Chronic prurigo is itself a common condition, but it can also occur secondary to a large number of diseases. Management is challenging as historically chronic prurigo has been poorly defined and very few treatments are available. Clinically, it presents as excoriated, hyperkeratotic lesions. When chronic prurigo is suspected, a comprehensive differential diagnosis is essential. New diagnostic criteria have appeared in recent years and new drugs have been developed. Although no truly effective treatment is yet available, patients will benefit from a greater understanding of this condition.
El prurigo crónico es una entidad con una apariencia clínica común pero que puede ser secundaria a un gran número de patologías. Históricamente ha sido una enfermedad no bien definida y con escasas terapias disponibles, por lo que su manejo es muy complejo. Clínicamente se caracteriza por lesiones escoriadas e hiperqueratósicas en el contexto de un paciente con prurito crónico. Ante la sospecha de un prurigo crónico, es fundamental realizar un buen diagnóstico diferencial e identificar todas sus posibles causas. En los últimos años se han producido importantes avances con la aparición de nuevos criterios diagnósticos y con el desarrollo de nuevos fármacos. Un mayor conocimiento de esta patología redundará en el beneficio de unos pacientes que hasta el momento carecen de un tratamiento claramente efectivo.
Chronic prurigo (CPG) is a disease characterized by intense itching and lesions secondary to scratching. Although CPG is a specific clinical entity, it may also be secondary to numerous underlying conditions. CPG has a significant impact on quality of life. Both the intensity and frequency of itching and pain are greater in CPG than in either psoriasis or atopic dermatitis.1 CPG patients’ higher scores on the Dermatology Life Quality Index (DLQI) also reveal greater disease-related impairment.2 Anxiety is experienced by 37% of individuals with this diagnosis, depression by 29%, and suicidal ideation by 19%.3 The condition also has considerable economic impact, requiring more medical visits than other skin diseases.4
Opinions still differ as to what constitutes a case of prurigo5 and what the optimal treatment is, given that few studies provide an evidence base for treatment. The recent recognition of CPG as an independent condition is a first step toward more appropriate diagnosis and treatment of patients with this complaint. Recent years have seen important advances relevant to CPG. Discoveries related to immunology and the development of new drugs require our full attention and understanding if our patients are to benefit from them. From this situation stems the interest of this review of recent publications on CPG.
DefinitionsConfusion as to how to define prurigo begins with terminology. Two spellings, and hence pronunciations, of the Spanish term coexist: prúrigo and prurigo. Only the latter, however, is accepted by the Royal Academy's Dictionary of the Spanish Language (DLE in its Spanish abbreviation).6 Meanwhile, the Spanish adjective form pruriginoso (pruriginous) is often used to qualify conditions that are pruritic — ie, that cause itching, or pruritus — even though the DLE reserves the qualifier pruiginoso to refer to anything related to the nature of the disease prurigo or that causes it.6 According to Pereira & Ständer,7 the English language literature offers 2 adjectives — itchy and pruritic — to refer to the main symptom (as the Spanish adjective prurítico does) as well as the adjective pruriginous to indicate a relation to prurigo. This distinction may seem unimportant, but it becomes relevant when we try to define the lesions of prurigo. In this review, the lesions typically associated with CPG will be termed pruriginous, while pruritic will refer to itching caused by any condition, including the lesions of prurigo.
Hippocrates recorded the first descriptions of CPG in the third century CE, and the term prurigo seems to have first appeared in the United Kingdom at the end of the 18th century.8 Since then it has been used to refer to many unrelated conditions that cause itching,7 and because of the variety of clinical presentations involved, multiple diagnostic terms have emerged. One example is prurigo nodularis of Hyde. Another is Besnier prurigo, the presentation associated with atopic dermatitis.9 Geographic variations have also emerged. For example, Japanese guidelines refer to 2 clinical subtypes — prurigo nodularis and prurigo chronica multiformis10 — a distinction not made in the Western literature.
With the goal of introducing clarity, experts belonging to the European Prurigo Project published a consensus paper in 2018 to propose definitions, classifications, and terminology.11 The paper states that CPG is “a distinct disease defined by the presence of chronic pruritus and multiple localized or generalized pruriginous lesions.” This entity, the group notes, “occurs due to a neuronal sensitization to itch and the development of an itch-scratch cycle [and CPG] can be of dermatological, systemic, neurologic, and psychiatric/psychosomatic, multifactorial or undetermined origin” (p. 1064, Table 1 of the reference).11 Cases of CPG therefore have a common clinical appearance but their origin is potentially heterogeneous. An underlying dermatologic condition is present in up to half of the cases. The consensus group further described pruriginous lesions as “excoriated, scaling and/or crusted nodules and/or plaques, often with a whitish or pink centre and hyperpigmented border” (p. 1064, footnote to Table 1). Types of CPG have also been established, as follows: papular, nodular, plaque, umbilicated (Table 1 of the same reference), and linear (Pereira et al.12). Pruriginous lesions of different morphologies may be present in the same patient simultaneously, and the features of these lesions can change over the course of the disease.
Diagnostic Criteria for CPG: the 3 main Criteria Must be Present to Support the Diagnosis.
| Main criteria | - Chronic itching (>6 wk). Itching must be the first symptom. |
| - History and/or signs of scratching (e.g., excoriations) | |
| - Multiple localized or generalized pruriginous lesions | |
| Associated criteria | - Signs |
| Symmetrical distribution of lesions into areas where the skin can be reached and scratched | |
| Normal or lichenified skin between lesions | |
| Possibility of other lesions caused by scratching | |
| Face and palms rarely involved | |
| Pruriginous lesions are persistent. | |
| - Symptoms | |
| Itching precedes skin lesions. | |
| Possibility of burning, stinging, or other pain | |
| Signs of chronicity: very intense, continuous itching; alloknesis, hyperknesis, and the propagation of pruriginous lesions | |
| - Functional aspects | |
| Quality of life deterioration | |
| Loss of sleep | |
| Lost work days | |
| Obsessive–compulsive behavior | |
| - Emotional aspects | |
| Depression | |
| Anxiety | |
| Anger | |
| Disgust | |
| Embarrassment | |
| Impotence | |
| - Pathophysiology | |
| Chronic pruritus may induce neuronal sensitization. | |
| Itch–scratch cycle | |
| Various conditions may cause chronic pruritus. |
Abbreviation: CPG, chronic prurigo.
