Desmoplastic melanoma (DM) accounts for 0.4% to 4% of all melanomas. These skin tumors are mainly formed by amelanotic spindled melanocytes immersed in an abundant collagen stroma and are classified as pure when the desmoplastic component accounts for at least 90% of the invasive tumor and as mixed or combined otherwise. DMs are more common in men (male to female ratio, 1.7 to 2:1), and the mean age at diagnosis is 66 to 69 years. The tumors tend to occur in chronically sun-exposed areas, often in association with lentigo maligna, and are difficult to recognize because they can resemble a scar, presenting as a firm, unpigmented papule or plaque with poorly defined borders. DMs also have a strong tendency to recur locally, and pure variants rarely spread to the lymph nodes. Nonetheless, recently published series suggest that patients with DM have a similar prognosis to those with nondesmoplastic melanoma of the same thickness. The clinical management of DM varies in certain aspects from that of other melanomas and is reviewed in this article.
El melanoma desmoplásico (MD) representa entre el 0,4-4% de todos los melanomas. Se presenta como un tumor constituido predominantemente por melanocitos fusiformes amelanóticos inmersos en un estroma colágeno abundante. Se clasifica en MD puro o mixto, basándose en la proporción de melanoma desmoplásico frente a la del melanoma no desmoplásico presente en el tumor infiltrante. En el MD puro el componente desmoplásico representa más del 90% del melanoma infiltrante mientras que, en el MD combinado o mixto, el componente desmoplásico representa menos del 90%.
El MD es más frecuente en varones (ratio 1,7-2 :1); la edad media al diagnóstico oscila entre 66-69 años, y suele localizarse en áreas de fotoexposición crónica, a menudo asociado a un lentigo maligno. Su reconocimiento clínico es difícil ya que se presenta como una pápula o placa no pigmentada, indurada y de bordes mal definidos, que recuerda a una cicatriz.
El MD es un tumor con una alta tendencia a la recurrencia local y en el caso del MD puro, una baja tendencia a la diseminación ganglionar. Sin embargo, en las series más contemporáneas, su pronóstico global parece ser similar al de melanomas no desmoplásicos (MND) del mismo grosor. Su abordaje clínico posee algunos matices diferenciales, en comparación al resto de melanomas, que se revisan en el presente trabajo.
Desmoplastic melanoma (DM) is a rare variant of melanoma that has distinct histologic features and biological behavior to conventional melanoma. It was described by Conley et al.1 in 1971 as a paucicellular tumor composed of spindle cells with little atypia and abundant collagenous stroma.1 In terms of prognosis, there is some controversy regarding the risk of lymph node spread and its impact on survival.2–4 This review offers updated, practical information on how to manage DM.
Material and MethodsWe conducted a literature search in PubMed, EMBASE, and Google Scholar databases using the search term “desmoplastic melanoma” and additional terms depending on the subsection studied. We also scanned the reference lists of selected articles to identify other potentially relevant articles.
