Cutaneous T-cell lymphomas (CTCL) such as mycosis fungoides (MF) and Sézary syndrome (SS) are rare lymphomas with varying prognoses. The aim of the study was to describe the survival of a cohort of patients with MF/SS and evaluate the prognostic factors impacting disease survival.
Materials and MethodsAll cases of MF/SS diagnosed from 2008 through 2022 were retrospectively analyzed. The demographic variables, histological parameters, and analytical data were analyzed too. Progression-free survival (PFS) and disease-specific survival (DSS) were calculated.
ResultsA total of 148 cases were included. A total of 121 (82%) and 27 cases were diagnosed with MF, and SS, respectively. A total of 37 patients (25%) experienced progression at some point disease progression. The median PFS and median DSS were 127 and 135 months, respectively. Age >60 years, diagnosis of SS, the presence of large cell transformation (LCT) at diagnosis, folliculotropism in early stages, high Ki-67 expression, the presence of the clonal T-cell receptor (TCR) in blood, elevated LDH and B2M levels, and advanced stages (IIB, IVA, T3, T4, N3/Nx) were associated with worse prognosis across the entire cohort.
ConclusionsStage IVA and the presence of LCT at diagnosis stood out as independent factors of unfavorable prognosis. LCT was the variable that most significantly impacted the patients’ survival and was closely associated with tumor skin involvement and stage IIB.
Los linfomas cutáneos de células T (LCCT) como la micosis fungoide (MF) y el síndrome de Sézary (SS) son linfomas poco comunes con pronósticos variables. El objetivo del estudio fue describir la supervivencia de una cohorte de pacientes con MF/SS y evaluar aquellos factores pronósticos con impacto en la supervivencia de la enfermedad.
Material y métodosSe analizaron retrospectivamente todos los casos diagnosticados de MF/SS entre 2008 y 2022. Se evaluaron variables demográficas, parámetros histológicos, y datos analíticos. Se calcularon la supervivencia libre de progresión (PFS) y la supervivencia específica de la enfermedad (DSS).
ResultadosSe incluyeron un total de 148 casos. Ciento veintiún casos (82%) fueron diagnosticados de MF y 27 casos de SS. Treinta y siete pacientes (25%) progresaron en algún momento de la evolución. La mediana de PFS fue de 127 meses y la mediana de DSS de 135 meses. La edad >60 años, el diagnóstico de SS, la presencia de transformación a célula grande (TCG) al diagnóstico, el foliculotropismo en estadios iniciales, la elevación de Ki-67, la presencia de TCR clonal en sangre, niveles elevados de LDH y B2M, y estadios avanzados (IIB, IVA, T3, T4, N3/Nx) se asociaron con un peor pronóstico en la cohorte.
ConclusionesEl estadio IVA y la presencia de TCG al diagnóstico destacaron como factores independientes de pronóstico desfavorable. La TCG fue la variable que produjo una disminución más acentuada de la supervivencia de los pacientes estando estrechamente relacionada con la afectación cutánea tumoral y el estadio IIB.
Mycosis fungoides (MF) and Sézary syndrome (SS) are the most common subtypes of cutaneous T-cell lymphomas (CTCL).1,2 MF usually has an indolent course with prolonged survival and a good clinical response to skin-directed therapies. However, 20% up to 25% of patients progress to advanced tumor stages.3 These advanced forms of MF and SS are aggressive diseases of difficult treatment and a grim prognosis.1,2 The factors involved in the progression and the survival of these lymphomas is still to be elucidated.
This study describes the progression-free survival (PFS) and disease-specific survival (DSS) of a cohort of patients with MF/SS and aims to evaluate which prognostic factors have an adverse impact on the survival of these lymphomas.
Materials and MethodsWe analyzed all consecutive cases diagnosed with MF and SS from January 2008 through December 2022 at the Cutaneous Lymphoma Unit of Hospital Universitari de Bellvitge, Barcelona, Spain. Diagnosis was achieved based on the criteria established by the World Health Organization (WHO)-European Organization of Research and Treatment of Cancer (EORTC)2. Demographic data such as age, sex, diagnostic subtype, status at last follow-up, stage at diagnosis and at progression (TNMB staging was used based on the recommendation proposed by the International Society for Cutaneous Lymphoma [ISCL] – EORTC),4 histological parameters (large cell transformation [LCT], folliculotropism [FT], percentage of CD30+cells, Ki-67, clonal T-cell receptor [TCR] in the skin) and hematological parameters (LDH, beta2-microglobulin [B2M], leukocytes, lymphocytes, clonal TCR in blood) were collected. Ki-67 staining was considered intense in biopsies showing a Ki-67 proliferation index > 30% and mild if<30%. LDH and B2M were considered elevated if values were > 213 U/L and 2.4mg/L, respectively.