Few published studies have dealt with the epidemiology of CPG, a condition thought to be rare. Its prevalence is estimated to be around 72 cases per 100000 population,13 but in our experience, this complaint is considerably more common, especially in individuals aged between 50 and 60 years14 and Black patients.15 No differences between the sexes have been described.14
PathophysiologyVarious diseases cause itching in complex ways involving numerous pathways that are often imperfectly understood.16 Moreover, itching leads to scratching, which disrupts the skin barrier, prolonging inflammation.17 When itching becomes chronic, diverse mechanisms eventually cause peripheral and central nervous system sensitization to the symptom.18 Only a minority of patients with chronic pruritus develop pruriginous lesions, however. A genetic predisposition and other factors that are still unknown probably favor the development of CPG in an individual with chronic itching.
The pathogenesis of this chronic disease unfolds along 2 axes — nerve inflammation and nerve plasticity — as well as the interaction between them.19 Understanding the pathogenesis of CPG is the basis for finding an effective treatment.
Anatomically, CPG is characterized by nerve ending hyperplasia in the papillary dermis and hypoplasia in the epidermis.20 Hypoplasia occurs in the epidermis in both healthy and damaged skin,21 but its relevance is not entirely clear. One hypothesis is that hypoplasia is due to subclinical small-fiber neuropathy, but this anatomical abnormality does not necessarily manifest itself in functional changes.22 Furthermore, hypoplasia is corrected when lesions recover.23 A finding of hypoplasia, therefore, is believed to be a consequence of scratching, not its cause.
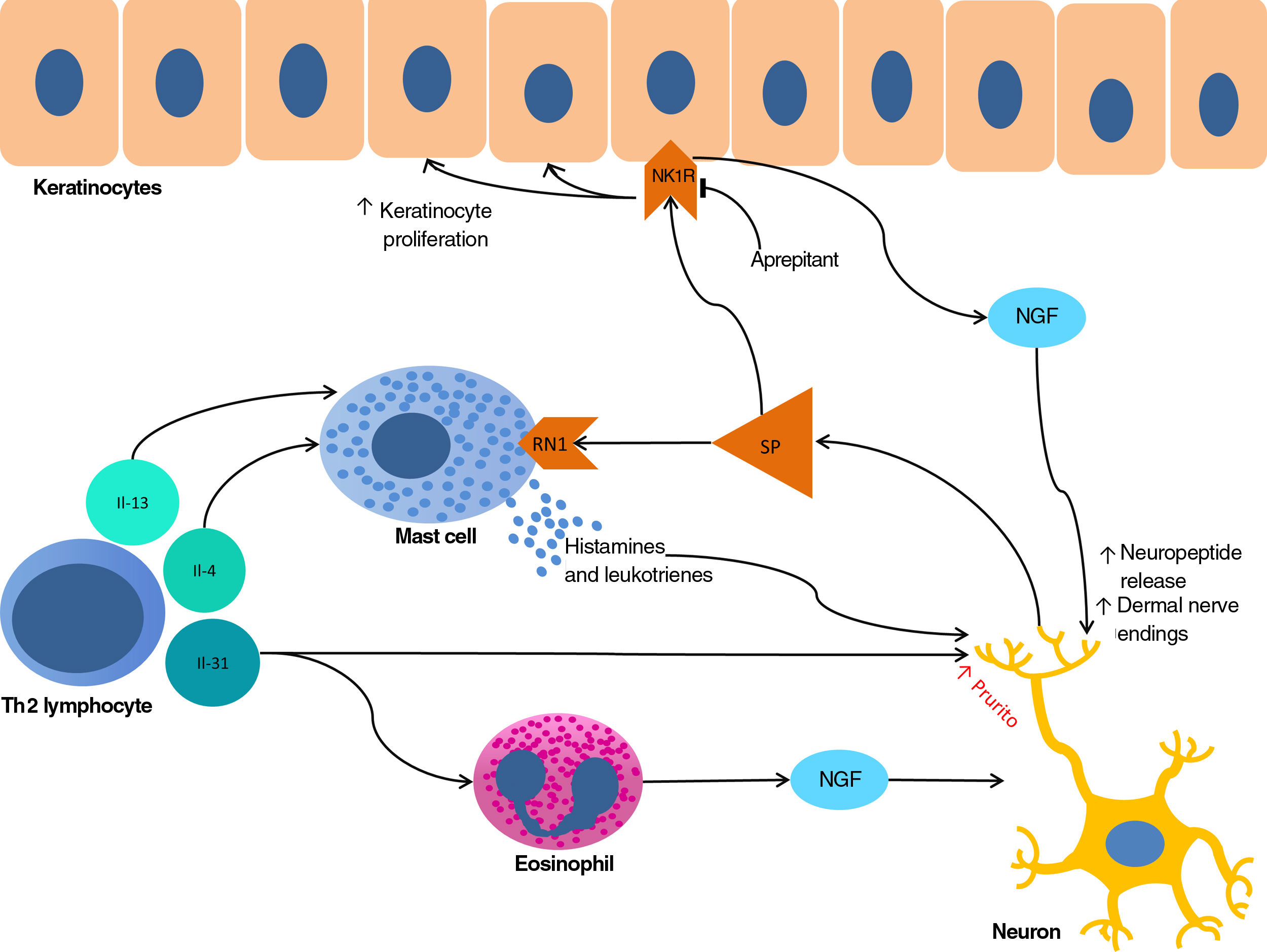
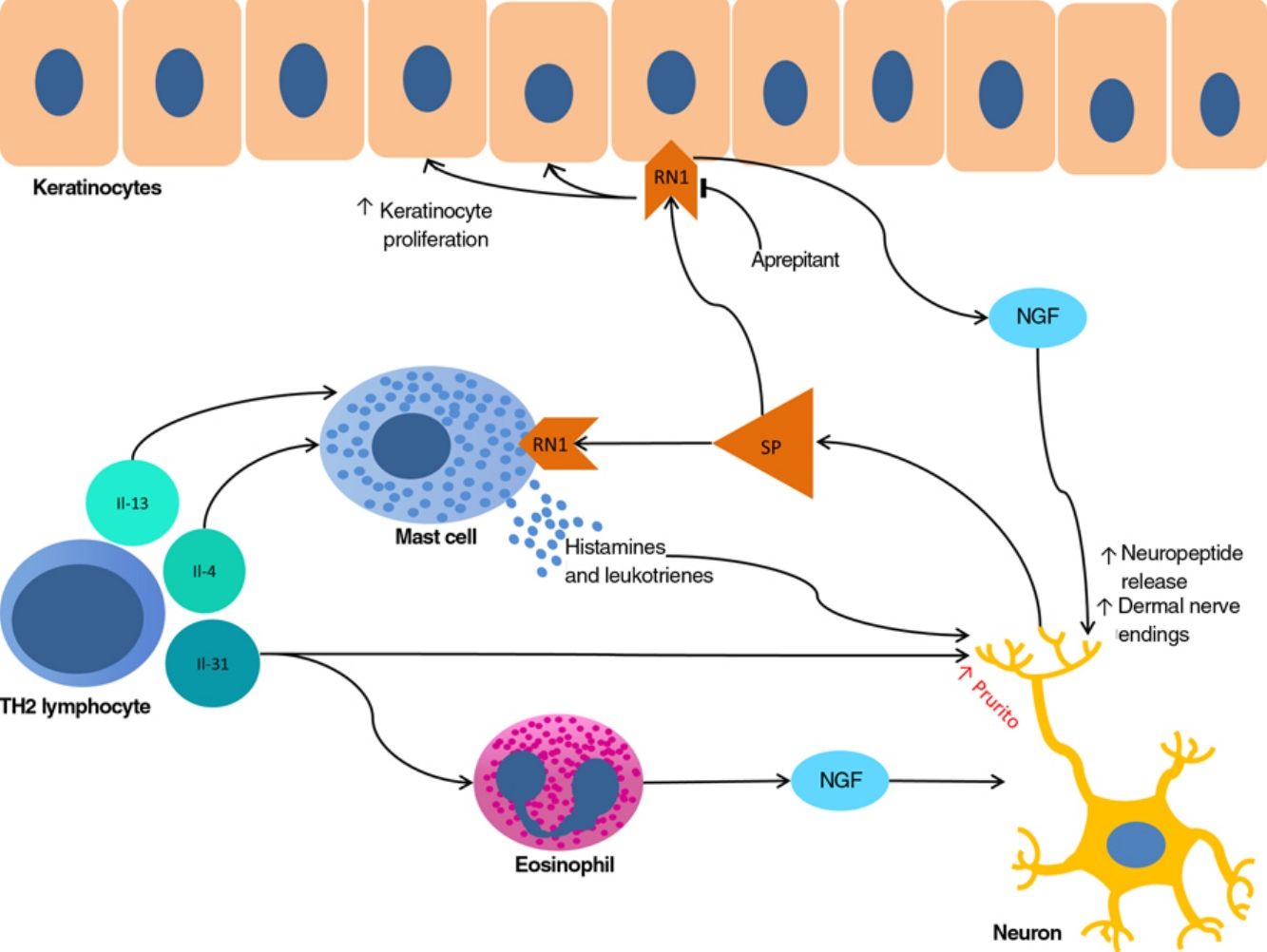
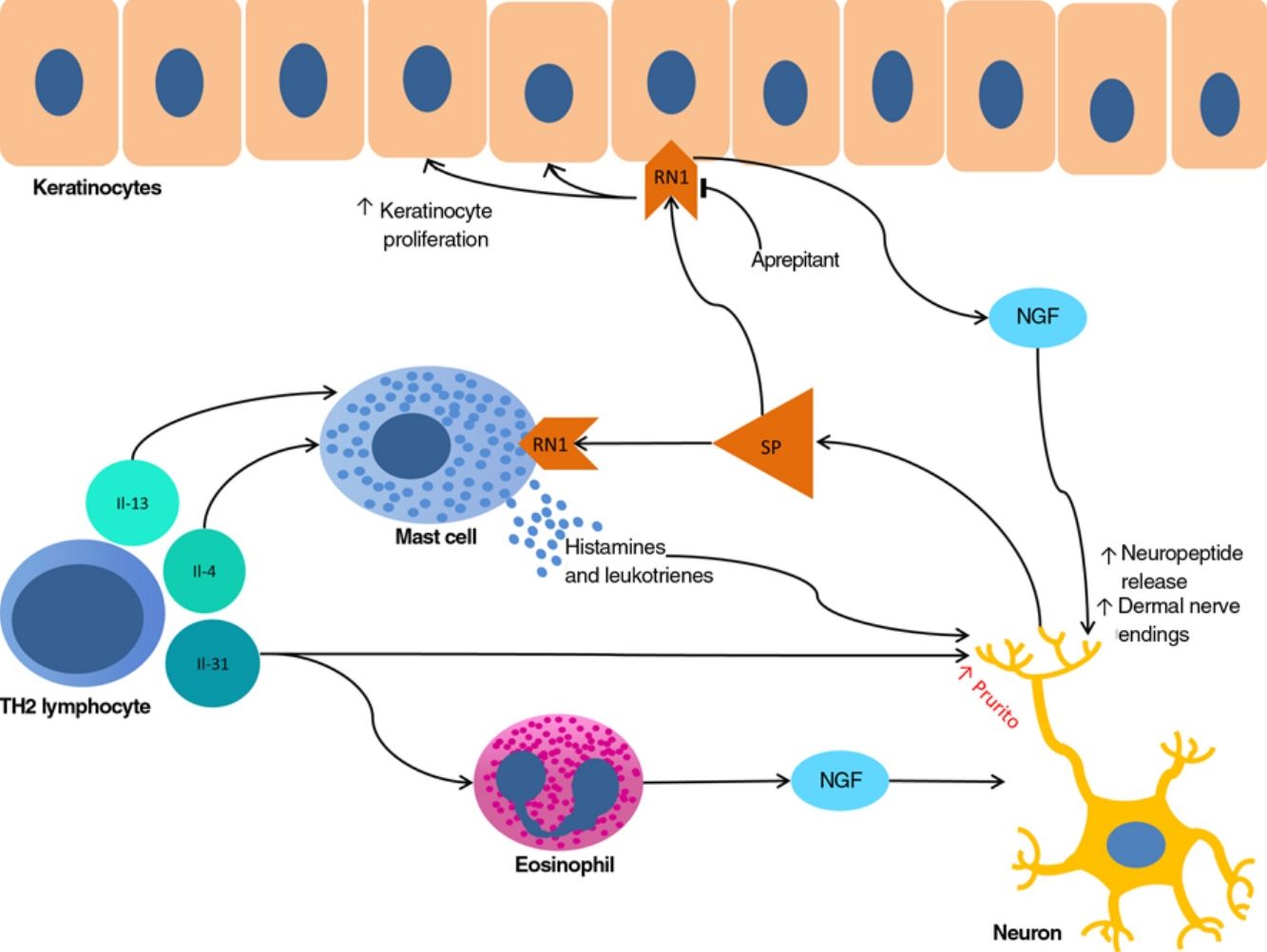
The proliferation of nerve endings in the dermis, on the other hand, seems to be related to increased local inflammatory activity, which is in turn promoted by proinflammatory substances secreted by the same endings. A positive feedback loop therefore emerges (↑local inflammation → ↑nerve inflammation → ↑proinflammatory substances → ↑ local inflammation), providing microscopic confirmation of the clinical itch-scratch cycle (Fig. 1).
