Acne vulgaris is a highly prevalent skin condition that can have a considerable impact on quality of life. Topical retinoids, benzoyl peroxide, different antibiotics, and isotretinoin have been the therapeutic pillars for decades. The recent approval of a new antibiotic by the US Food and Drug Administration for moderate to severe forms of acne, together with other new drugs that are being developed, may expand the therapeutic arsenal.
Sarecycline is a narrow-spectrum tetracycline-derived antibiotic (Table 1) with a lower probability of generating antibiotic resistance. The clinical trials SC1401 (n = 968) and SC1402 (n = 1034) included patients with moderate to severe acne, who were randomized to receive sarecycline or a placebo. The main parameter assessed was the Investigator’s Global Assessment (IGA) success, equivalent to a reduction of ≥2 points on the IGA and reaching a score of 0 (no lesions) or 1 (hardly any lesions) after 12 weeks. This criterion was achieved by 21.9% and 22.6%, respectively, in the group treated with sarecycline, compared to 10.5% and 15.3% in the placebo group (P < .0001 and P = .0038). The most common adverse effects were nausea, nasopharyngitis, and headaches1. Clinical trial PR-10411 (n = 285) compared the effectiveness of 3 different dosages of sarecycline (0.75, 1.5, and 3 mg/kg) to a placebo and assessed the reduction in lesions at week 12. Significant results were achieved in the groups receiving 1.5 and 3 mg/kg, with a reduction of lesions greater than 50%; no differences were observed between the 2 groups with higher dosages2. These studies led to the approval of sarecycline, the first systemic drug approved for acne by the US Food and Drug Administration in more than 40 years. Another alternative novel antibiotic is 4% topical minocycline, a tetracycline antibiotic with bactericidal action against Cutibacterium acnes, and practically no systemic absorption. Randomized trial FX2017-22 (n = 1488) compared minocycline with a placebo, with significant reduction in inflammatory lesions at week 12 (P < .0001) and improvement of the IGA score (P < .0001) in the intervention group3.
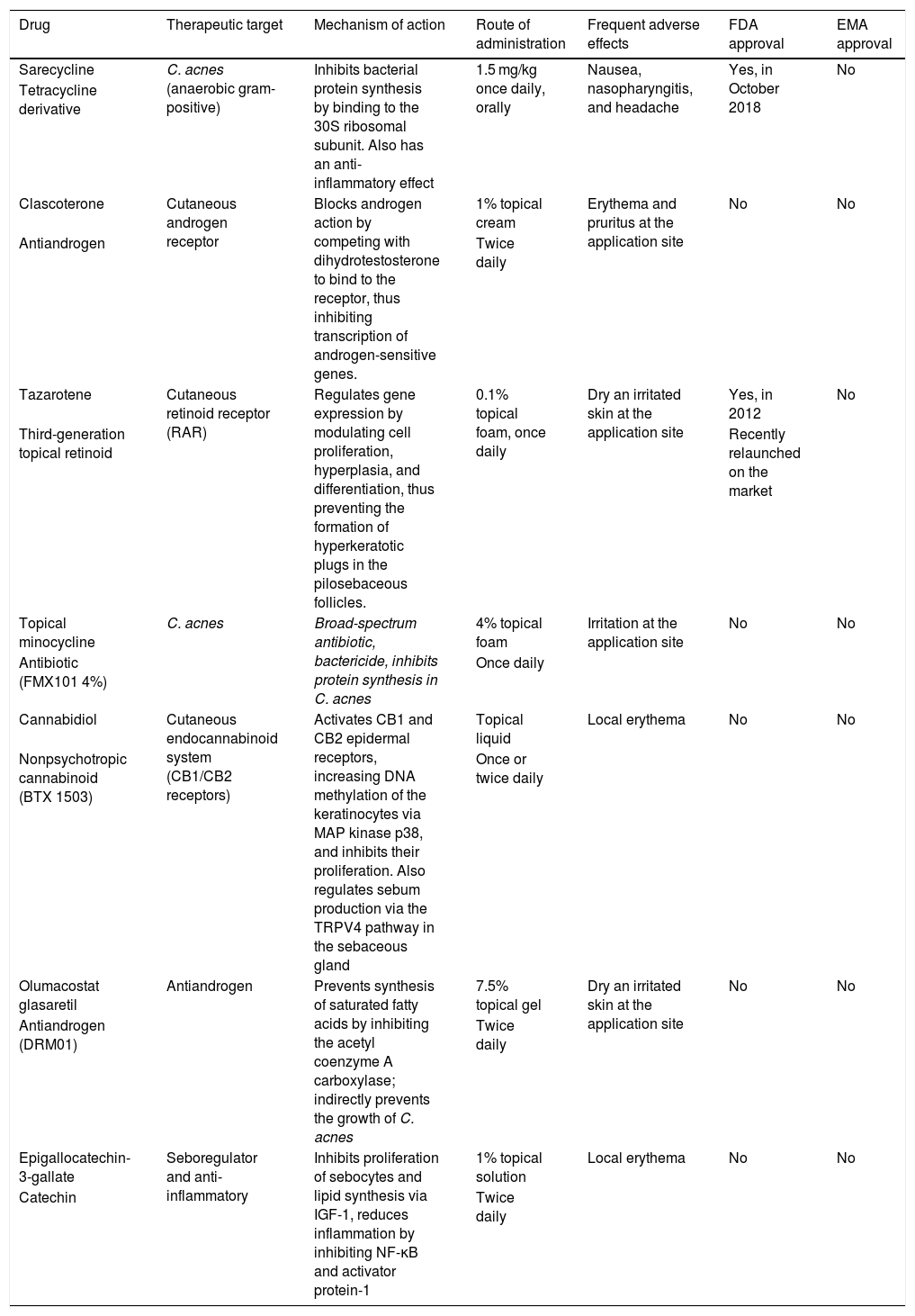
Characteristics of the new drugs approved or under development for the treatment of acne.