LCT was defined when the presence of large lymphocytes > 25% of the dermal infiltrate or following the formation of large cell nodules.5
PFS was defined as the time from the first objective response to treatment until relapse, progression, or death (in patients who progress) or until the date of the last follow-up (in patients who don’t); overall survival (OS) was defined as the time elapsed from the starting date of treatment to the all-cause mortality date; and DSS as the time elapsed from the starting date of treatment to the date of lymphoma-related death. Progression was defined as the change from one stage to a higher one.
Statistical analysisStatistical analysis was performed using SPSS Statistics software. A descriptive frequency analysis was conducted for categorical variables, and the normality of numerical variables was evaluated. Contingency tables and statistical tests were used to analyze relationships between variables. Fisher's exact test or the chi-square test were used, when appropriate, depending on the size of expected frequencies. The Mann-Whitney U test was used to compare numerical and categorical variables.
DSS and survival rates throughout years were estimated using the Kaplan-Meier method. The log-rank test was used for group comparison. Multivariate analyses were performed using Cox proportional hazards regression model, including significant variables from previous analyses.
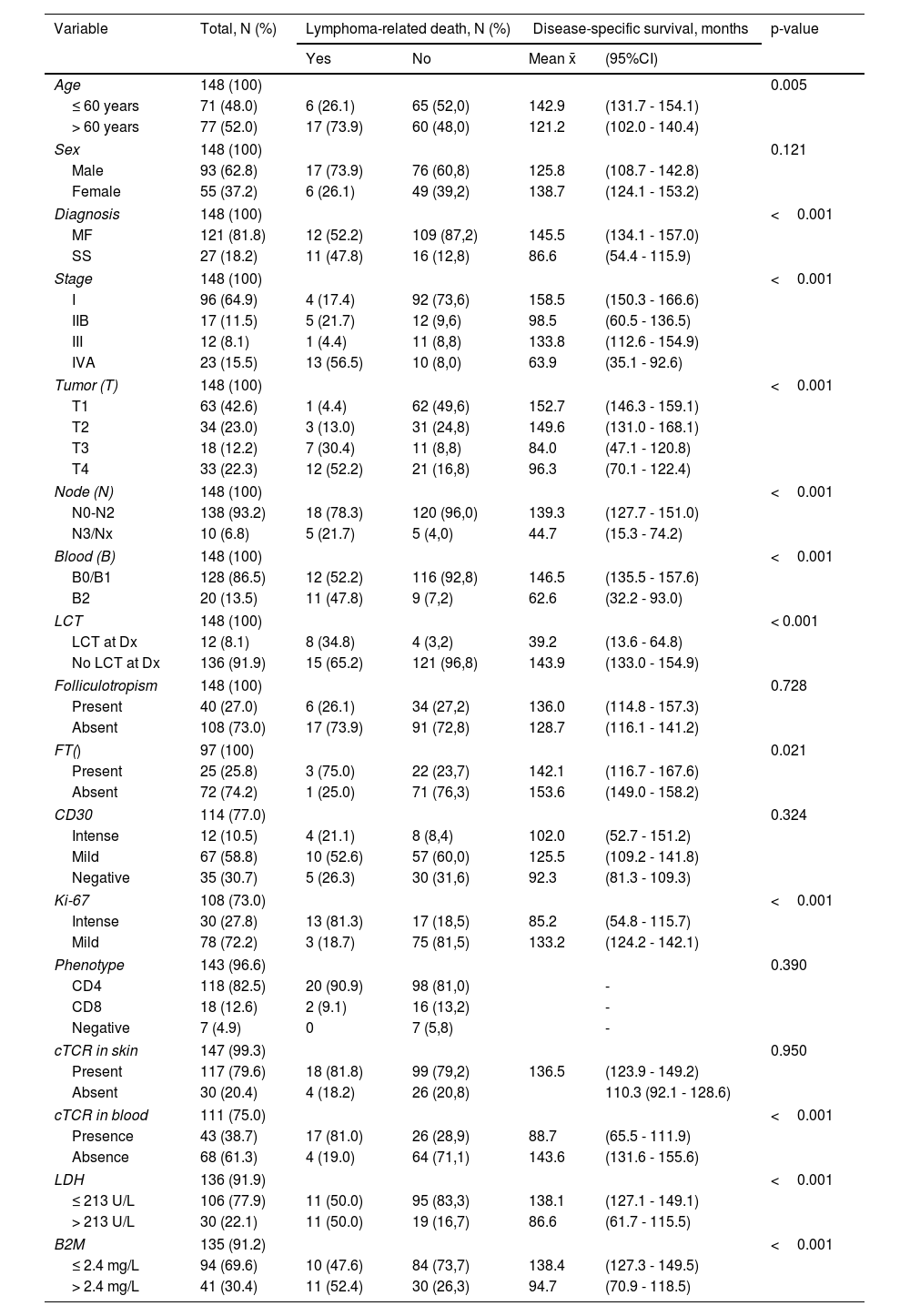
ResultsA total of 148 cases were included. Table 1 shows the main characteristics of patients with MF and SS. The median age was 62 years [49-70 years], and 63% of patients were men. A total of 121 cases (82%) were diagnosed with MF, and 27 with SS. At diagnosis, the initial stages (< IIA) accounted for 65% of cases. A total of 37 patients (25%) progressed at some point during the course of their disease. The median PFS was 127 months (129 months for MF and 95 months for SS). At the last follow-up, 53% of patients were alive with lesions, 24% in complete remission, and 23% had already died (16% due to lymphoma and 7% for other reasons). In 10% of MF cases, death was lymphoma-related vs 41% of the cases of SS. The median DSS for the entire cohort was 135 months. Table 1 also shows the results of the univariate Kaplan-Meier/log-rank survival analysis for the cohort for each of the variables detailed below.
