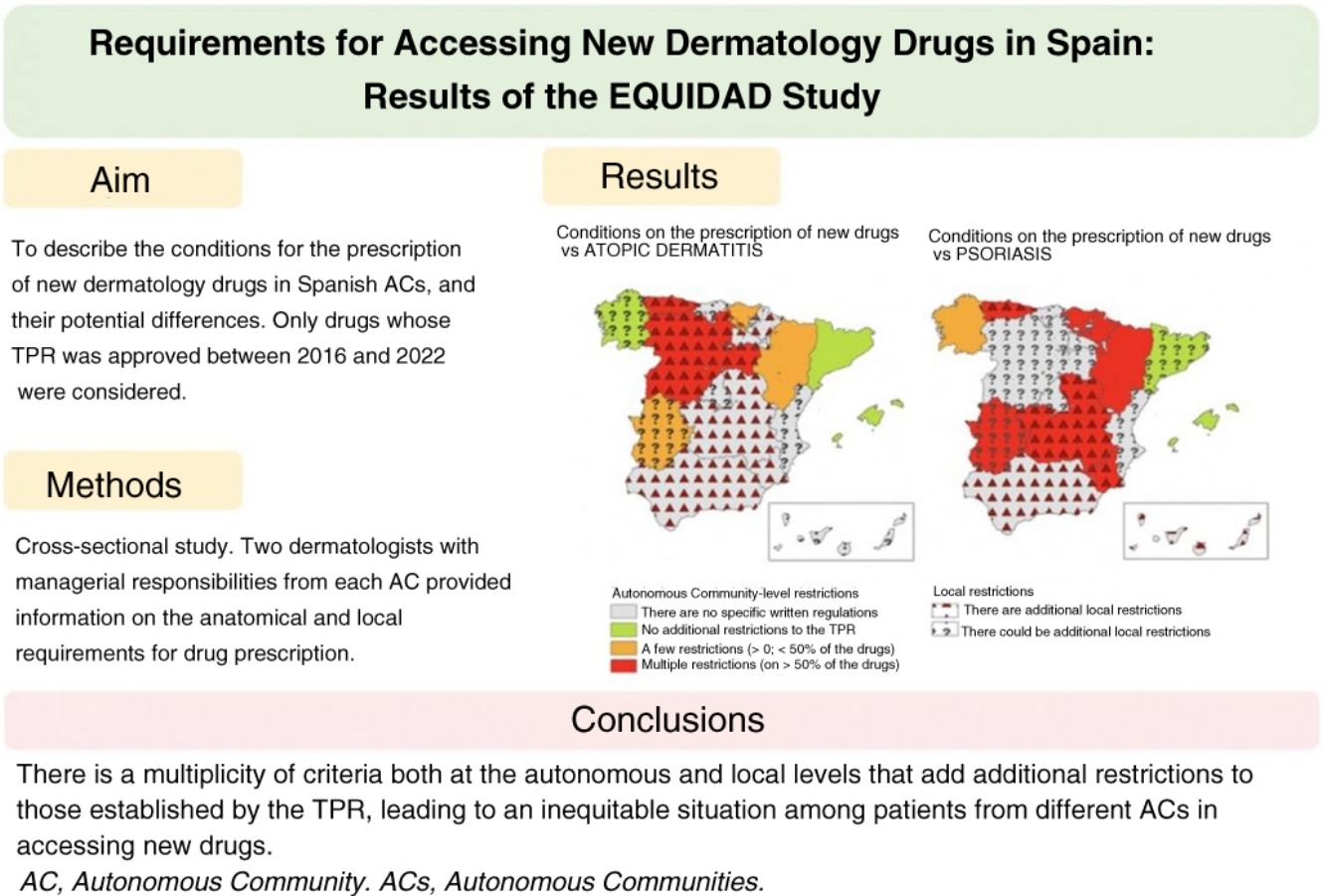
Although the Spanish Ministry of Health prepares national therapeutic positioning reports (TPRs) and drug reimbursement policies, each of the country's 17 autonomous communities (ACs) is responsible for health care services and prescription requirements in its territory. The aim of the EQUIDAD study was to describe and explore potential differences in prescription requirements for new dermatology drugs across the autonomous communities.
Material and methodsCross-sectional study conducted in April and May, 2023. Two dermatologists with management responsibilities from each autonomous community reported on territorial and more local prescription requirements for drugs covered by national TPRs issued between 2016 and 2022.