EpidemiologyDM is rare and accounts for less than 4% of all melanomas.5,6 According to a recent retrospective study, just 0.4% of all melanomas diagnosed in the Netherlands between 2000 and 2014 were DMs.7 In another study using data from the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) Program, the authors estimated an annual incidence of 0.2 cases of DM per 100000 inhabitants, with an annual increase of 4.6%.5 This increase was attributed to the relationship between DM and sun exposure, population aging, and improvements in diagnosis.
DM is more common in men, with a male to female ratio of approximately 1.7-2 to 1.5,8,9 Mean age at diagnosis is 66 to 69 years, which is considerably older than that described for nondesmoplastic melanoma (NDM) (approximately 60 years).5,8–10
Similarly to lentigo maligna (LM) and lentigo maligna melanoma (LMM), DM tends to occur in areas of chronic sun exposure. The most common location is the head and neck (50% of cases), followed by the trunk (20%-25%) and extremities (20%-25%). DM can, however, arise in areas not chronically exposed to the sun, such as mucous membranes11 and acral sites.12
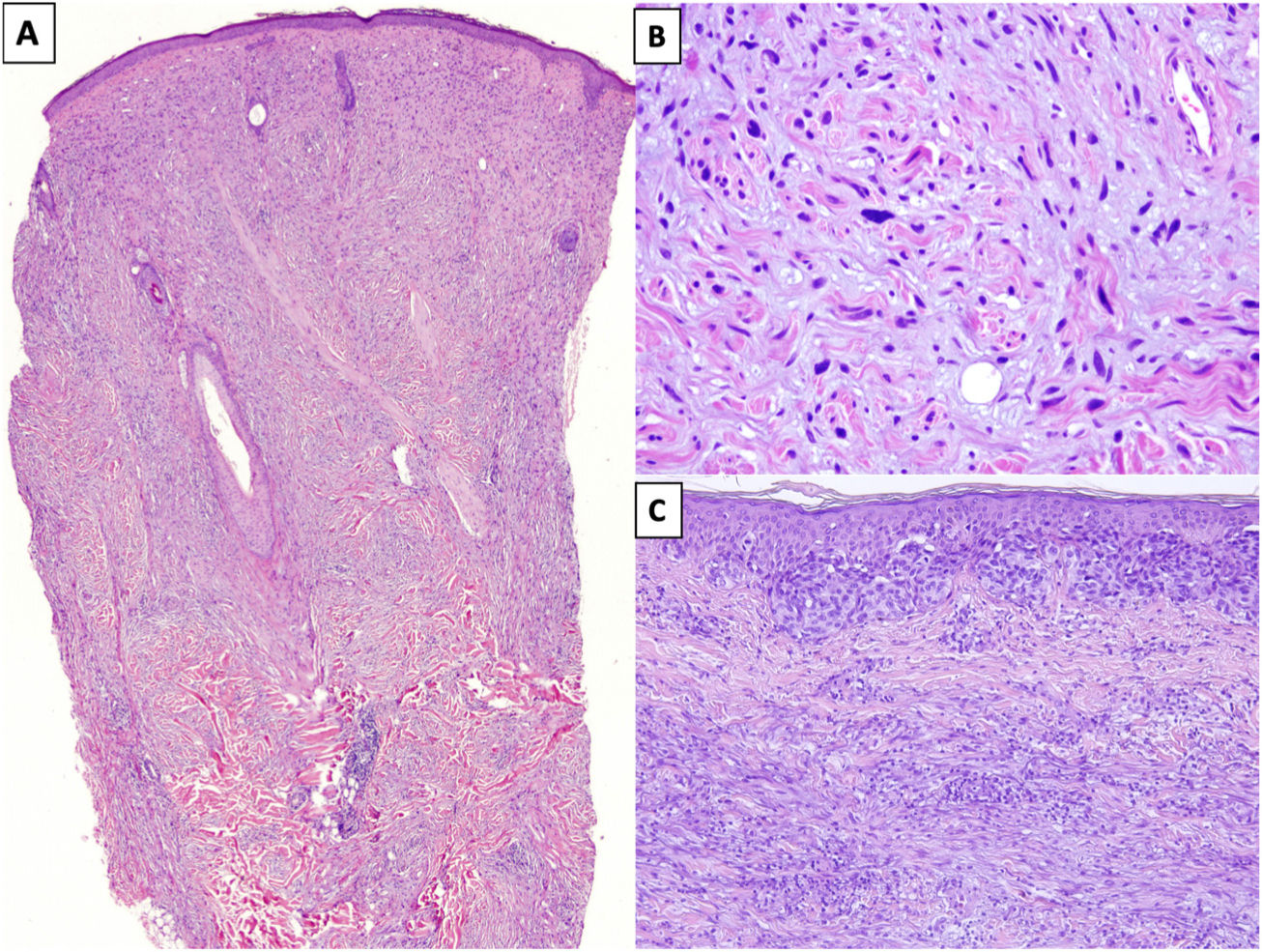
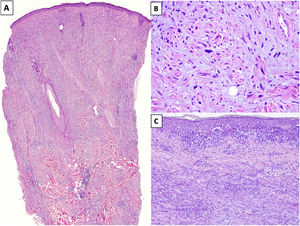
HistopathologyDM is an invasive melanoma primarily composed of amelanotic spindle-shaped melanocytes immersed in a highly collagenous stroma.1,8 Its characteristic morphologic appearance is that of a paucicellular dermal tumor with an irregular outline, a poorly defined contour, and a low to moderate density of melanocytes in a prominent collagenous stroma (Fig. 1A). Tumor cells are typically arranged in an isolated, disordered fashion among the collagen bundles.13 Melanocytes are usually spindle shaped and nonpigmented (similar to fibroblasts) and have poorly defined cytoplasms and cytoplasmic membranes. Cytologic atypia ranges from minimal to moderate; mitotic figures are uncommon (Fig. 1B).
A, Invasive desmoplastic melanoma (DM) extending into the deep reticular dermis (hematoxylin-eosin, original magnification ×20). B, DM with spindle-shaped melanocytes with several, large hyperchromatic nuclei arranged in an isolated, disordered fashion among a slightly fibromyxoid stroma (hematoxylin-eosin, original magnification ×200). C, DM associated with a melanoma in situ (hematoxylin-eosin, original magnification ×200).
The overlying epidermis shows few or no alterations in almost 50% of cases14 and the appearance is that of a fibrous or mesenchymal tumor. In the remaining cases, histology shows an atypical proliferation of melanocytes at the dermoepidermal junction or a melanoma in situ, usually LM15 (Fig. 1C). In these cases, the appearance may be that of a melanoma in situ or a junctional melanocytic lesion with a prominent underlying scar.16,17
The histologic diagnosis of DM presents challenges. Because of its inoffensive, deceptive appearance and the superficial nature of some biopsies, DM can go unnoticed or be mistaken for other lesions. The differential diagnosis should include benign tumors and lesions such as scars, dermatofibroma, neurofibroma, and desmoplastic nevus, in addition to malignant tumors, such as desmoplastic sarcomatoid carcinoma, atypical fibroxanthoma, dermatofibrosarcoma protuberans, fibrosarcoma, leiomyosarcoma, and malignant peripheral nerve tumor.