Description of demographic parameters, TNMB staging at diagnosis, histological and hematological parameters, and results of the univariate Kaplan-Meier/log-rank analysis of disease-specific survival for the entire cohort.
| Variable | Total, N (%) | Lymphoma-related death, N (%) | Disease-specific survival, months | p-value | ||
|---|---|---|---|---|---|---|
| Yes | No | Mean x̄ | (95%CI) | |||
| Age | 148 (100) | 0.005 | ||||
| ≤ 60 years | 71 (48.0) | 6 (26.1) | 65 (52,0) | 142.9 | (131.7 - 154.1) | |
| > 60 years | 77 (52.0) | 17 (73.9) | 60 (48,0) | 121.2 | (102.0 - 140.4) | |
| Sex | 148 (100) | 0.121 | ||||
| Male | 93 (62.8) | 17 (73.9) | 76 (60,8) | 125.8 | (108.7 - 142.8) | |
| Female | 55 (37.2) | 6 (26.1) | 49 (39,2) | 138.7 | (124.1 - 153.2) | |
| Diagnosis | 148 (100) | <0.001 | ||||
| MF | 121 (81.8) | 12 (52.2) | 109 (87,2) | 145.5 | (134.1 - 157.0) | |
| SS | 27 (18.2) | 11 (47.8) | 16 (12,8) | 86.6 | (54.4 - 115.9) | |
| Stage | 148 (100) | <0.001 | ||||
| I | 96 (64.9) | 4 (17.4) | 92 (73,6) | 158.5 | (150.3 - 166.6) | |
| IIB | 17 (11.5) | 5 (21.7) | 12 (9,6) | 98.5 | (60.5 - 136.5) | |
| III | 12 (8.1) | 1 (4.4) | 11 (8,8) | 133.8 | (112.6 - 154.9) | |
| IVA | 23 (15.5) | 13 (56.5) | 10 (8,0) | 63.9 | (35.1 - 92.6) | |
| Tumor (T) | 148 (100) | <0.001 | ||||
| T1 | 63 (42.6) | 1 (4.4) | 62 (49,6) | 152.7 | (146.3 - 159.1) | |
| T2 | 34 (23.0) | 3 (13.0) | 31 (24,8) | 149.6 | (131.0 - 168.1) | |
| T3 | 18 (12.2) | 7 (30.4) | 11 (8,8) | 84.0 | (47.1 - 120.8) | |
| T4 | 33 (22.3) | 12 (52.2) | 21 (16,8) | 96.3 | (70.1 - 122.4) | |
| Node (N) | 148 (100) | <0.001 | ||||
| N0-N2 | 138 (93.2) | 18 (78.3) | 120 (96,0) | 139.3 | (127.7 - 151.0) | |
| N3/Nx | 10 (6.8) | 5 (21.7) | 5 (4,0) | 44.7 | (15.3 - 74.2) | |
| Blood (B) | 148 (100) | <0.001 | ||||
| B0/B1 | 128 (86.5) | 12 (52.2) | 116 (92,8) | 146.5 | (135.5 - 157.6) | |
| B2 | 20 (13.5) | 11 (47.8) | 9 (7,2) | 62.6 | (32.2 - 93.0) | |
