliminating the learning effect.
In conclusion, the Spanish version of the PURE-4 questionnaire has been validated using rigorous methods in terms of sensitivity, specificity, feasibility, and construct validity for PsA screening. The optimal cutoff value was ≥2, with acceptable internal consistency. Both paper and electronic versions were highly rated by the patients, with similar proportions of patients preferring the paper, electronic, or the two versions.
Therefore, this questionnaire could guide early referral to rheumatology, reducing delay in PsA diagnosis and treatment. This tool should be considered as a supplement for dermatologists when exploring signs and symptoms of PsA in psoriatic patients.
FundingThis study was funded by Novartis Farmacéutica S.A., Spain, following good publication practices (GPP-2022).
Conflicts of interestIsabel Belinchón Romero has received consultant and/or speaker ‘s fees and participated in clinical trials sponsored by the following pharmaceutical companies whose therapeutic arsenal includes drugs used to treat psoriasis: Janssen Pharmaceuticals Inc, Almirall SA, Lilly, AbbVie, Novartis, Celgene, Biogen, Amgen, Leo-Pharma, Pfizer-Wyeth, BMS, UCB, and MSD.
Ana López-Ferrer has received fees for her participation as a member of scientific committees, consultant and/or speaker's fees, and research grants or participated in clinical trials sponsored by the following pharmaceutical companies (unrelated to this manuscript though): AbbVie, Almirall, Amgen, Janssen, LEO Pharma, MSD, Eli Lilly, Novartis, and UCB Pharma.
Marta Ferrán i Farrés has received consultant and/or speaker's fees and participated in clinical trials sponsored by the following pharmaceutical companies: Janssen Pharmaceuticals Inc, Eli Lilly, Novartis, Pfizer-Wyeth, MSD, AbbVie, Celgene, and Almirall SA.
Raquel Rivera Díaz has received consultant and/or speaker's fees and participated in clinical trials sponsored by the following pharmaceutical companies: AbbVie, Almirall SA, Celgene, GSK, Janssen Pharmaceuticals Inc, Eli Lilly, Leo-Pharma, MSD, Novartis, Pfizer-Wyeth, and UCB.
David Vidal Sarro has received consultant and/or speaker's fees and participated in clinical trials sponsored by the following pharmaceutical companies: AbbVie, Celgene, Eli Lilly, Janssen Pharmaceuticals Inc, Novartis, Gebro Pharma, Leo-Pharma, and UCB.
Lourdes Rodríguez Fernández-Freire has received consultant's fees and/or fees from the following pharmaceutical companies: AbbVie, Janssen Pharmaceuticals Inc, MSD, Pfizer-Wyeth, Novartis, Celgene, Almirall SA, Eli Lilly, and Leo-Pharma.
Pablo de la Cueva Dobao has received consultant and/or speaker's fees and participated in clinical trials sponsored by the following pharmaceutical companies: AbbVie, Almirall SA, Astellas Pharma, Biogen Inc., Boehringer Ingelheim, Celgene, Janssen Pharmaceuticals Inc, LEO Pharma, Eli Lilly, MSD, Novartis, Pfizer, and UCB.
Jorge Santos Juanes has received consultant and/or speaker's fees from the following pharmaceutical companies: Novartis, Eli Lilly, Janssen Pharmaceuticals Inc, AbbVie, Amgen, and Sanofi.
Vicenç Rocamora Duran has received consultant and/or speaker's fees from the following pharmaceutical companies: Janssen Pharmaceuticals Inc, Eli Lilly, AbbVie, Almirall, Amgen, and Novartis.
Víctor Martín Vázquez and Lara Gómez Labradror are employees of Novartis in Spain.
Ruben Queiro has received consultant and speaker's fess and works as project coordinator of the following pharmaceutical companies: Abbvie, Lilly, UCB, Janssen, Pfizer, Amgen, Novartis. Additionally, he has received grants from Janssen, Novartis, and Abbvie.
The authors wish to thank Anabel Herrero, PhD, on behalf of Springer Healthcare, for her editorial support and assistance during manuscript writing.