Immunohistochemistry can be very useful in the differential diagnosis, but on occasions it is of no help. In such cases, it is important to check for morphologic features often seen in DM. While these features are not specific, they can help establish a diagnosis.
- -
DM often spreads to deep layers, in many cases occupying the entire dermis and extending into the subcutaneous tissue.18
- -
Fibromyxoid stroma is common.19
- -
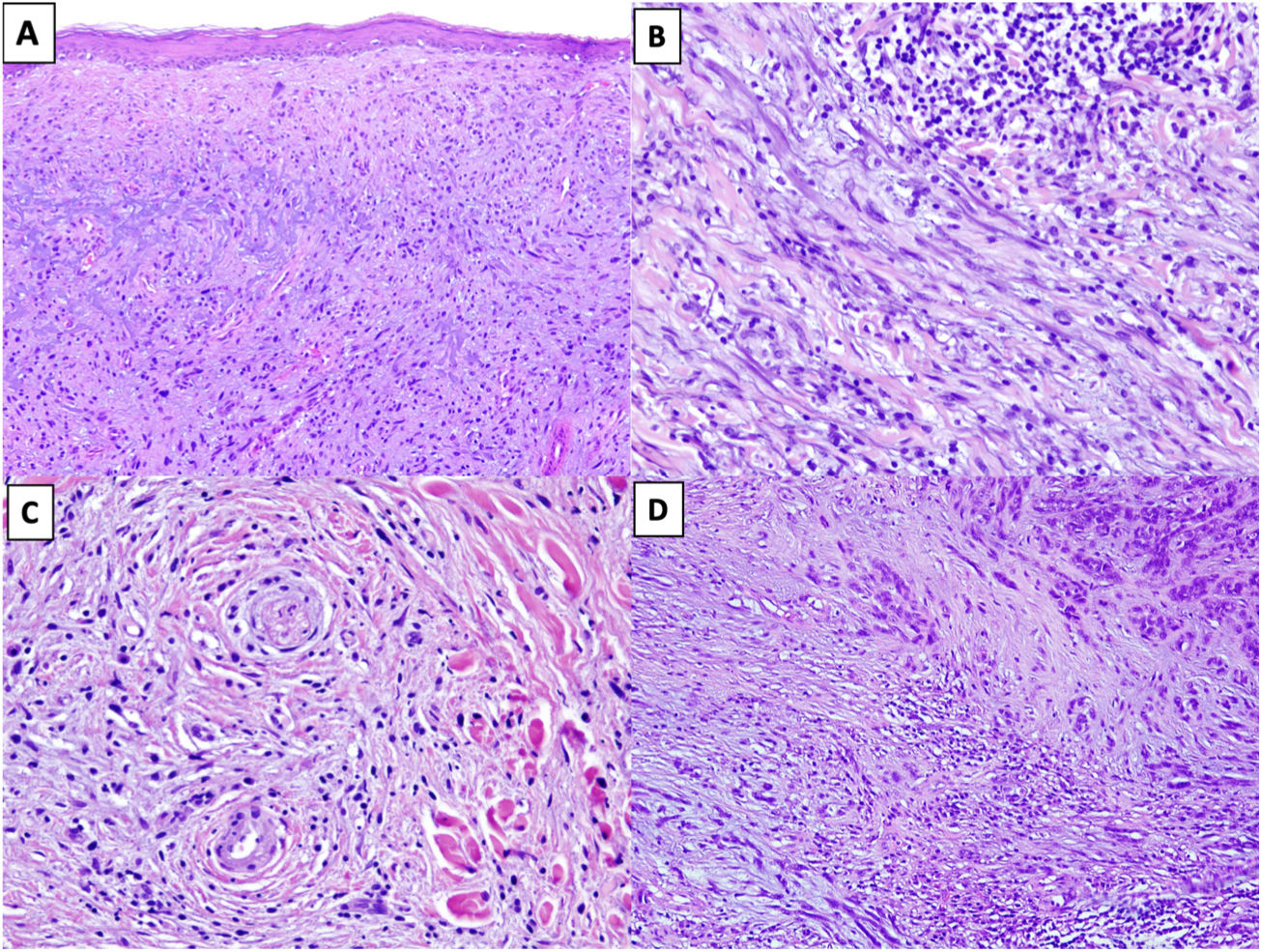
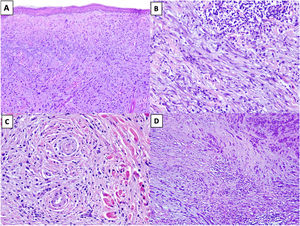
Solar elastosis is observed in the superficial dermis in approximately 80% of tumors.20 Observation of clumps of elastotic material trapped in the core of the tumor, even in its deeper regions, offers important diagnostic information as these clumps are not seen in other entities in the differential diagnosis (Fig. 2A).16
Figure 2.A, DM with actinic elastosis and trapped elastolytic material (hematoxylin-eosin, original magnification ×100). B) DM with a nodular aggregate of lymphocytes (hematoxylin-eosin, original magnification ×200). C, DM with perineural invasion (hematoxylin-eosin, original magnification ×200). D, Mixed DM. Note the nondesmoplastic component formed by compact nests of epithelioid melanocytes in the top right corner and the desmoplastic component occupying less than 90% of the invasive tumor in the lower part of the image (hematoxylin-eosin, original magnification ×100).
- -
The presence of small peripheral or perineural lymphoid aggregates can also help raise suspicion of DM in paucicellular tumors with minimal atypia (Fig. 2B).21
- -
Neurotropism (perineural or intraneural invasion) is observed in approximately 30% of DMs (Fig. 2C).22 DM can sometimes exhibit neural transformation, which is currently considered to be a form of neurotropism.23
- -
Melanocytes with large, hyperchromatic nuclei are always present, even if focally.
- -
DM is a predominantly amelanotic tumor; diffuse pigmentation is very unusual.
In 2004, Busam et al.13 proposed classifying DM as pure or mixed (combined) depending on the proportion of the invasive tumor occupied by the desmoplastic component. For a DM to be classified as pure, at least 90% of the invasive component had to be desmoplastic and be accompanied by a fibrous stroma. Mixed DM, by contrast, had a smaller desmoplastic component (<90%) and was accompanied by an NDM component comprising cohesive groups of epithelioid and/or spindle-shaped melanocytes without an intercellular fibrous stroma (Fig. 2D).
Histologic subtypes of DM also show other microscopic differences. Mixed DM tends to be more cellular and has greater cytologic atypia, a higher mitotic rate, and a higher proliferative index (KI67). Neurotropism and neural differentiation, by contrast, are more common in pure DM13,24.
Busam et al.13 also showed that the above histologic distinction might have prognostic and treatment implications, highlighting the importance of correct classification. In some cases, particularly in more cellular tumors, the desmoplastic component can be difficult to detect using hematoxylin-eosin staining, leading to staging errors.
