The 7-item RECAP (Recap of Atopic Eczema) questionnaire is used to assess the control of different degrees of eczema severity in patients of all ages. Long-term control of eczema is one of the 4 core outcome domains to be assessed in clinical trials of eczema therapies. After the RECAP was developed in the United Kingdom, it was translated into Chinese, German, Dutch, and French.
ObjectivesTo produce a validated Spanish version of the RECAP questionnaire and, secondarily, to test its content validity in a group of Spanish patients with atopic eczema.
Material and methodsIn a 7-step process we produced 2 forward translations and 1 back translation of the RECAP questionnaire. Experts then held two meetings to reach consensus and draft a Spanish version of the questionnaire. Fifteen adult patients with atopic eczema were interviewed to evaluate the comprehensibility, comprehensiveness, and relevance of the drafted items. These patients also completed the Atopic Dermatitis Control Tool (ADCT), the Dermatology Life Quality Index (DLQI), and the Patient-Oriented Eczema Measure (POEM). Stata software (version 16) was then used to explore the correlations between the patients’ scores on these tools and the RECAP.
ResultsThe patients found the Spanish version of the RECAP to be comprehensible and easy to answer. We observed a strong correlation between results on the Spanish RECAP and the ADCT, and highly significant correlations between the RECAP and the DLQI and POEM tools.
ConclusionsThe culturally adapted Spanish version of the RECAP is linguistically equivalent to the original version of the questionnaire. RECAP scores correlate highly with other patient-reported outcome measures.
RECAP es un cuestionario de siete ítems diseñado para capturar la experiencia del control del eccema atópico en todas las edades y severidades. El control a largo plazo del eccema es uno de los cuatro dominios de resultados principales para los ensayos de eccema atópico. Ha sido desarrollado en el Reino Unido y traducido al chino, al alemán, al holandés y al francés.
ObjetivosEl propósito fue generar una versión española del cuestionario RECAP, y como objetivo secundario, validarlo lingüísticamente y probar su validez de contenido en la población española con eccema atópico.
Material y métodosLlevamos a cabo un proceso de 7 pasos. El cuestionario se tradujo dos veces hacia delante y una hacia atrás. Se celebraron dos reuniones de consenso entre expertos para obtener una versión en español del RECAP. Entrevistamos a 15 pacientes adultos con eccema atópico para evaluar los criterios de comprensibilidad, exhaustividad y relevancia. Al mismo tiempo, proporcionamos a los pacientes los cuestionarios ADCT, DLQI y POEM para realizar la correlación entre ellos y el RECAP, con las herramientas informáticas adecuadas utilizando Stata v.16.
ResultadosLos participantes en el estudio consideraron que la versión española del RECAP era comprensible y fácil de responder. Encontramos una fuerte correlación entre la versión española del cuestionario RECAP y la ADCT, y una correlación muy significativa con el DLQI y el POEM, respectivamente.
ConclusionesLa versión española del RECAP y su adaptación transcultural es lingüísticamente equivalente a la versión original. Muestra una alta correlación con otros PROM existentes.
Atopic eczema, also known as atopic dermatitis, is a pruritic skin disease that generally begins during infancy and follows a clinical course with periods of recurrence and remission. Up to 20% of children and 3% of adults have eczema.1
No tool to assess the patient's experience of control of this condition was available until the end of 2019, with the appearance of the validated RECAP questionnaire to “capture” a patient's perceptions of eczema control.2
A wide range of experts have been attempting to harmonize the use of tools to assess patient-reported outcome (PRO) measures of atopic dermatitis. At the seventh meeting of HOME (Harmonising Outcome Measures for Eczema) in Tokyo, the RECAP questionnaire and the Atopic Dermatitis Control Tool (ADCT)3,4 were selected to use as basic tools for long-term use in this clinical setting.