Nerve growth factor (NGF), one of the principal proteins implicated in prurigo, is secreted by mast cells and eosinophils and plays a role in the proliferation of nerve endings and keratinocytes, leading to the anatomical changes seen in CPG.19 TH2 lymphocytes are important in the synthesis of NGF, through the secretion of IL-4 and IL-13.24,25 The importance of the TH2 pathway could explain why atopic dermatitis is found in association with CPG in many patients.
Substance P, one of the main mediators of neurogenic inflammation, is secreted by nerve endings. On binding to NK-1R (neurokinin 1 receptor) and M4GPRX2 (mas-related G-protein coupled receptor member X2) on cutaneous mast cells,26 substance P evokes degranulation and the release of such mediators of pruritus as histamine and leukotrienes.27 NK-1Rs are also present in keratinocytes, which release other pruritogenic substances (partially explaining the inability of antihistamines to achieve complete control over itching) as well as other inflammatory mediators such as IL-128 and NGF itself.19 Neurogenic inflammation stimulates increased release of neuropeptides by type-C afferent nerve fibers, increasing their sensitization and spontaneous activity, and perpetuating chronic pruritus.29
IL-31 is another significant mediator in the neuroimmune system. This cytokine, which is important in various conditions, is upregulated 50-fold in nodular-type prurigo30 and is able to evoke intense pruritus in animal models.30 Produced mainly by TH2 lymphocytes, IL-31 binds to a receptor present in a subpopulation of sensory neurons in the spinal ganglia that are positive for TRPV1 and TRPA1 — the transient receptor potential cation channel (subfamilies V and A) member 1.31
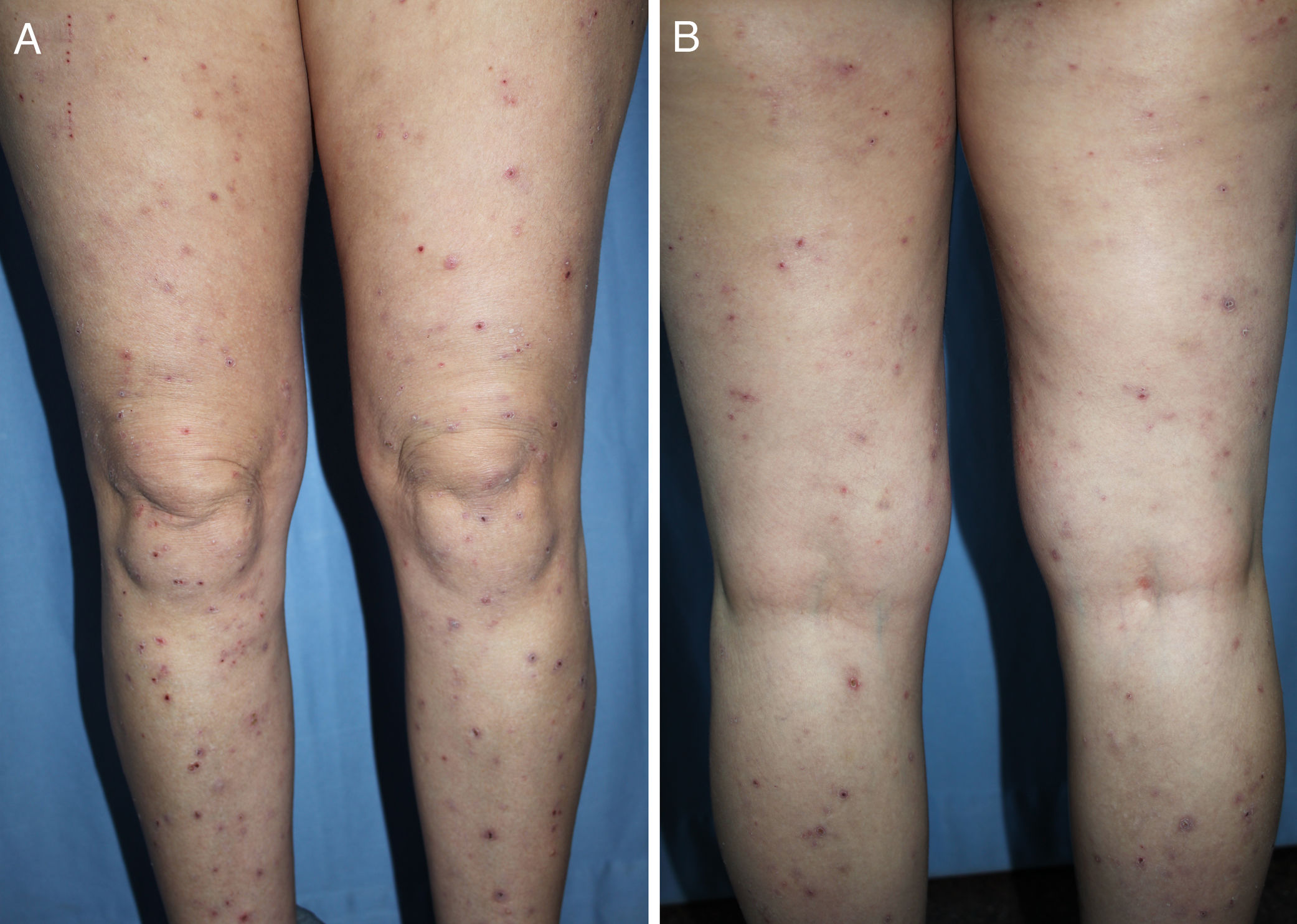
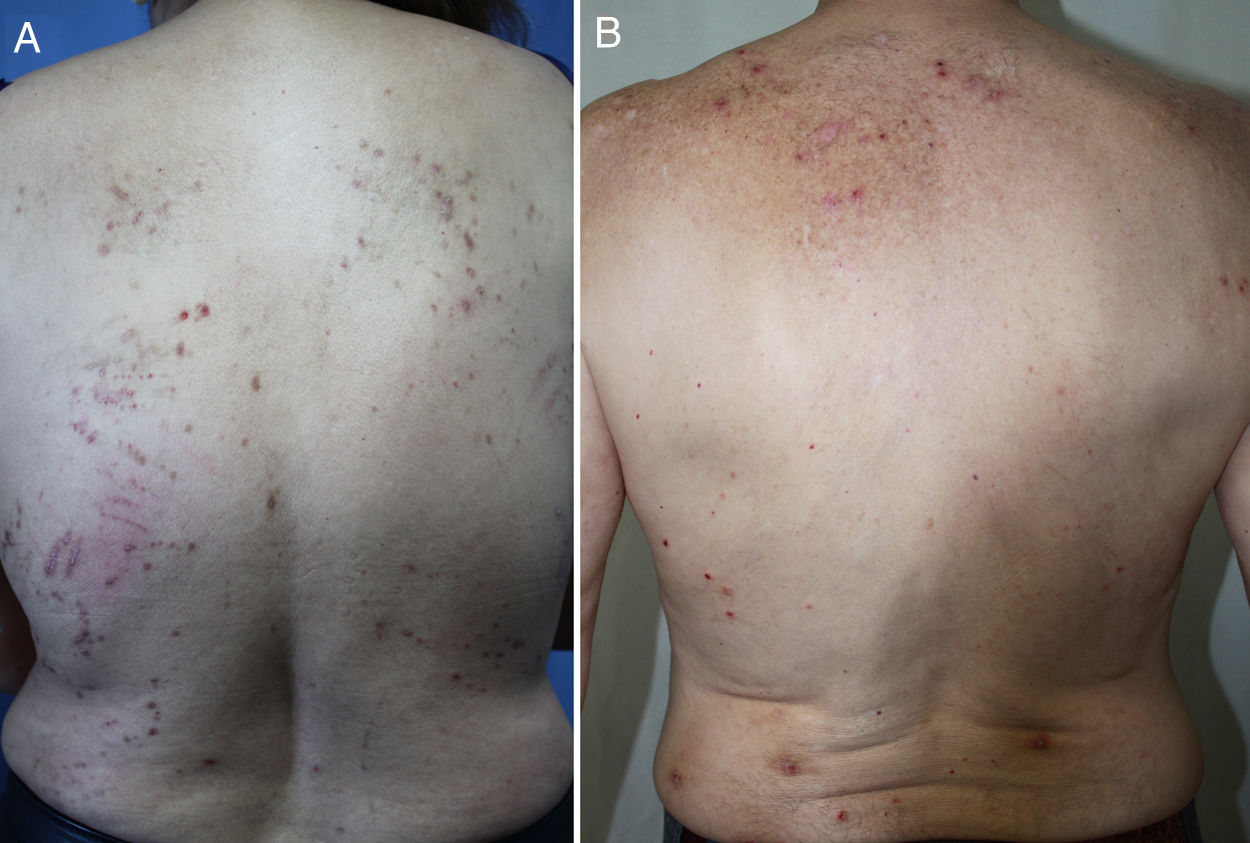
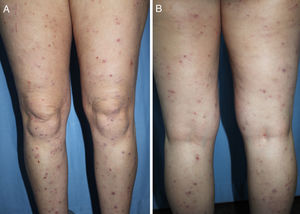
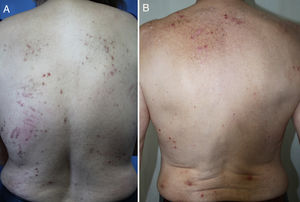
DiagnosisCPG is a clinical diagnosis based on a thorough physical examination and a detailed medical history. A patient usually presents complaining of intense itching and has signs of scratching (excoriated lesions) that are generalized in most cases but spare the face, palms and soles (Fig. 2). There is usually no excoriation in the interscapular area and center of the back, where patients cannot reach, such that the lesions create the so-called butterfly sign (Fig. 3). Diagnosis is not always quite