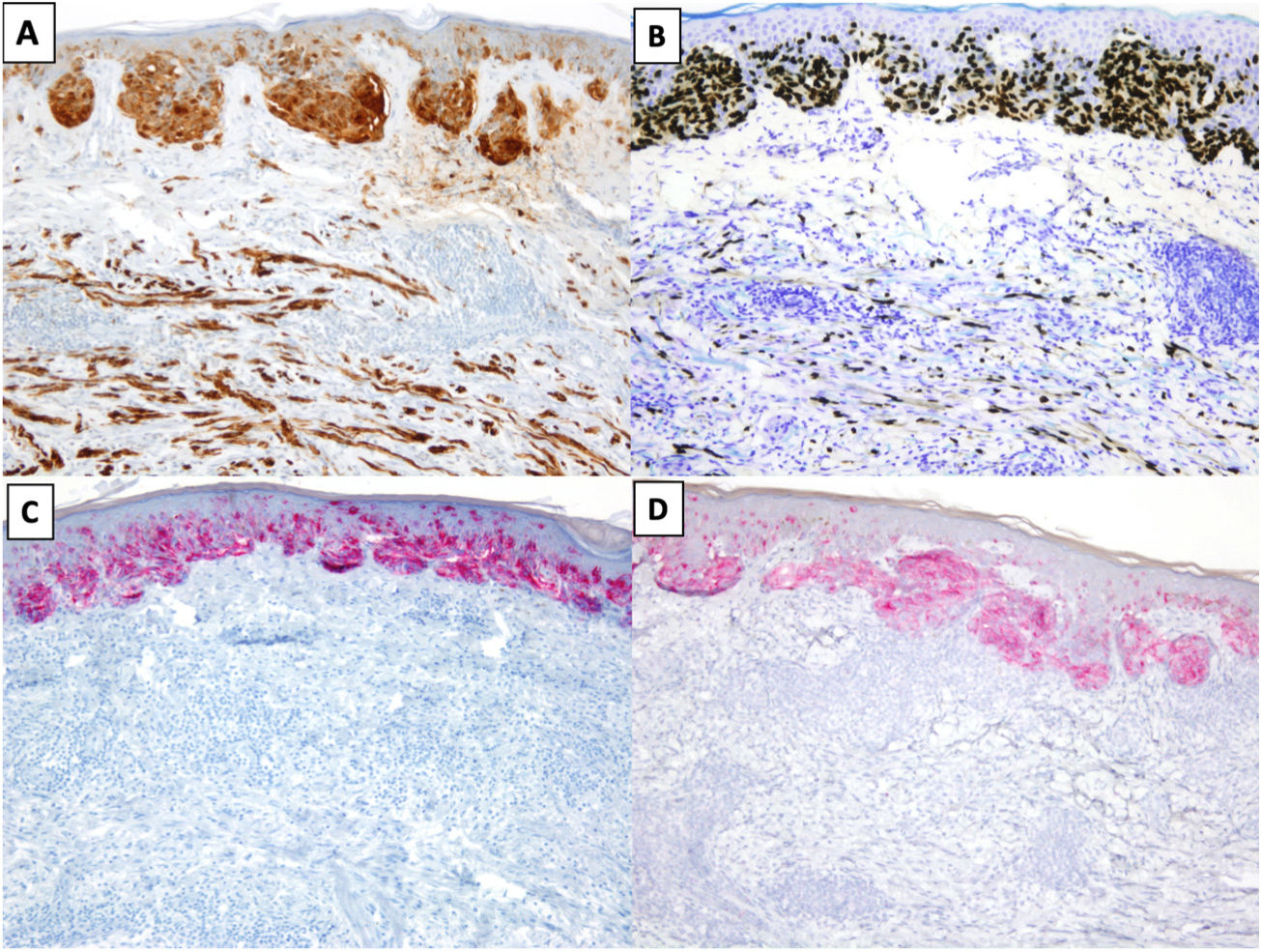
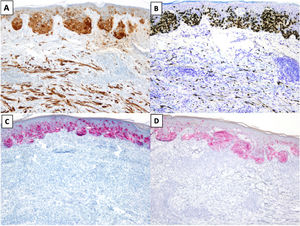
The most common immunohistochemical profile for DM is positive staining with S100, SOX10, and nerve growth factor receptor (NGFR) (75%) and negative staining with the melanocytic differentiation markers human melanoma black 45 (HMB45), tyrosinase, microphthalmia-associated transcription factor (MITF), and melan antigen recognized by T cells 1 (Melan-A or MART-1) (Fig. 3).25 It is important to correlate positive results with morphologic findings, especially in re-excision specimens, as S10026 and SOX-1027 may also be expressed in stromal and inflammatory cells, while NGFR is seen in myoepithelial cells, fibroblasts, reactive myofibroblasts, and nerve fibers.28 Before ruling out DM, it is important to bear in mind that some tumors express minimal or no SOX10.29 Unlike melanocytes in DM, those in melanoma in situ or in the NDM component of mixed DM show positive staining with HMB45, tyrosinase, MITF, and Melan-A and do not express NGFR. PReferentially expressed Antigen in MElanoma (PRAME) is more common in other variants of melanoma than in DM, where it is expressed in just 35% of cases.30
Desmoplastic melanoma (DM) associated with superficial spreading melanoma. A and B, Immunohistochemical staining with S100 (×100) and SOX10 (original magnification ×100). Both stains were positive for DM and melanoma in situ cells. C and D, Immunohistochemical staining with melanoma antigen (original magnification ×100) and human melanoma black 45 (original magnification ×100): Both stains were positive for melanoma in situ cells and negative for DM cells.
Most DMs in severely sun-damaged skin have very high mutational burden with a strong UV radiation signature. Activating mutations in the MAPK signaling pathway (e.g., the BRAF V600E mutation), which are relatively common in other types of melanomas, are usually absent in DM. Mutations in NF1 (55%)31, TP53 (48%), and CDKN2A (47%), however, are common. Other less common activating mutations in the MAPK pathway may also be seen, such as amplifications of the receptor tyrosine kinase gene (EGFR, MET, and ERBB2) and loss of CBL.32 Some of these alterations could be potential therapeutic targets.