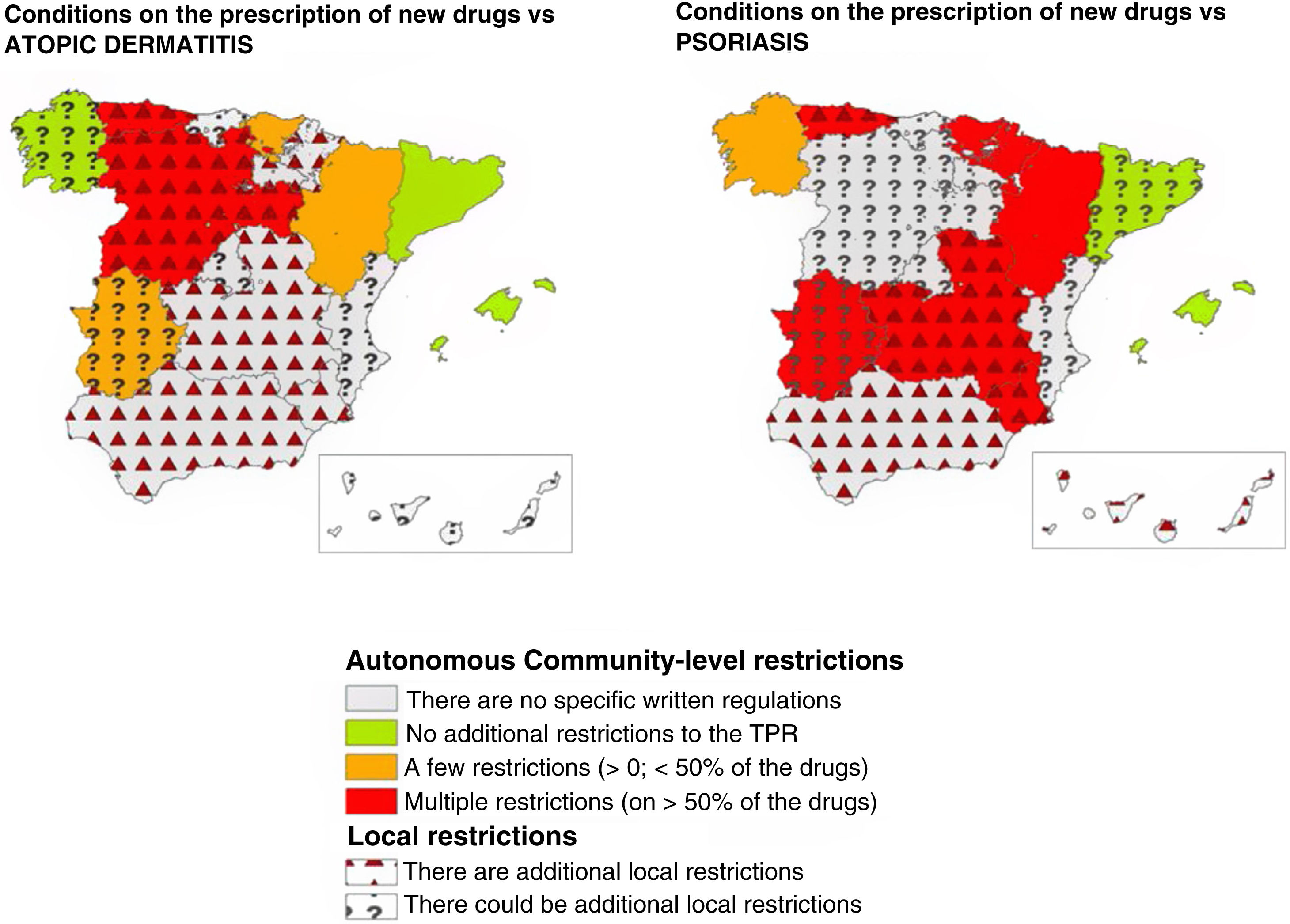
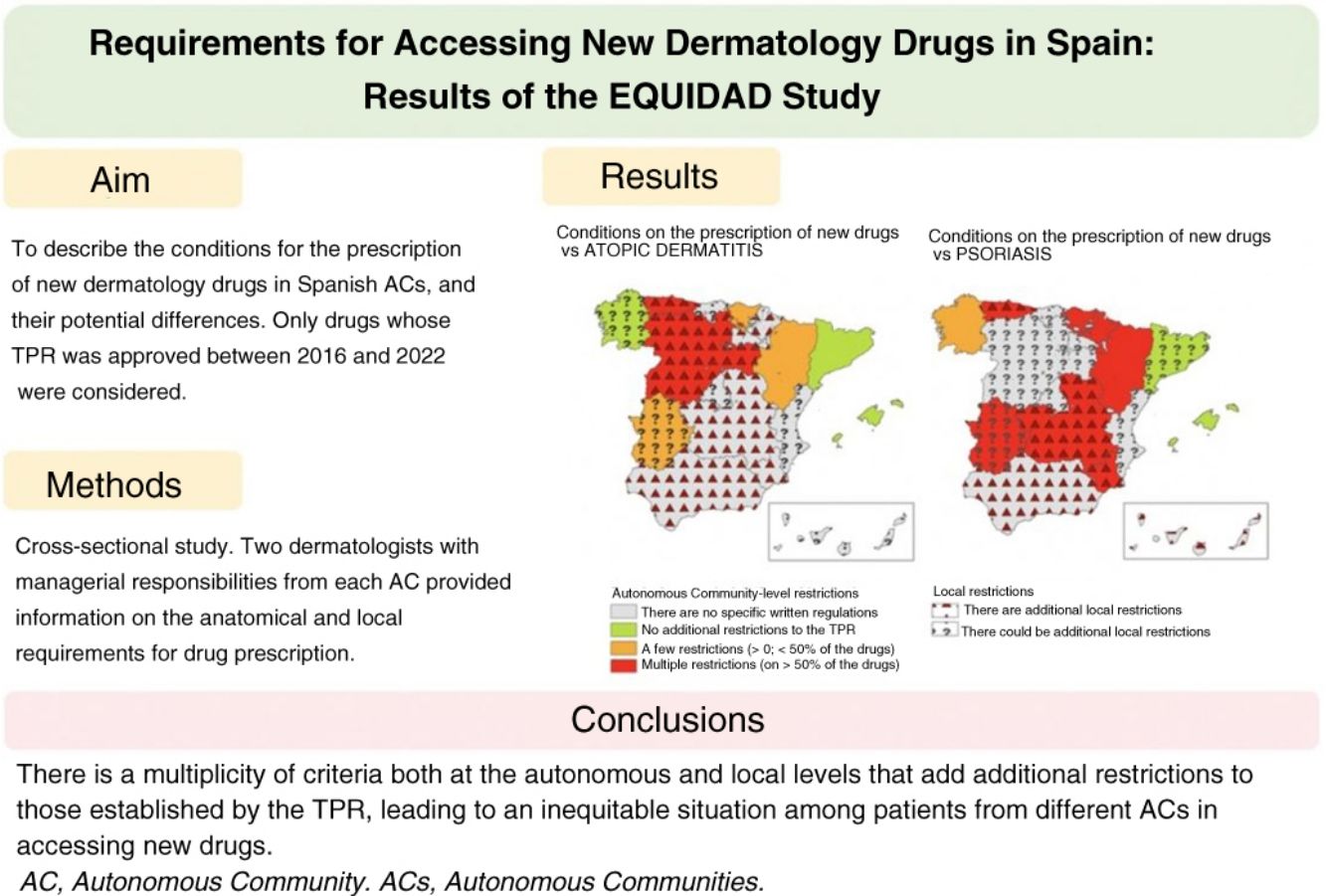
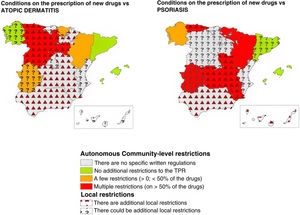
ResultsThirty-three researchers from 17 autonomous communities participated. The data submitted revealed between-community inequities in access to new drugs. Overall, 64.7% of the regions imposed additional prescription requirements to those mentioned in the TPRs for psoriasis. This percentage was lower for atopic dermatitis (35.3%) and melanoma (11.8%). The most common requirement for accessing a new drug was a previous prescription for another drug. Differences and additional requirements were also detected at the local level (i.e., differences between hospitals within the same autonomous community).
ConclusionsSpain's autonomous communities have multiple regional and local prescription requirements that are not aligned with national TPR recommendations. These differences result in inequitable access to new drugs for both patients and practitioners across Spain.
En España, aunque el Ministerio de Sanidad elabora el informe de posicionamiento terapéutico (IPT) y las condiciones de reembolso de los fármacos, las Comunidades Autónomas (CC. AA.) gestionan los servicios de salud y deciden sobre las condiciones de prescripción en su ámbito territorial. El objetivo del estudio EQUIDAD fue describir los condicionantes para la prescripción de los nuevos fármacos en Dermatología en las CC. AA. y sus posibles diferencias.
Material y métodosEstudio transversal realizado en abril-mayo del 2023. Dos dermatólogos con responsabilidades directivas de cada Comunidad Autónoma (C. A.) informaron sobre los condicionantes autonómicos y locales en la prescripción de los fármacos cuyo IPT para el tratamiento de enfermedades dermatológicas fue publicado en los años 2016-2022. Los datos fueron recogidos mediante un cuestionario online.