so simple, however, and dermatologists vary considerably in how they make decisions. Diagnostic criteria were proposed by the European Prurigo Project11 (Table 1). We should emphasize that the necessary initial symptom is itching, given that the other criteria listed may also appear in various other skin diseases, which must be ruled out. The patient's cooperation is important for establishing whether the itching began prior to the appearance of the pruriginous lesions or afterwards. The project's criteria are useful for distinguishing CPG from neurotic excoriations, in which the patient does not report itching but admits to scratching, and dermatitis artefacta, in which the patient denies a hand in causing the lesions.
In short, the associated criteria are not requisites. However, they facilitate diagnosis, and strict adhesion to them will allow the clinician to rule out other diseases when evaluating a diagnosis of CPG. Diseases to rule out are shown in Table 2.
Differential Diagnosis of CPG.
| Stings |
| Cutaneous lymphomas |
| Dermatitis herpetiformis |
| Hypertrophic lichen planus |
| Scabies |
| Neurotic excoriations and dermatitis artefacta |
| Sporotrichoid infections |
| Mycobacterial infections |
| Multiple keratoacanthomas |
| Multiple dermatofibromas |
| Sarcoidosis |
| Bullous pemphigoid |
CPG lesions have characteristic histologic features. The epidermis typically presents orthohyperkeratosis and acanthosis nigricans that can occasionally progress to pseudoepitheliomatous hyperplasia. The so-called hairy palms sign can be recognized as hyperkeratosis similar to acral peeling skin, but with hair follicles. Fibrosis in the papillary and reticular dermis is marked by a proliferation of fibroblasts and a predominantly perivascular and interstitial inflammatory infiltrate with lymphocytes and macrophages.32 Many of these features are similar to those of lichen simplex chronicus, another condition caused by scratching.32
Dermoscopy can help differentiate between CPG and other diseases, especially hypertrophic lichen planus. In nodular-type CPG there are pearly white areas, red globules, glomerular vessels, and reddish-brown crusts.33–35 Also typical are peripheral white striations forming a starburst pattern.33–35
We will next focus on 2 entities that can be confusing and are the subject of debate. They should be included in the differential diagnosis of CPG.