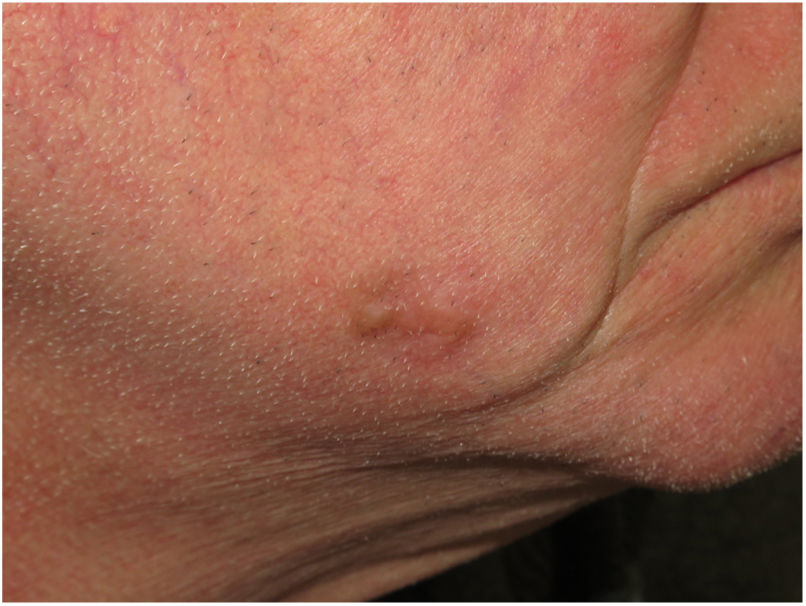
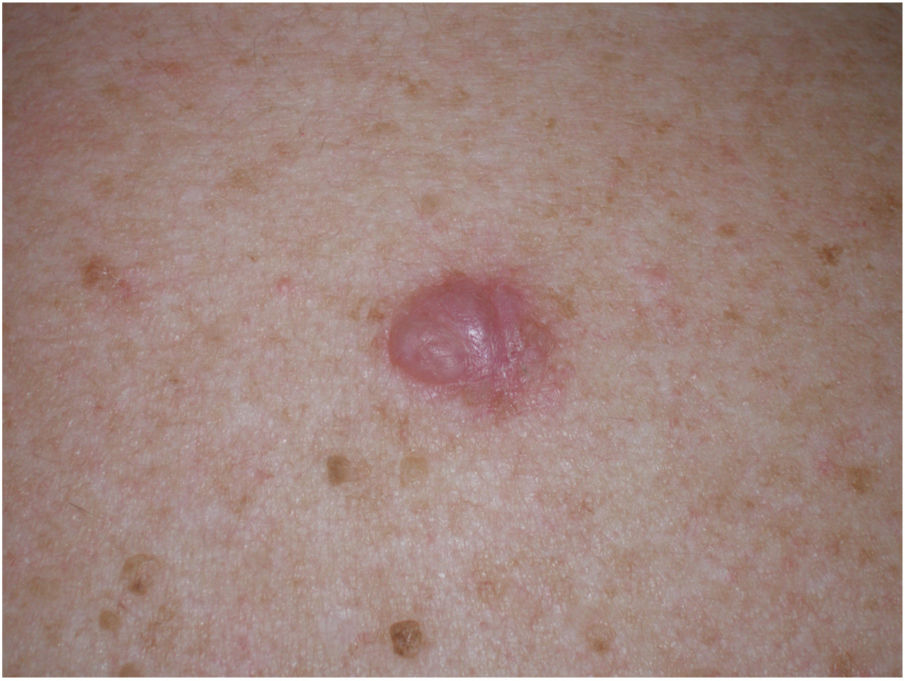
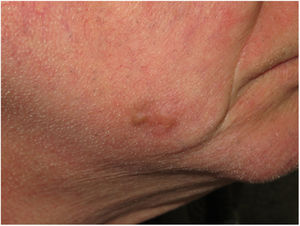
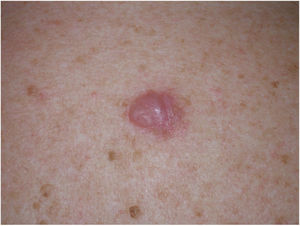
Clinical PresentationDM usually presents as a firm, nonpigmented papule or plaque with poorly defined borders in sun-damaged skin (Fig. 4). Malignant melanoma is suspected initially in just 27% of cases.20 Clinically, DM is often confused with a benign skin lesion, such as scar tissue, dermatofibroma, neurofibroma, and intradermal melanocytic nevus, or with a malignant nonmelanocytic skin tumor such as basal cell carcinoma and squamous carcinoma (Fig. 5).
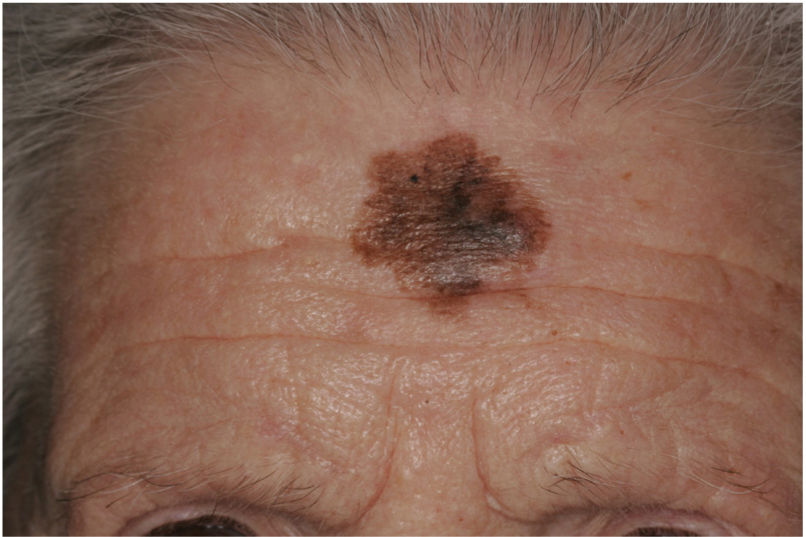
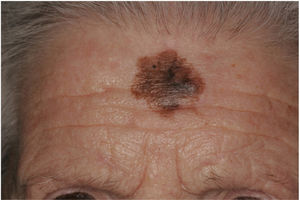
There are also clinical differences between pure and mixed DMs.13 An epidermal component in the form of LM, LMM, or superficial spreading melanoma appears to be present in 80% to 100% of mixed DMs. Lesions suspicious for LMM should therefore be palpated to check for a firm subcutaneous nodule indicative of DM (Fig. 6).15
Associated epidermal lesions are less common in pure DM (63%-80% of cases), which usually presents as a nodule or indurated subcutaneous plaque without superficial changes, explaining why its diagnosis is often delayed and why it is thicker at diagnosis than the mixed variant.20,33 Both pure and mixed DMs are thicker at diagnosis than conventional melanomas, with a mean Breslow thickness of 2.5 to 6.5 mm and in most cases a Clark level of IV or V.5,10,20,34
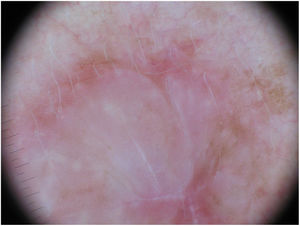
Dermoscopy can be a useful tool for the diagnosis of DM. Absence of a pigment network and observation of regression structures with off-white scar areas, granules (peppering), and atypical vascular patterns should raise suspicion.35 Jaimes et al.,33 in a study of 37 DMs, reported that the most common dermoscopic characteristics were vascular blush, polymorphous vessels, peppering, and asymmetric perifollicular hyperpigmentation. Just 43% of the tumors had features specific to melanocytic lesions, such as globules (44%), a pigment network (38%), a pseudonetwork (25%), and a negative network (6%) (Fig. 7). In other series, however, all the tumors analyzed showed at least 1 melanoma-specific characteristic.15,33,36 As expected, mixed DMs, which more often have an epidermal component, show a greater number and variety of melanoma-specific characteristics and dermoscopic findings associated with LM, such as the annular-granular pattern and polygonal lines.33
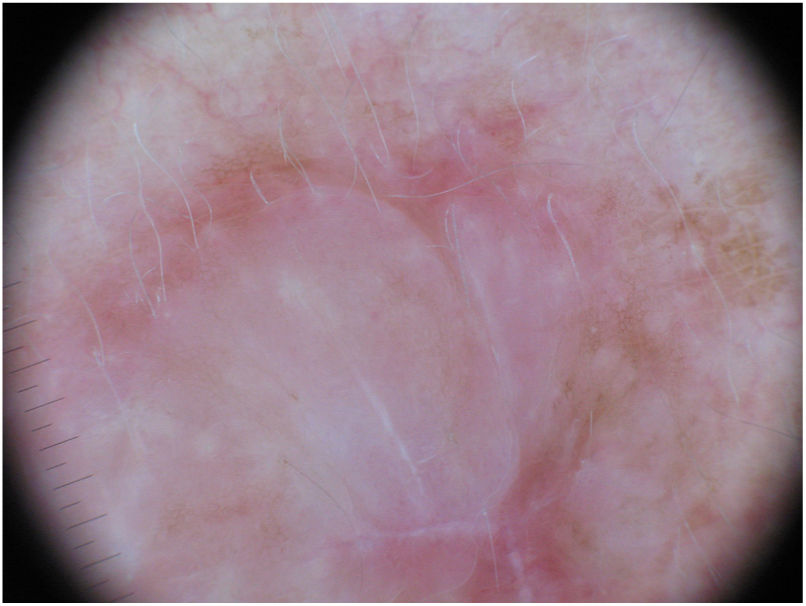
Dermoscopic image of the tumor with a palpable pink component shown in Fig. 4. Observation of a pigment network in several areas of the lesion indicated a melanocytic lesion.
There is still little evidence to support the usefulness of confocal reflectance microscopy (CRM) in the diagnosis of DM. In a study of 14 cases analyzed by CRM followed by histopathologic analysis, Maher et al.36 found that CRM detected a similar frequency of melanoma-specific features (pagetoid cells, cells with atypia and nucleated cells in the dermis) to that observed in other subtypes of melanoma. By contrast, abundant spindle cells interspersed with collagen fibers in the superficial dermis appeared to be more specific to DM.