Facilitating the uptake of the RECAP questionnaire internationally requires linguistically equivalent translations that preserve the content validity of the original version. Content validity, which is to say the degree that a PRO questionnaire faithfully reflects the concepts it purports to measure,5 is considered a tool's most important property. Items in a PRO measurement tool, along with the possible responses and the instructions, must be relevant, complete, and comprehensible in the context of a construct of interest in a specific target population.6
The RECAP questionnaire contains 7 items for a patient or caregiver to answer to capture the patient's experience of eczema control. The patient-reported and the caregiver-reported versions were developed simultaneously to guarantee that the instrument could be used in all age groups. This questionnaire, which was developed in the United Kingdom, can be completed quickly and is freely available to use.2
MethodsThe translation process followed the principles of good practice developed by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) for the cultural adaptation of questionnaires referring to PRO measures.7
The first step was to ensure respect of copyright by contacting the University of Nottingham to obtain permission to use and translate the RECAP. We also invited the developers to participate.
The second step involved obtaining 2 independent forward translations (to peninsular Spanish) from 2 native Spanish speakers with experience in translating PRO measurement tools.
The third step was to discuss discrepancies between the 2 translations, reconcile them, and create a single Spanish version ready for back translation (to English).
In the fourth step a reconciled English back translation was produced, based on 2 back translations by native English speakers who had been living in a Spanish speaking country for many years and worked independently.
The fifth step involved the team's review of the back translation to ensure the conceptual equivalence between the Spanish translation and the original English version.
The sixth step harmonized all the available RECAP translations to ensure the equivalence of concepts between all of them and the original.
The seventh and final step involved a cognitive review of the resulting Spanish version of the RECAP with 15 subjects representing the target population of adults with atopic eczema. This review assessed equivalence and checked the group's perceptions of language issues that might not have been resolved during the translation process. During face-to-face meetings with each subject, we also collected responses not only to the Spanish versions of the RECAP but also to 3 additional questionnaires: the Dermatology Life Quality Index (DLQI), the ADCT, and the Patient-Oriented Eczema Measure (POEM). We used a set of questions (Table 1) to elicit their opinions about the comprehensibility of the RECAP items. The purpose of the cognitive review of the RECAP with adults with atopic eczema was to capture the patients’ opinions of possible difficulties they had when responding to the questions and to use their comments to improve the translation.