Nodular bullous pemphigoid is considered a rare clinical variant of pemphigoid that causes intense pruritus. Bullous and pruriginous lesions are both present in this diagnosis,36 and some authors consider it to be an independent disease.37 However, the morphology of lesions and the clinical course would also seem to be compatible with a diagnosis of CPG secondary to the itching caused by bullous pemphigoid. It is therefore important to examine the patient with chronic pruritus to discover whether there is a prior history of blisters and if necessary proceed to obtain biopsy specimens of perilesional skin for direct immunofluorescence.
Based on clinical and histological similarities, some authors consider acquired reactive perforating dermatoses to be forms of umbilicated CPG that are a response to long-term stimulus in conditions like diabetes mellitus and renal insufficiency.38
Some authors describe a prurigo nodularis-like phenotype in atopic dermatitis,39,40 which can be truly difficult to distinguish from nodular-type CPG secondary to atopic dermatitis. The pruritic lesions we find in reactive perforating dermatoses and those reported in the aforementioned prurigo nodularis-like condition should be considered true cases of CPG. Reactive perforating dermatoses would be CPG secondary to some systemic disease. The “nodular-like” condition would be CPG secondary to atopic dermatitis.
ManagementAfter a confirmed diagnosis of CPG, potential underlying causes should be investigated and ruled out to find the origin of pruritus. The causes of CPG are multiple in 90% of cases.14Table 3 lists the most frequent ones. In up to half of patients the origin is a dermatologic condition,14,41 most often atopic dermatitis, which is also the most common single cause. The onset of CPG tends to come at an earlier age when atopic dermatitis is present.41,42 Consequently, a complete dermatologic medical history and an exhaustive physical examination are necessary. If a patient reports that CPG lesions appeared over inflamed skin, biopsy is recommended.43 Biopsy is also recommended if the clinician suspects skin diseases other than prurigo that can begin without specific lesions. Examples of such diseases are bullous pemphigoid and dermatitis herpetiformis. CPG cases associated with dermatitis due to contact allergy have also been described, so the clinician should evaluate whether or not to order patch tests. However, the evidence is still scarce regarding the usefulness of patch testing.44,45
Most frequent causes of CPGa.
| Dermatologic conditions |
| - Atopic dermatitis |
| - Scabies |
| - Bullous pemphigoid |
| - Mycosis fungoides |
| Systemic diseases |
| - Renal insufficiency (uremic pruritus) |
| - Diabetes mellitus |
| - Hematologic cancers |
| - HIV infection |
| - Cirrhosis |
| Neuropathic causes |
| - Brachioradial pruritus |
| - Postherpetic neuralgia |
| Psychiatric disorders |
| - Depression |
| - Anxiety |
| Unknown |
a Most cases have multiple causes, but when there is a single cause it is usually a dermatologic disorder, mainly atopic dermatitis.
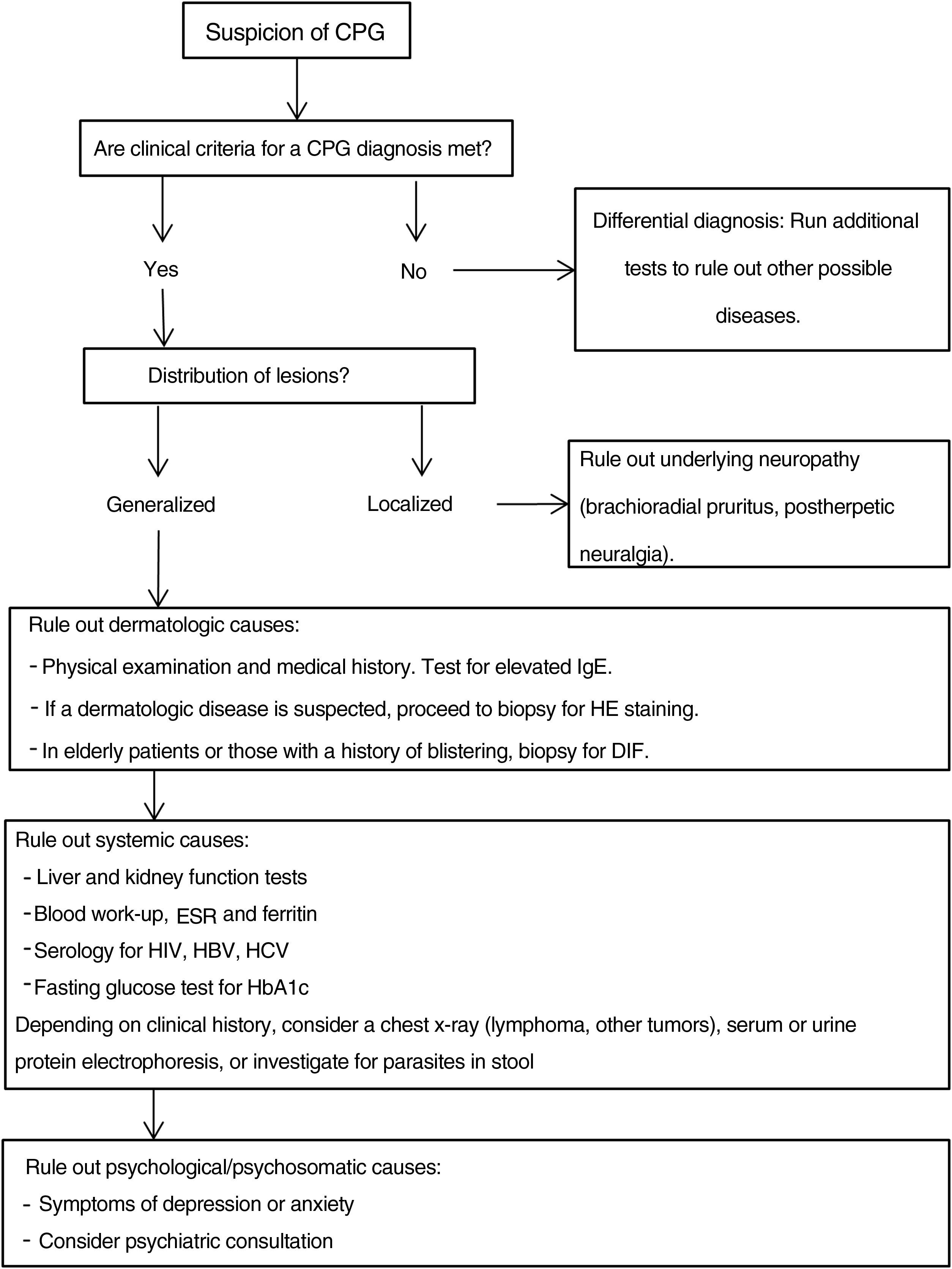
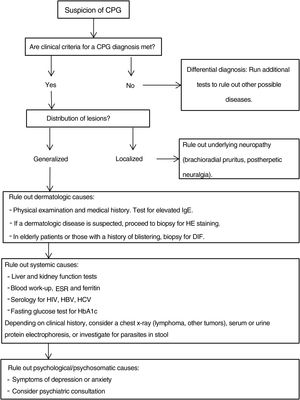
Additional tests may be needed to rule out the causes of the patient's symptoms and to complete the differential diagnosis.20,43Fig. 4 proposes a diagnostic algorithm for CPG.