PrognosisCurrent evidence suggests that DM behaves differently to conventional melanoma.3,37 It appears to be associated with a higher risk of local recurrence and a lower rate of lymph node metastasis.3,34,38 The risk of lymph node involvement seems to be lower than in NMDs of a similar thickness3,37; variable rates have been reported for sentinel lymph node involvement (0% to 18.2% depending on the series).3 It is difficult to accurately predict the prognosis of DM, as conflicting data have been reported and many studies do not distinguish between pure and mixed variants. Most recent studies, however, have not found significant differences in survival between patients with DMs and NDMs of a similar thickness.3,6,20,37,39,40 The impression that pure DM is less likely than mixed DM to spread to distant sites and therefore has better survival rates has not been consistently demonstrated. Maurichi et al.39 observed significant differences in overall survival between patients with mixed and pure DM (61.3% vs. 79.5%), but their findings have not been corroborated by subsequent studies.7,41 Distant metastases, which are mostly located in the lung, have been linked to previous recurrences and deep lesions.34
Most studies have shown that DM has a high risk of local recurrence (approximately 10%-14%)37,39,42,43, particularly in the case of mixed DMs.39 Some authors have attributed the more local aggressive nature of pure DM to its later diagnosis (it is a rare tumor with an atypical presentation) and to the high frequency of perineural invasion and inadequate surgical margins.20,37
A number of factors might explain the more aggressive behavior of DM. Shi et al.,38 in a retrospective study of 3657 DM cases, found that male sex and an age of older than 68 years were independent predictors of worse overall and disease-free survival. Although these findings have some support in the literature,31,36,40 other authors have not detected any differences in disease-free survival.37,44,45 Perineural invasion has also been proposed as a poor prognostic factor in DM6,46 and has been seen to significantly correlate with greater Breslow thickness.20
Treatment Strategies in DMSurgeryNumerous studies have shown that wide excision (with margins of ≥ 2 cm) does not improve survival in primary cutaneous melanoma with a Breslow thickness ≤ 2 mm.39,47 DMs, however, tend to be thicker than conventional melanoma at diagnosis and more often need excision with 2-cm margins. Wide excision is especially important considering the higher local recurrence rates described for DM.6,34,42
In one Australian series, an excision margin of ≥ 2 cm was associated with fewer recurrences than one of < 1 cm.6 Maurichi et al.39 also evaluated prognosis according to DM subtype and surgical margins and found that patients with pure DM and a Breslow thickness ≤ 2 mm had higher recurrence rates and worse overall 5-year survival when 1-cm rather than 2-cm margins were used. Prognosis, however, was similar when 2-cm margins were used to treat patients with pure DM and a Breslow thickness of ≤ 2 mm or > 2 mm. Margin size did not significantly influence prognosis in patients with mixed DM.