| LCT | 148 (100) | < 0.001 | ||||
| LCT at Dx | 12 (8.1) | 8 (34.8) | 4 (3,2) | 39.2 | (13.6 - 64.8) | |
| No LCT at Dx | 136 (91.9) | 15 (65.2) | 121 (96,8) | 143.9 | (133.0 - 154.9) | |
| Folliculotropism | 148 (100) | 0.728 | ||||
| Present | 40 (27.0) | 6 (26.1) | 34 (27,2) | 136.0 | (114.8 - 157.3) | |
| Absent | 108 (73.0) | 17 (73.9) | 91 (72,8) | 128.7 | (116.1 - 141.2) | |
| FT() | 97 (100) | 0.021 | ||||
| Present | 25 (25.8) | 3 (75.0) | 22 (23,7) | 142.1 | (116.7 - 167.6) | |
| Absent | 72 (74.2) | 1 (25.0) | 71 (76,3) | 153.6 | (149.0 - 158.2) | |
| CD30 | 114 (77.0) | 0.324 | ||||
| Intense | 12 (10.5) | 4 (21.1) | 8 (8,4) | 102.0 | (52.7 - 151.2) | |
| Mild | 67 (58.8) | 10 (52.6) | 57 (60,0) | 125.5 | (109.2 - 141.8) | |
| Negative | 35 (30.7) | 5 (26.3) | 30 (31,6) | 92.3 | (81.3 - 109.3) | |
| Ki-67 | 108 (73.0) | <0.001 | ||||
| Intense | 30 (27.8) | 13 (81.3) | 17 (18,5) | 85.2 | (54.8 - 115.7) | |
| Mild | 78 (72.2) | 3 (18.7) | 75 (81,5) | 133.2 | (124.2 - 142.1) | |
| Phenotype | 143 (96.6) | 0.390 | ||||
| CD4 | 118 (82.5) | 20 (90.9) | 98 (81,0) | - | ||
| CD8 | 18 (12.6) | 2 (9.1) | 16 (13,2) | - | ||
| Negative | 7 (4.9) | 0 | 7 (5,8) | - | ||
| cTCR in skin | 147 (99.3) | 0.950 | ||||
| Present | 117 (79.6) | 18 (81.8) | 99 (79,2) | 136.5 | (123.9 - 149.2) | |
| Absent | 30 (20.4) | 4 (18.2) | 26 (20,8) | 110.3 (92.1 - 128.6) | ||
| cTCR in blood | 111 (75.0) | <0.001 | ||||
| Presence | 43 (38.7) | 17 (81.0) | 26 (28,9) | 88.7 | (65.5 - 111.9) | |
| Absence | 68 (61.3) | 4 (19.0) | 64 (71,1) | 143.6 | (131.6 - 155.6) | |
| LDH | 136 (91.9) | <0.001 | ||||
| ≤ 213 U/L | 106 (77.9) | 11 (50.0) | 95 (83,3) | 138.1 | (127.1 - 149.1) | |
| > 213 U/L | 30 (22.1) | 11 (50.0) | 19 (16,7) | 86.6 | (61.7 - 115.5) | |
| B2M | 135 (91.2) | <0.001 | ||||
| ≤ 2.4 mg/L | 94 (69.6) | 10 (47.6) | 84 (73,7) | 138.4 | (127.3 - 149.5) | |
| > 2.4 mg/L | 41 (30.4) | 11 (52.4) | 30 (26,3) | 94.7 | (70.9 - 118.5) | |
B2M, beta-2-microglobulin; cTCR, clonal T-cell receptor; Dx, diagnosis; FT, folliculotropism; LCT, large cell transformation; LDH, lactate dehydrogenase; MF, mycosis fungoides; SS, Sézary syndrome.