Proposed diagnostic algorithm for CPG. It is important to emphasize that most cases of CPG are multifactorial in origin, such that confirming a cause does not mean that additional possible causes should not be investigated. CPG refers to chronic prurigo; HE, hematoxylin–eosin; DIF, direct immunofluorescence, HbA1C, glycated hemoglobin; ESR, erythrocyte sedimentation rate; HBV, hepatitis B virus; HCV, hepatitis C virus.
Severity scales and quality of life questionnaires may be useful for objectively evaluating the efficacy of treatments. The DLQI and visual analog scales are available for patient reporting of symptoms, and there is also a version of the Investigator Global Assessment (IGA) of severity that is specific to nodular-type CPG. The IGA's severity classification relies on physician estimation of the approximate number of lesions present at a given moment. Five levels are defined: clear (0 nodules), almost clear (1–5 nodules), mild (6–19 nodules), moderate (20–100 nodules), and severe (more than 100 nodules).43
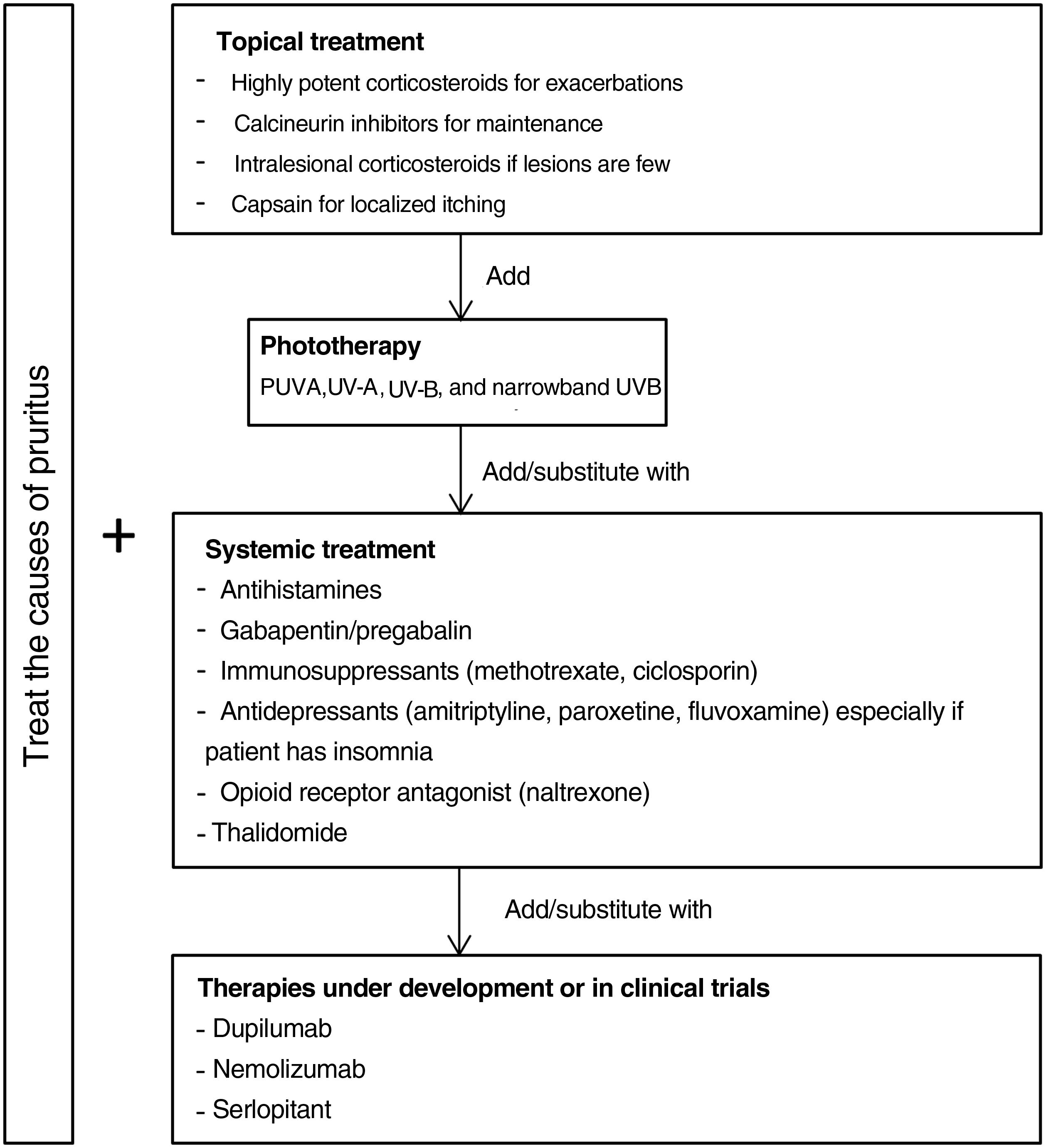
TreatmentThe treatment of CPG is highly complex because there are no specific therapeutic targets. A large number of medications are prescribed,5 but their efficacy is generally low. The aim of therapy should be 2-fold: to attenuate itching and reduce the number of lesions.43 It is essential to first treat the underlying pruritic disease that is the basis for the clinical picture. However, since the lesions of CPG are self-perpetuating, they may persist.7 Most patients also require specific treatment for pruriginous lesions. Additional difficulties are that most treatments have not been evaluated in randomized controlled trials,46 that the trials done have highly heterogeneous endpoints, and that nodular-type CPG is the clinical setting in all the trials published so far.