A surgical margin of 2 cm thus should be considered for the excision of pure DMs, even in tumors thinner than 2 mm. This recommendation is less clear for tumors thinner than 1 mm, as no studies have analyzed outcomes with 1-cm margins.3 In conclusion, wide excision should be prioritized whenever possible in pure DMs to avoid local recurrence, although this may not always be possible as many lesions are located in anatomically complex areas, such as the head and neck.15
Sentinel Lymph Node BiopsyThe value of sentinel lymph node (SLN) biopsy in DM is controversial. In melanoma, this procedure is currently used to identify patients with a worse prognosis who could benefit from adjuvant therapy. Evidence of its usefulness in DM is based on data from retrospective case series, several of which have shown clear differences in melanoma-specific survival according to SLN status.5,37,40,44
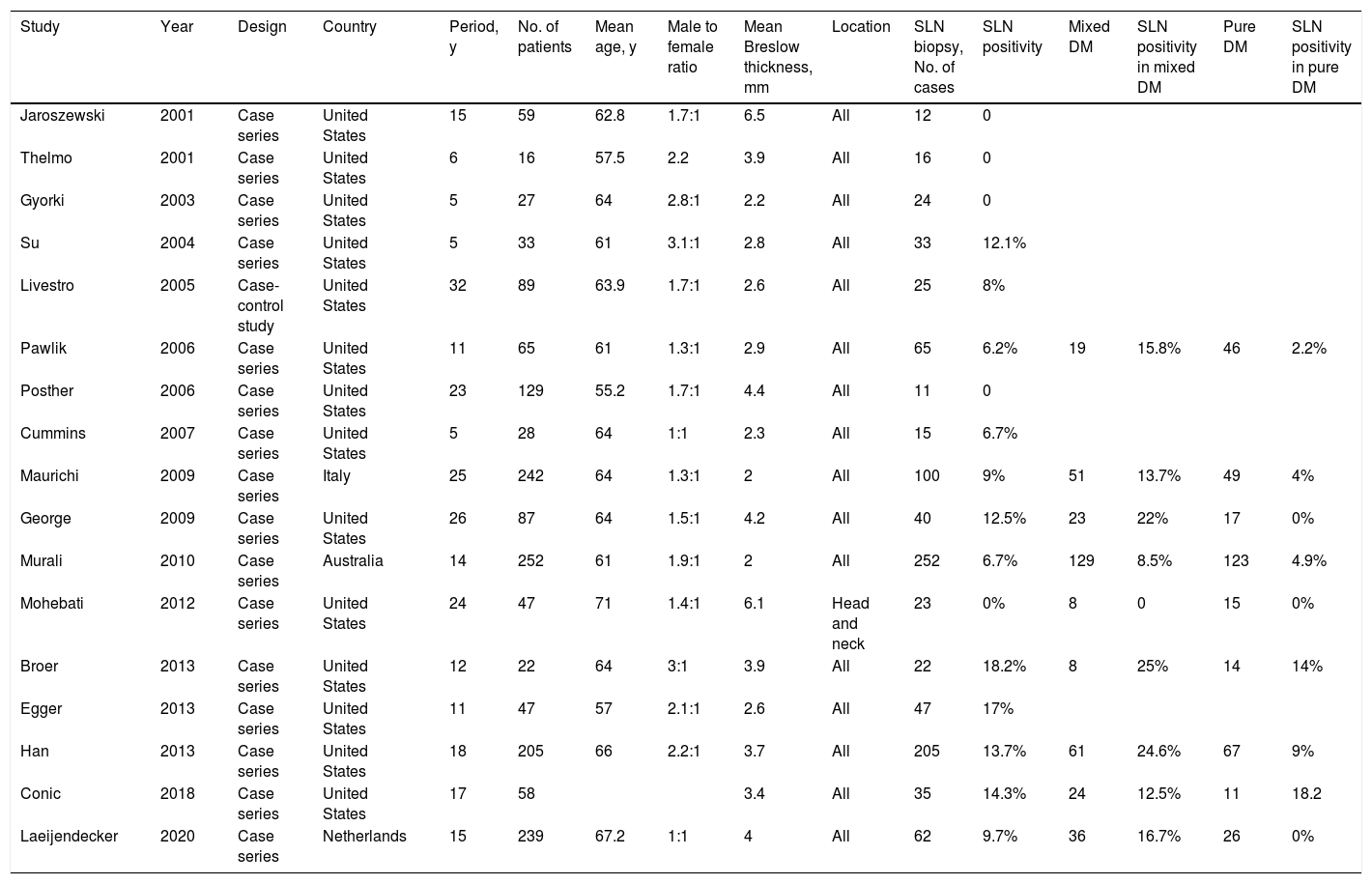
A 5% probability of SLN positivity is the usual threshold for considering SLNB.48 According to the most recent series of DM, SLN positivity rates range between 0% and 18.2%7,37,39–43,49–58 (Table 1), and the 5 series that reported a rate of 0% all had fewer than 25 patients.42,43,49,50,55 Dunne et al.59 reported a rate of 6.5% in a systematic review published in 2017.
Case Series Evaluating SLN Biopsy in DM.
| Study | Year | Design | Country | Period, y | No. of patients | Mean age, y | Male to female ratio | Mean Breslow thickness, mm | Location | SLN biopsy, No. of cases | SLN positivity | Mixed DM | SLN positivity in mixed DM | Pure DM | SLN positivity in pure DM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jaroszewski | 2001 | Case series | United States | 15 | 59 | 62.8 | 1.7:1 | 6.5 | All | 12 | 0 | ||||
| Thelmo | 2001 | Case series | United States | 6 | 16 | 57.5 | 2.2 | 3.9 | All | 16 | 0 | ||||
| Gyorki | 2003 | Case series | United States | 5 | 27 | 64 | 2.8:1 | 2.2 | All | 24 | 0 | ||||
| Su | 2004 | Case series | United States | 5 | 33 | 61 | 3.1:1 | 2.8 | All | 33 | 12.1% | ||||
| Livestro | 2005 | Case-control study | United States | 32 | 89 | 63.9 | 1.7:1 | 2.6 | All | 25 | 8% | ||||
| Pawlik | 2006 | Case series | United States | 11 | 65 | 61 | 1.3:1 | 2.9 | All | 65 | 6.2% | 19 | 15.8% | 46 | 2.2% |
| Posther | 2006 | Case series | United States | 23 | 129 | 55.2 | 1.7:1 | 4.4 | All | 11 | 0 | ||||
| Cummins | 2007 | Case series | United States | 5 | 28 | 64 | 1:1 | 2.3 | All | 15 | 6.7% | ||||
| Maurichi | 2009 | Case series | Italy | 25 | 242 | 64 | 1.3:1 | 2 | All | 100 | 9% | 51 | 13.7% | 49 | 4% |
| George | 2009 | Case series | United States | 26 | 87 | 64 | 1.5:1 | 4.2 | All | 40 | 12.5% | 23 | 22% | 17 | 0% |
| Murali | 2010 | Case series | Australia | 14 | 252 | 61 | 1.9:1 | 2 | All | 252 | 6.7% | 129 | 8.5% | 123 | 4.9% |
| Mohebati | 2012 | Case series | United States | 24 | 47 | 71 | 1.4:1 | 6.1 | Head and neck | 23 | 0% | 8 | 0 | 15 | 0% |
| Broer | 2013 | Case series | United States | 12 | 22 | 64 | 3:1 | 3.9 | All | 22 | 18.2% | 8 | 25% | 14 | 14% |
| Egger | 2013 | Case series | United States | 11 | 47 | 57 | 2.1:1 | 2.6 | All | 47 | 17% | ||||
| Han | 2013 | Case series | United States | 18 | 205 | 66 | 2.2:1 | 3.7 | All | 205 | 13.7% | 61 | 24.6% | 67 | 9% |
| Conic | 2018 | Case series | United States | 17 | 58 | 3.4 | All | 35 | 14.3% | 24 | 12.5% | 11 | 18.2 | ||
| Laeijendecker | 2020 | Case series | Netherlands | 15 | 239 | 67.2 | 1:1 | 4 | All | 62 | 9.7% | 36 | 16.7% | 26 | 0% |
Abbreviations: DM, desmoplastic melanoma; SLN, sentinel lymph node.