| Drug | Therapeutic target | Mechanism of action | Route of administration | Frequent adverse effects | FDA approval | EMA approval |
|---|---|---|---|---|---|---|
| Sarecycline | C. acnes (anaerobic gram-positive) | Inhibits bacterial protein synthesis by binding to the 30S ribosomal subunit. Also has an anti-inflammatory effect | 1.5 mg/kg once daily, orally | Nausea, nasopharyngitis, and headache | Yes, in October 2018 | No |
| Tetracycline derivative | ||||||
| Clascoterone | Cutaneous androgen receptor | Blocks androgen action by competing with dihydrotestosterone to bind to the receptor, thus inhibiting transcription of androgen-sensitive genes. | 1% topical cream | Erythema and pruritus at the application site | No | No |
| Antiandrogen | Twice daily | |||||
| Tazarotene | Cutaneous retinoid receptor (RAR) | Regulates gene expression by modulating cell proliferation, hyperplasia, and differentiation, thus preventing the formation of hyperkeratotic plugs in the pilosebaceous follicles. | 0.1% topical foam, once daily | Dry an irritated skin at the application site | Yes, in 2012 | No |
| Third-generation topical retinoid | Recently relaunched on the market | |||||
| Topical minocycline | C. acnes | Broad-spectrum antibiotic, bactericide, inhibits protein synthesis in C. acnes | 4% topical foam | Irritation at the application site | No | No |
| Antibiotic (FMX101 4%) | Once daily | |||||
| Cannabidiol | Cutaneous endocannabinoid system (CB1/CB2 receptors) | Activates CB1 and CB2 epidermal receptors, increasing DNA methylation of the keratinocytes via MAP kinase p38, and inhibits their proliferation. Also regulates sebum production via the TRPV4 pathway in the sebaceous gland | Topical liquid | Local erythema | No | No |
| Nonpsychotropic cannabinoid (BTX 1503) | Once or twice daily | |||||
| Olumacostat glasaretil | Antiandrogen | Prevents synthesis of saturated fatty acids by inhibiting the acetyl coenzyme A carboxylase; indirectly prevents the growth of C. acnes | 7.5% topical gel | Dry an irritated skin at the application site | No | No |
| Antiandrogen (DRM01) | Twice daily | |||||
| Epigallocatechin-3-gallate | Seboregulator and anti-inflammatory | Inhibits proliferation of sebocytes and lipid synthesis via IGF-1, reduces inflammation by inhibiting NF-κB and activator protein-1 | 1% topical solution | Local erythema | No | No |
| Catechin | Twice daily |
Abbreviations: C. acnes indicates Cutibacterium acnes; EMA, European Medicines Agency; FDA, US Food and Drug Administration; IGF-1, growth factors similar to type-1 insulin; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; RAR, retinoic acid receptor.
Greater understanding has recently been achieved of sebum production, which is modulated by androgens, the endocannabinoid system, and multiple proinflammatory mediators such as histamine and leukotrienes, and this has opened up new areas of therapeutic research. Among topical treatments, clascoterone, a competitive antagonist of the androgen receptor, showed superiority compared to placebo in 2 phase-III trials, evaluated by means of the IGA score4. Clascoterone would be the first antiandrogen without systemic hormonal effects and could be used in men. Cannabidiol is a nonpsychotropic cannabinoid with anti-inflammatory, sebostatic, and antimicrobial action, which has been shown to be well tolerated in phase-Ib trials. A phase-II trial (drug BTX1503) is currently finishing that evaluated the effectiveness of topical cannabidiol in acne. In systemic treatments, a randomized clinical trial (n = 100) showed that the combination of levocetirizine (a nonsedating antihistamine) and isotretinoin was superior and had fewer adverse effects than treatment with isotretinoin alone5. Similar results have been obtained using desloratidine6. Antihistamines modulate sebum production and reduce the adverse effects of the retinoids.
The development of these new drugs may change the treatment of acne. It is essential to be aware of them and to achieve greater efficacy and safety in the treatment of this condition.
Please cite this article as: Alamon-Reig F, Bois MC, Morgado-Carrasco D. FR - Nuevos fármacos para el manejo del acné. Actas Dermosifiliogr. 2022;113:86–88.