Translated questions about the comprehensibility of the Spanish language version of the RECAP questionnaire to capture patient-reported control of eczemaa
| • Overall, the questionnaire seems to be | ||||
|---|---|---|---|---|
| Very easy to understand | Easy to understand | Neither easy nor hard to understand | Difficult to understand | Very difficult to understand |
| • Mark which questions were difficult to understand | |||||||
|---|---|---|---|---|---|---|---|
| None | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| • Mark the phrase that correctly describes why the question(s) weren’t easy to understand | ||
|---|---|---|
| The language in the question(s) was too complicated. | An answer (or answers) didn’t seem me to be properly written. | I didn’t understand the question(s). |
| • If you answered either the second or third question referring to any item in the questionnaire, how would you write the item(s) so that other patients could understand it better?...................................................................................... |
a Translator's note: These translations are to facilitate the reader's comprehension of this article. They are not validated for use with patients.
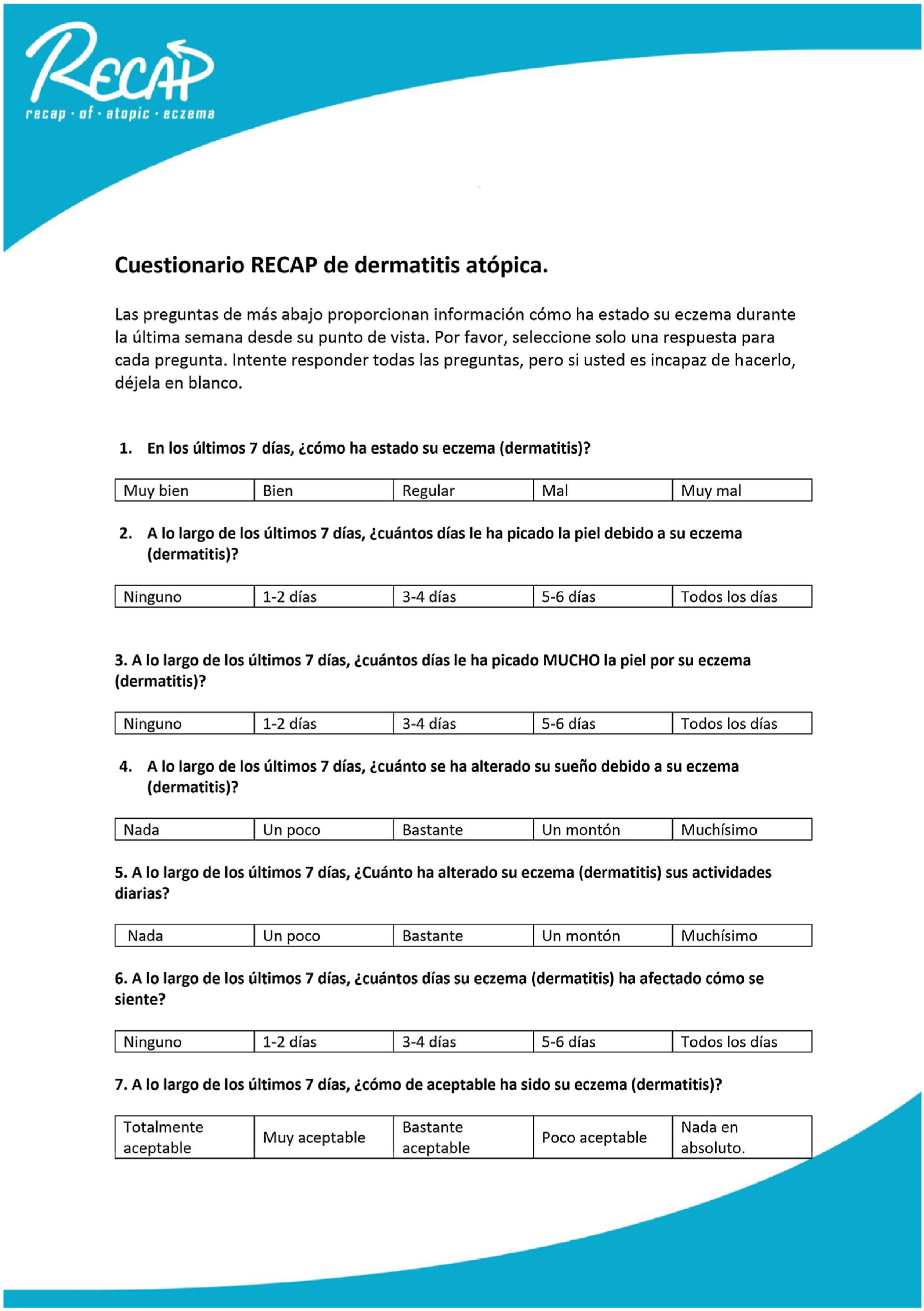
The findings were reviewed and discussed among members of the research team and a final version of the Spanish RECAP (Fig. 1) was produced. Once the definitive Spanish version was set, we registered copyright, including intellectual property rights.
Spanish version of the RECAP questionnaire to capture the adult patient's perception of control of atopic eczema.
Translator's note: For the purposes of reading this article (not for use with English-speaking patients, for which the published original should be used10), this note provides literal translations of the 7 Spanish items as follows: 1. In the last 7 days, how has your eczema (dermatitis) been? (very good, good, so-so, bad, very bad) ■ 2. During the last 7 days, how many of the days has your skin itched because of your eczema (dermatitis)? (none, 1-2 days, 3-4 days, 5-6 days, all the days) ■ 3. How many of the last 7 days has your skin itched A LOT because of your eczema (dermatitis)? (none, 1-2 days, 3-4 days, 5-6 days, all the days) ■ 4. During the last 7 days, how much has your sleep been affected by your eczema (dermatitis)? (not at all, a little, quite a bit, a great deal, enormously) ■ 5. During the last 7 days, how has your eczema (dermatitis) affected your activities of daily living? (not at all, a little, quite a bit, a great deal, enormously) ■ 6. During the last 7 days, how many days has your eczema (dermatitis) affected how you feel? (none, 1-2 days, 3-4 days, 5-6 days, all the days) ■ 7. During the last 7 days, how acceptable has your eczema (dermatitis) been? (completely acceptable, very acceptable, quite acceptable, not very acceptable, absolutely unacceptable).
Statistical correlations between the RECAP scores and those of the ADCT, DLQI, and POEM were also analyzed. We checked the normality of score distributions with the Shapiro–Wilk test; mean (SD) scores were calculated for use as descriptive statistics.