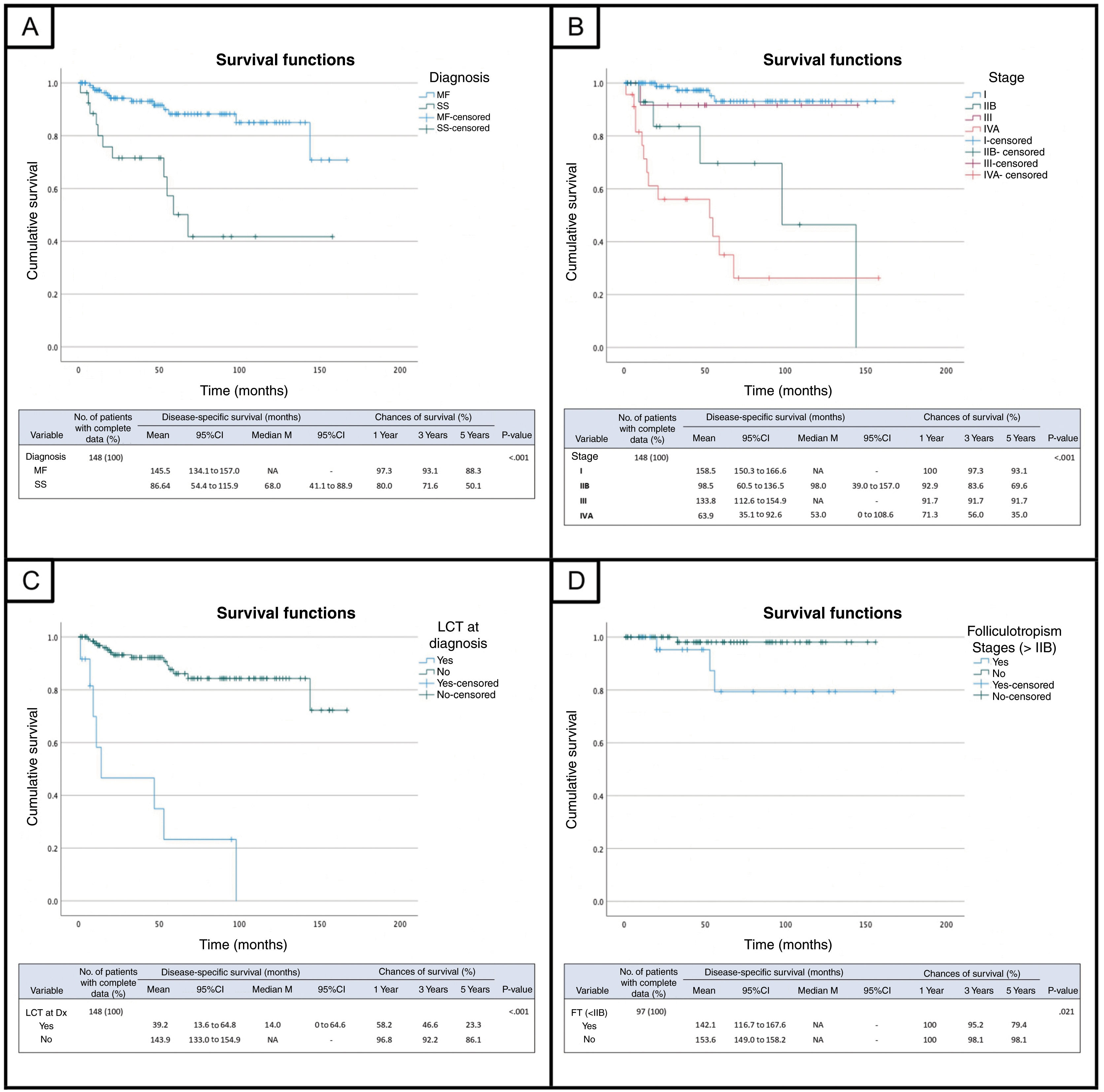
In the univariate analysis, age > 60 years (52% of cases) was associated with poorer DSS with a mean survival of 121 months. No differences were found based on sex. Regarding diagnosis, SS had worse outcomes with a mean survival of 86.6 months and a 5-year survival rate of 50% vs 88% in the MF group (fig. 1A). However, no survival differences were found when comparing SS patients to the advanced MF group. Regarding stage at diagnosis, Figure 1B indicates the mean DSS and the 1-, 3-, and 5-year survival rates for each stage. No statistically significant differences were seen either between stages I and III, yet differences were seen compared to stages IIB and IVA. Regarding IVA stage—which includes IVA1 with peripheral blood involvement B2 (median DSS, 55 months) and IVA2 with nodal involvement N3 (median DSS, 14 months)—no differences were found between the 2 groups. However, when comparing survival of N3 and/or Nx (abnormal peripheral nodes without histological confirmation) with N1 and/or N2, differences became evident. Differences in survival were also found between patients with B2 involvement (IVA)—who had a worse prognosis (62.6 vs 146.5 months; p <0.001)—compared to those with B0 or B1.