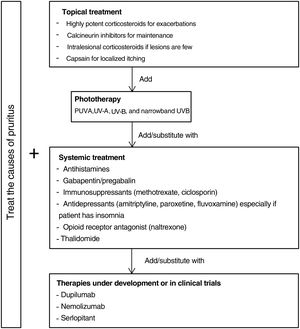
Fig. 5 proposes a therapeutic algorithm. Most cases require several simultaneous treatments. Therapy should be multimodal and always attempt to target the primary cause along with lesions.
Topical TreatmentsTopical preparations are generally the first line of therapy in mild cases of CPG. They are also used to complement systemic therapy. Topical corticosteroids act by regulating the immune response through T lymphocytes and cytokines. In 1 clinical trial, both pruritus and the number of lesions decreased significantly with application of a 0.1% betamethasone cream under occlusion versus application of a comparator.47 Our own experience suggests that application of a very high-potency corticosteroid cream such as 0.05% clobetasol propionate can be more useful given the difficulty medications have in penetrating the typical hyperkeratosis of pruriginous lesions. Studies providing a lower grade of evidence have reported improvement with intralesional application of corticosteroids.48,49 This technique can be especially useful for treating small numbers of refractory lesions. Topical calcineurin inhibitors are a safe alternative for achieving results similar to corticosteroid treatment in longterm therapy.50 Calcipotriol was also effective in a single small study.51 Capsaicin seems to produce an antipruritic effect upon binding to TRPV1, desensitizing nerve endings. A transient side effect of capsaicin application is a burning sensation that limits its use in CPG to local treatments in cases of neuropathic pruritus.52,53
Systemic TreatmentsPhototherapy can be useful mainly in patients with generalized CPG who are not candidates for systemic immunosuppressants because of advanced age or comorbidity. Psoralen plus UV-A therapy (PUVA) and other modalities using UV-A, UV-B, and narrowband UV-B light have been found efficacious.54–59 A combination of a UV-B 308nm excimer light and a PUVA bath proved superior to PUVA alone in a small randomized trial,60 but no other trials have compared phototherapeutic modalities.
Antihistamines are often used to control itching even though they do not usually provide sufficient relief by themselves.61 A small case series found that combining antihistamines with leukotriene inhibitor therapy was efficacious.62
Gabapentinoids seem to act by inhibiting synaptic secretion of glutamate, which is an excitatory neurotransmitter. These drugs are particularly useful when the central nervous system plays a role, as they can stabilize mood and reduce the urge to scratch.63 Although good results in CPG have been reported based on open trials and case series,64–66 caution is advisable. Doses should be increased gradually given that gapentinoids have adverse effects such as nausea, sleepiness, and dizziness.67
Antidepressants such as amitriptyline,68 paroxetine, and fluvoxamine69 have been efficacious in the treatment of both pruritus and pruriginous lesions. Because these drugs can induce drowsiness they are especially useful in patients who report insomnia due to pruritus.43
Good results have been reported in CPG of diverse origins for the use of μ-opioid receptor antagonists like naltrexone.70,71 A limitation is that they often cause adverse effects such as nausea and vomiting.72 One phase 2 clinical trial underway includes the study of the effect on pruritus of nalbuphine, a κ-receptor agonist and μ-receptor antagonist.73 This drug has appeared to reduce pruritus mediated by IL-31 in animal models.74
Another effective alternative is thalidomide.75,76 This drug has serious adverse effects, including peripheral neuropathy (in 20% of patients) in addition to its teratogenic potential.77 A low-dose regimen (under 100mg) may avoid such adverse effects yet maintain thalidomide's antipruritic efficacy.78,79
The main systemic immunosuppressants used in CPG are methotrexate and ciclosporin. Case series have demonstrated their efficacy.80–82 A decision to opt for one over the other should be made based on their different side effects. Ciclosporin is generally reserved for younger patients without kidney disease or for scenarios that require a faster onset of action.
Therapies Under DevelopmentAdvances in our understanding of the pathophysiology of CPG and the role of certain interleukins have led to the development of new drugs that may prove effective.
Dupilumab is a monoclonal antibody that binds to the α receptor of IL-4, inhibiting both IL-4 and IL-13 signaling.83 Several uncontrolled studies and case series (with 70 patients in total) support its efficacy.39,40,84–91 Treatment leads to improvement and is well tolerated in most cases. Conjunctivitis is the only adverse effect. Since dupilumab is authorized to treat atopic dermatitis, patients who are not atopic might be expected to improve less. However, 2 studies that compared patients with and without atopy did not see significant differences in clinical improvement between them.92,93 One of the studies did find a more rapid response to treatment in the patients with atopic dermatitis.93 Current evidence therefore suggests that dupilumab may be effective. Confirmation may come on publication of the results of a phase 3 trial that is still running.94
A phase 2 trial of nemolizumab in moderate to severe nodular CPG (defined by the presence of more than 20 lesions) and severe pruritus (score of more than 7 on a numerical scale of 0 to 10) was recently published.95 Nemolizumab is a monoclonal antibody that blocs receptor A of IL-31. The authors reported a significant effect on pruritus, the primary endpoint. Itching decreased by 53% in patients on nemolizumab by week 4, versus 20% in patients on placebo (P<.001). Improvement was unrelated to a patient's history of atopy. Treatment with nemolizumab was associated with abdominal pain, diarrhea, and musculoskeletal symptoms.95 A phase 3 trial is currently underway.96
A phase 2 placebo-controlled trial of oral aprepitant revealed no advantage of the active treatment for improving pruritus in CPG.97 Aprepitant is a selective NK-1R antagonist. However, serlopitant, another drug in development that has the same mechanism of action, did improve pruritus in a phase 2 trial.98
Vixarelimab, an oncostatin M-receptor inhibitor, showed promising results in controlling pruritus in a phase 2 trial.99
A small clinical study (10 patients) found that apremilast led to no improvement.100
ConclusionsCPG is an independent clinical entity associated with neuronal sensitization that causes a secondary itch-scratch cycle. The management of CPG is complex because of highly varied underlying causes and clinical presentations. However, the ability to correctly identify and classify CPG will facilitate better management. Promising medical therapies that are under development may improve the management of this difficult-to-treat disease.
Conflicts of InterestThe authors declare that they have no conflicts of interest.