Associations between results from the questionnaires were studied with the Spearman correlation coefficient (ρ). Linear or linearizable models were drawn. We calculated 95% CIs for all statistics.
Stata software (version 16 for Windows) was used for all calculations. The significance level was set at 5%.
ResultsThe mean age of participants in the target population sample was 37.4years (range, 18–59years). Four of the 15 participants were women (26.7%). Four had had atopic eczema since their first year of life; the condition appeared in early childhood (around age 3years) for 6 participants, in puberty (around age 12years) for 2, and in adulthood for 3.
A sample size of 4–6 participants would have been considered sufficient, whereas a sample of 7 or more participants is considered very good according to a group that developed standards for the systematic review of PRO studies (the COSMIN study group for Consensus-based Standards for the Selection of Health Measurement Instruments).8 The individual interview sessions with participants lasted between 15 and 30minutes and included filling out the RECAP questionnaire as well as the additional PRO measurement tools. The patients had generally positive impressions of the RECAP. None suggested that it had important problems. Nor did any of them express negative opinions about any of the questions.
Most of the questions were easy for patients in the target sample to understand. A few changes were introduced to make the phrasing more appropriate for Spanish readers. Examples are changes from the phrase “the last week” (ultima semana in several items) to the more specific phrase of los últimos siete dias (last 7 days) to reflect a cultural difference in how these expressions for the time frame are understood. One patient thought the term aceptable (acceptable) was difficult to understand in one item; that patient would have preferred tolerable and also thought that using the verb tolerar (tolerate) instead of the adjective form would have been better. One participant wanted to include an additional question — “¿En qué zona sintió picor?” (In which area did you feel itching?). However, we concluded that the addition would not be relevant to what the item was measuring.
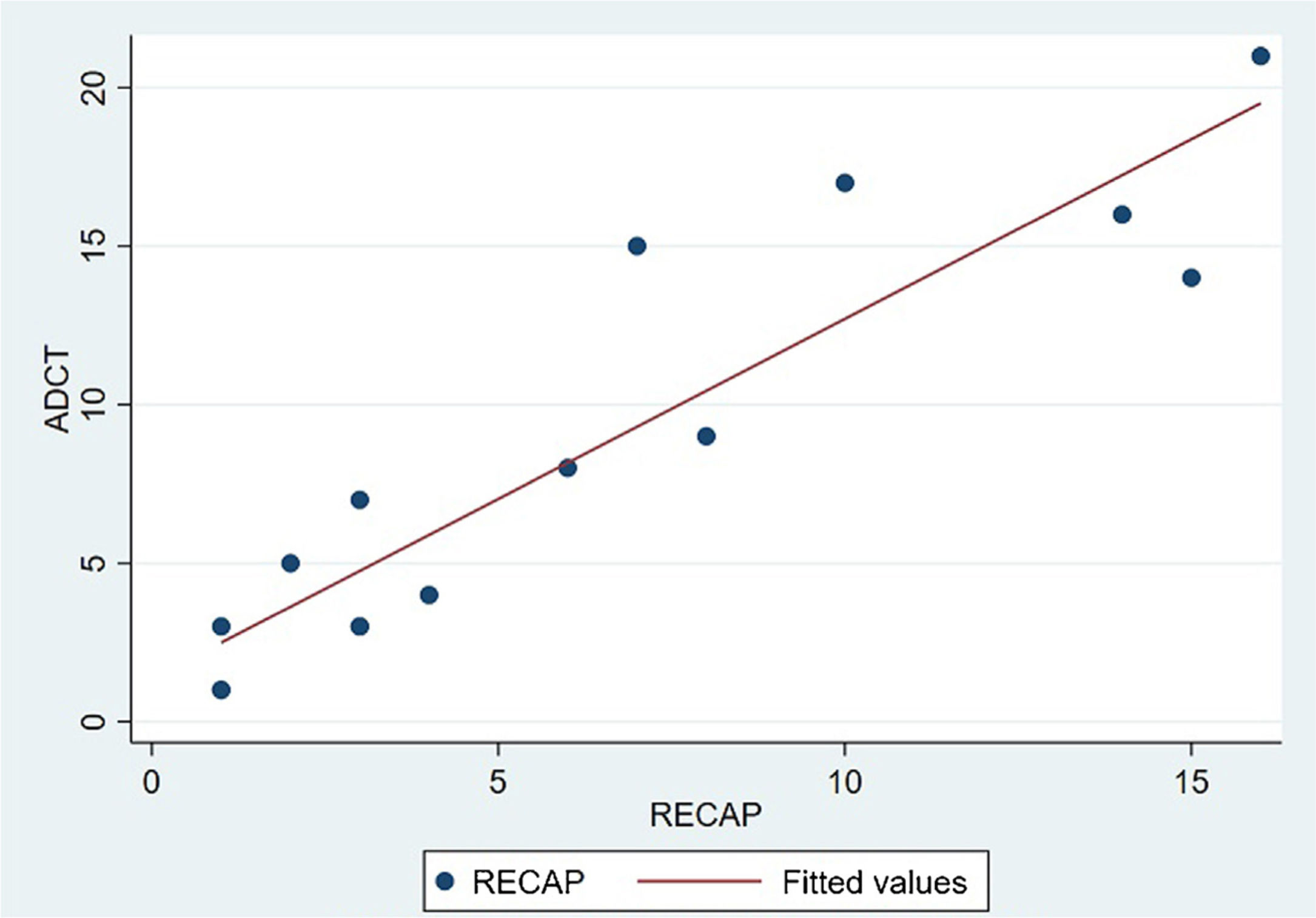
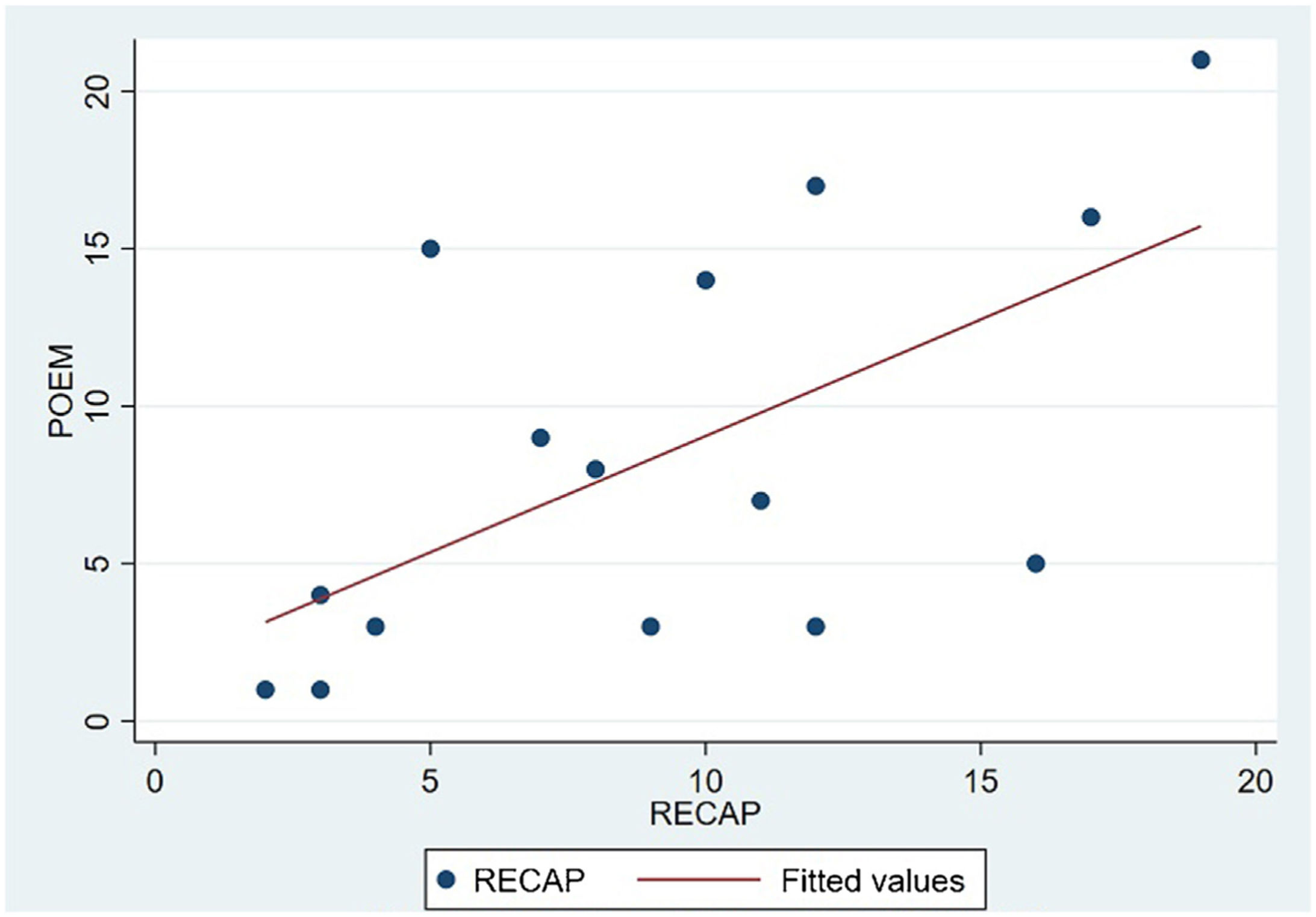
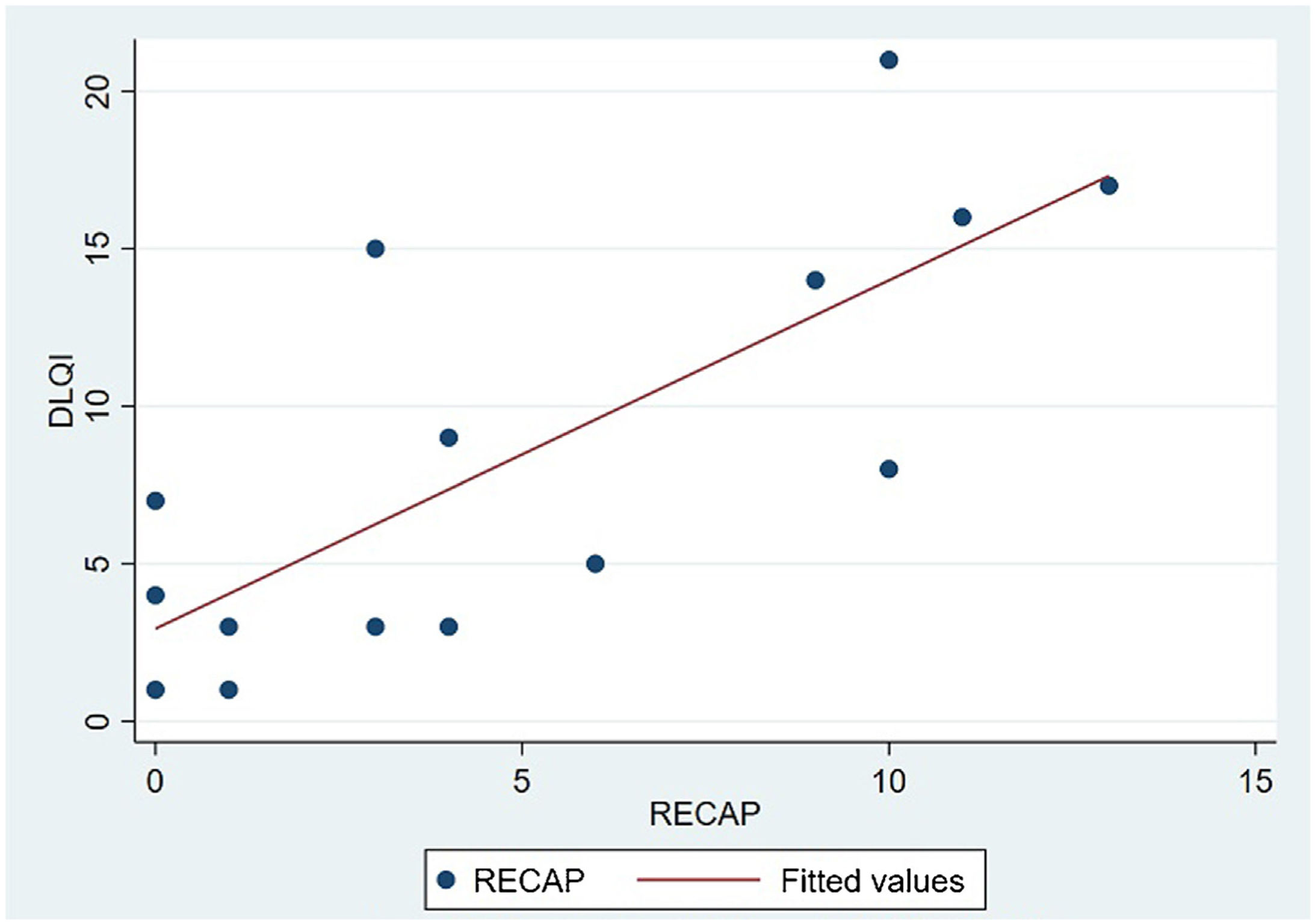
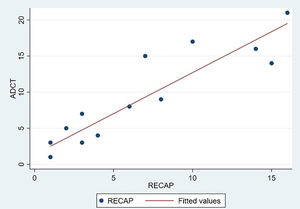
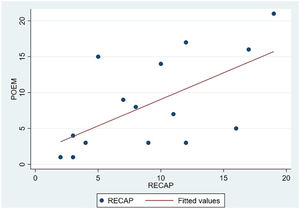
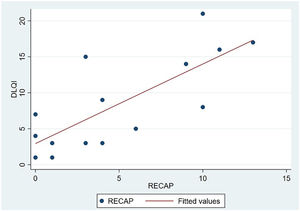
The analysis of statistical associations between scores on the different PRO questionnaires showed that they correlated highly with the RECAP. The highest association was between the ADCT and the RECAP (Spearman's ρ=0.9146), indicating that a higher score on the RECAP was related to a higher score on the ADCT and that both tools measured the same phenomenon (Fig. 2). The correlations between the RECAP and the POEM (ρ=0.6078) and the RECAP and the DLQI (ρ=0.763) were less strong (Figs. 3 and 4, respectively).