Disease-specific survival (DSS) curves and tables for the cohort of patients with MF/SS comparing:
A) DSS between patients with mycosis fungoides (MF) and patients with Sézary syndrome (SS). B) DSS across different stages. C) DSS between patients with large cell transformation (LCT) at diagnosis and those without it. D) DSS for the variable folliculotropism (FT) among patients with stages < IIB.
Regarding histological parameters, LCT was reported in 19% of patients during the course of their disease, and 43% of these cases were reported at diagnosis. The presence of LCT at diagnosis had a significant adverse effect on these patients’ survival (fig. 1C). The presence of FT (27% of cases) was not associated with poorer survival across the entire cohort, but when analyzed in the population that started in early stages <IIB, patients with FT had significantly lower survival rates vs those without FT (fig. 1D). Conversely, the analysis of the population with stages > IIB showed that those without FT had even lower survival rates. Intense Ki-67 staining, found in 28% of the 108 cases studied, was also associated with poorer survival, with a mean of 85.2 months and a 5-year survival rate of 50.6% vs 92.4% in the group with mild Ki-67 (p <0.001); no such association was found with CD30 expression. The predominant phenotype in the series was CD4+(83% of cases), while CD8+and double negative CD4-/CD8- cases accounted for 13% and 5%, respectively. No differences were found between the different phenotypes. Regarding clonality studies, skin clonality documented in 80% of cases was not associated with unfavorable outcomes, while patients with positive blood clonality (39% of cases) had poorer DSS vs those without it (88.7 vs 143.6 months; p <0.001). Regarding analytical data, elevated LDH (22%) and B2M (30%) were also associated with worse survival rates in the cohort (p <0.001).
In the multivariate analysis, LCT at diagnosis (Hazard ratio [HR], 10.41) and the IVA stage (HR, 6.29) were the variables that showed an independently significant effect on DSS (p <0.001).
DiscussionFor years, efforts have been made to elucidate factors or markers of poor prognosis associated with high mortality rates, low survival, or a higher risk of lymphoma progression.6,7 In 2013, the CLIPI (Cutaneous Lymphoma International Prognostic Index) was proposed—the first prognostic index for cutaneous lymphoma—which included factors associated with poor prognosis in the early (male sex, age > 60 years, plaques, FT, N1/X) and advanced stages (male sex, age > 60 years, stages B1/B2, N2/N3, visceral involvement), attributing low, intermediate, or high risk based on the score.8 In 2015, the CLIC (Cutaneous Lymphoma International Consortium) embarked on a large-scale project called ProCLIPI (Prospective Cutaneous Lymphoma International Prognostic Index) which was designed to create an international CTCL database with the long-term goal of developing a prognostic index to identify patients with unfavorable outcomes.9,10 Some prospective studies have already been published, such as the one conducted by Scarisbrick et al., which included advanced cases of MF/SS, developing a prognostic index model with the variables stage IV, age > 60 years, LCT, and elevated LDH6. Most of these factors, and these prognostic indices, have not yet been validated in clinical practice, and so far, stage (TNM) has been the prognostic marker with the most predictive value.4,11
This study aims to evaluate which factors are associated with poorer prognosis and a higher risk of progression in a series of 148 patients with MF/SS from a reference center on the management of cutaneous lymphoma.