It could be helpful to distinguish between DM subtypes when evaluating the risk of SLN involvement and the value of SLN biopsy. SLN involvement is more likely in mixed DMs (8.5%-25%) than pure DMs (0-18.2%).7,39,41,52,54–56,58,60 The respective rates reported by Dunne et al.59 in their systematic review were 13.8% and 5.4%.59 Just 1 study has reported a higher risk of SLN involvement in pure DMs.41
To sum up, the value of SLN biopsy is clearer in mixed DM than in pure DM. It is also important, however, to consider other factors such as age, comorbidities, and primary tumor location. Old age and a head and neck location have traditionally been considered to be associated with a lower risk of SLN involvement.61
Few studies have reported positivity rates for non-SLN lymph nodes in patients with DM who have undergone complete lymph node dissection after a positive SLN biopsy. Two studies with over 200 DM patients each reported non-SLN lymph node positivity rates of 16.7% and 23.5%,37,58 which are similar to those reported for NDM.62 Although the evidence is limited, it would seem sensible to apply the same algorithm as that used in conventional melanoma to manage DM patients with a positive SLN biopsy.63
RadiotherapyRadiotherapy may be potentially useful in DM considering the high rates of local recurrence described.37,64 Unlike in conventional melanoma, which is relatively resistant to radiotherapy, several studies have shown that this treatment may be a useful adjunct for achieving local control in DM.3,34,65
A number of studies have also shown the possible benefits of adjuvant radiotherapy in patients with DM and associated risk factors (perineural invasion, extensive desmoplasia, positive margins, and recurrent disease).38,66,67 Two more recent studies confirmed that adjuvant radiotherapy improved local control in DM. Guadagnolo et al.,68 in a study of 130 patients with DM, found that 24% of patients treated exclusively with surgery and 7% of those treated with surgery plus adjuvant radiotherapy developed local recurrence. They also detected a significant association between adjuvant radiotherapy and superior local control in the multivariate analysis. Strom et al.69 also found that adjuvant radiotherapy was a significant predictor of better local control in their multivariate analysis. In particular, 14% of patients with positive resection margins treated with adjuvant radiotherapy developed recurrent disease compared with 54% of those who underwent excision only. The authors also described several prognostic factors that could be used to select DM patients with negative margins who might benefit from adjuvant radiotherapy: head and neck location, Breslow thickness > 4 mm, and Clark level V. Oliver et al.,70 in a more recent retrospective study, evaluated 100 patients with DM treated with surgery, surgery plus adjuvant radiotherapy, or surgery plus salvage radiotherapy for postoperative recurrences. They found 100% local control rates in the 7 patients treated with salvage radiotherapy and the 10 treated with adjuvant radiotherapy.
The current evidence, however, is based on retrospective studies. Prospective randomized trials are needed. One trial currently underway (NCT00975520)71 is comparing surgery alone versus surgery plus adjuvant radiotherapy in patients with DM excised with wide negative margins.
Systemic TherapyImmunotherapy with anti-PD1 drugs has demonstrated efficacy in the treatment of metastatic DM. A recent retrospective multicenter study of 60 cases of metastatic DM treated with anti-PD1 drugs reported objective tumor responses in 70% of patients over a mean follow-up of 22 months, and 32% of the patients achieved complete response.72 These rates, which are even higher than those observed in NDM, are probably due to the high mutational burden induced by UV radiation in DM. It has been proposed that immunotherapy might be more effective in tumors with a high mutational burden.73 These promising results need to be confirmed in prospective clinical trials. A phase II trial (NCT02775851)74 is currently recruiting patients to evaluate the efficacy of pembrolizumab in DM. BRAF inhibitors are not useful in DM as most patients do not have BRAF mutations.
ConclusionsDM is a rare variant of melanoma. It behaves differently to conventional melanoma and therefore requires different diagnostic and treatment strategies. Its diagnosis presents challenges for both clinicians and pathologists. Histologic classification of DM into pure and mixed variants appears to offer important information on tumor behavior and should be taken into account when taking treatment decisions. Pure DMs have a desmoplastic component that occupies at least 90% of the invasive tumor. Mixed DMs have a smaller desmoplastic component accompanied by a nondesmoplastic component.
Wide excision is essential for preventing recurrence and improving survival. SLN status appears to have prognostic value in DM, and SLN biopsy should be considered in mixed variants. Its usefulness in pure DM is less clear. Adjuvant radiotherapy to the tumor bed may be useful in patients with associated risk factors. Current evidence suggests that immune checkpoint inhibitors are associated with good response rates in metastatic DM.
FundingNo funding was received for this study.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We thank Professor Carlos Ferrándiz for critically reviewing this version of the manuscript.
Please cite this article as: Boada A, Quer Pi-Sunyer A, Richarz N, Jaka-Moreno A. Actualización en el diagnóstico y manejo del melanoma desmoplásico. Actas Dermosifiliogr. 2022;113:47–57.