The RECAP questionnaire was translated to German and validated by Gabes et al.,9 but the authors did not analyze statistical correlations between their German version and other PRO measurement tools.
The RECAP website10 reports that the questionnaire has also been translated to Chinese, by the Atopic Dermatitis Team of Guangdong Provincial Hospital of Traditional Chinese Medicine; to Dutch, by a team from the University of Groningen and the Erasmus University Medical Center; to French, for Belgium; and to German for Austria by TransPerfect, courtesy of Medidata Solutions. We were unable to locate articles reporting the processes by which these 4 language versions were produced.
We evaluated the content validity of our Spanish translation of the RECAP in 15 adults with atopic dermatitis. The results showed that it is valid regarding content and is linguistically equivalent to the original version already available. This Spanish version is now ready for assessment of its psychometric properties.
We only identified minor cultural issues during our validation, and they were resolved. All of the RECAP items were considered comprehensible, pertinent, and complete, generally speaking.
The instructions and response options were also easily understood by the group of patients from the target population, most of whom were very open when expressing themselves and enjoyed the test experience.
Our use of the COSMIN criteria for this step and the high correlations between the RECAP and other PRO measurement tools, especially the ADCT, support user confidence in the content validity of our translation and cultural adaptation to peninsular Spanish. As far as we know, this is the first Spanish version of the RECAP. This study only targeted cultural adaptation for native peninsular Spanish speakers; one patient of Chinese descent was included, but he was born and educated in Spain. This Spanish version of the RECAP might have received lower assessments of comprehensibility and/or equivalence from Spanish-speaking patients from countries other than Spain. If that were the case, a cultural adaptation of the translation would have to be done for speakers of different varieties of Spanish.
ConclusionThe process we followed in this study produced a Spanish version of the RECAP questionnaire that is linguistically equivalent to the original version for use in our practice setting. The Spanish RECAP is available for use provided a request is sent to the corresponding author. Correlations between the RECAP and the Spanish versions of the ADCT, DLQI, and POEM tools clearly demonstrate the RECAP's validity.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Many thanks to Dr. Ignacio García Doval, director of the Research Section of the Spanish Academy of Dermatology and Venereology (AEDV), for his support for and help with this project. We also thank Noelle Acheson and Joan Guitar for their help with translation.