Advanced age (> 60 years) had already been associated with poorer prognosis, especially in advanced stages of the disease.3,6,12 However, in this cohort, other factors such as comorbidities and limited treatment options could not be evaluated. Despite the predominance of male patients in the study, no significant survival differences were found between sexes.
The diagnosis of SS was associated with a worse course of the disease compared with MF. However, no differences in DSS were found between advanced MF and SS, suggesting that initial stage impacts prognosis more than the type of diagnosis. In line with these data, Figure 1B shows that statistically significant differences in survival were seen when comparing early stages with stage IIB and IVA. These differences resemble previously published data in which clear dissimilarities are observed between the median OS of early stages (IA-IIA) and more advanced stages of the disease (> IIB).1,3
Among patients with erythroderma, no differences in survival were seen between patients with low-level blood involvement (IIIB) vs those without it (IIIA). In contrast, and consistent with previously published data,3,6,13 patients with B2 involvement (IVA) showed a worse prognosis (p <0.001) vs those with B0 or B1.
Furthermore, no survival differences were seen in patients without nodal involvement and those with N1 and N2 involvement. However, patients with partial or total disappearance of nodal architecture (N3) showed a worse outcome. We also saw that cases with pathological nodes without staging (Nx) had a higher mortality risk—which is similar to the risk of N3—thus confirming recently published data showing that the increased size of the lymph node confirmed only by clinical evaluation (Nx) was associated with a higher risk of progression,12 highlighting the importance of performing biopsies of palpable nodes for proper staging.
The presence of LCT at diagnosis turned out to be an independent histological marker of poor prognosis, with an even worse survival than that reported by other studies.3,6,13,14 Additionally, as previously described,15 LCT was more prevalent in advanced stages, predominantly among patients with stage IIIB, specifically those with tumor-form skin involvement. Although FT phenomenon did not show any significant survival differences in the cohort studied, its effect seems to vary based on the stage of the disease, which is consistent with previously published data that showed FT acted as a predictor of poor outcome in early stages8,16.
Ki-67 and CD30 values were also evaluated, with intense Ki-67 being associated with poorer prognosis, while CD30 did not show any significant effects. Conversely, Scarisbrick et al.6 found that CD30 positivity was associated with poorer DSS in patients with a T3 tumor phase, which is closely associated with LCT.
T-cell clonality in the skin did not have a prognostic effect on survival; however, it was indeed associated with poorer prognosis when clonal TCR was found in blood (median survival, 68 months; p <0.001). Similarly, Scarisbrick et al.6 observed a 49% clonal match in their cohort of advanced MF/SS and a non-significant statistical trend towards poorer survival in patients with blood clones (49.8 months).
Elevated levels of LDH and B2M were associated with poorer prognosis across the entire cohort. In MF, several studies have found that LDH levels are associated with poor prognosis in advanced stages;6,13,17,18 however, predictive value in the early stages of the disease remains unclear.19 In our cohort, no significant differences regarding survival were found between CD4+, CD8+, or double-negative phenotypes.
ConclusionsIn conclusion, this study reviewed 148 cases diagnosed with MF/SS and found that age > 60 years, SS diagnosis, presence of LCT at diagnosis, FT in early stages, intense Ki-67, clonal TCR in blood, elevated LDH and B2M, and stages IIB, IVA, T3, T4, and N3/Nx were all factors associated with poorer disease prognosis. Stage IVA and the histological presence of initial LCT were the 2 independent predictive factors of adverse prognosis for MF/SS. Additionally, LCT was the variable that most significantly reduced patient survival and was closely associated with tumor skin involvement and stage IIB. However, further research is still needed to better understand the impact of these factors on patient survival.
LimitationsThis was a single-center, retrospective study, and results may lack generalizability and not even be applicable to other populations or geographic regions due to potential differences in demographics and health care. Additionally, MF and SS are rare diseases, which makes it difficult to obtain a representative sample, which could eventually impact the representativeness of the results. The lack of consideration for variability in treatment regimens is another significant limitation, as these treatments can affect disease progression and patient survival.
Conflicts of interestNone declared.