ResultadosUn total de 33 investigadores de 17 CC. AA. participaron en el estudio. Se observaron inequidades entre CC. AA. para el acceso a los nuevos fármacos. Existieron condicionantes autonómicos adicionales al IPT en psoriasis en el 64,7% de las CC. AA., siendo este porcentaje menor en dermatitis atópica (35,3%) o melanoma (11,8%). El más frecuente fue el requisito de un orden de prescripción previo para el uso del fármaco. En algunas CC. AA. se detectaron además variaciones y condicionantes locales (diferencias entre centros de una misma C. A.).
ConclusionesExiste una multiplicidad de criterios tanto a nivel autonómico como local que añade restricciones adicionales a las establecidas por los IPT y que plantean una situación de inequidad entre los pacientes y los profesionales de las diferentes CC. AA. en el acceso a los nuevos fármacos.
The advancements made in the pathogenic understanding of numerous dermatoses have impacted the development, approval, and commercialization of new dermatological drugs in recent years,1,2 which is a significant move forward in the management of patients with skin diseases. The introduction of newly funded drugs has a tremendous impact on spending for the Spanish National Health System.3
To guarantee the safety, efficacy, and sustainability of drug use, the process of approving new drugs follows a well-structured series of steps in Spain.4 After the drug is approved by the European Medicines Agency, a decision-making process on its price and funding in the Spanish National Health System is initiated, along with its potential inclusion in the routine clinical practice.5 The Therapeutic Positioning Report (TPR) is precisely drafted during this process. The TPR outlines the criteria on the use and monitoring of the drug,6 while placing it in relation to the therapeutic alternatives available based on efficiency and safety criteria, after the Inter-ministerial Committee on Pricing of Medicines and Healthcare Products (CIMP) has set the top price allowed for approved drugs, which is funded by the health services of the various Autonomous Communities (ACs).
Former studies conducted within the Spanish health care system have shown differences in access to drugs across various ACs.7,8 The reasons for these differences can vary, including the existence of additional criteria beyond the TPR regarding drug funding in some ACs. or, directly, the non-approved prescription of certain drugs in specific regions of the national territory, even when their use has been approved by the TPR.
Finally, we should mention that, in addition to regional factors affecting drug prescription, there are various local factors involved. The individualized management of each hospital pharmacy unit allows for the establishment of specific protocols at local level in some regions, which could lead to stricter restrictions to access to new dermatology drugs in our setting.9,10
The objective of the EQUITY trial was to describe the requirements at each Autonomous Community (AC) level for the prescription of new drugs whose TPR was published by the Spanish Ministry of Health and the Spanish Agency of Medicines and Medical Devices (AEMPS) from 2016 through 2022 regarding dermatological indications in Spain, and the existence of possible geographical differences regarding access to these drugs across the Spanish territory.
Materials and methodsDesign: this was a cross-sectional study. Therefore, an online questionnaire was created and sent through the Spanish Academy of Dermatology and Venereology (AEDV) back in March 2023. Researchers were asked on the prescription requirements of the drugs included in the study. The researchers responsible for each AC independently reviewed and filled out the information available from their respective AC from April through May 2023. Any discrepancies found within each AC were resolved through joint reevaluation of the questionnaire with the researchers involved.
Drugs included in the study: the study included drugs for which a financing resolution was issued in the TPR on the management of dermatological diseases from 2016 through 2022. During this period, drugs were approved for the following dermatological diseases: atopic dermatitis, psoriasis, basal cell carcinoma, Merkel cell carcinoma, and melanoma.
Participating researchers: a total of 2 participants from each AC studied were included. Participant researchers were selected by the study coordinators on the following criteria: a) physicians currently practicing dermatology, preferably chiefs of staff, or alternatively specialist physicians in their respective AC, and b) with enough knowledge on drug financing at regional level.
Variables of interest: the following variables on the existing requirements for pharmacological prescription of the aforementioned drugs were collected:
Specific requirements for prescription across various ACs for each of the drugs under study. The following options were taken into consideration: the requirement of strict compliance with the TPR by the different ACs, or the existence of additional restrictions beyond the TPR, or the absence of requirements for drug prescription in cases where no specific official documents existed. In case of additional restrictions beyond those imposed by the TPR, inquiries were made about their nature (prescription order, drug unavailability, limited prescription to non-dermatologist specialists, or others).
The existence of specific requirements for prescription at local level in some hospitals across various ACs. The following options were considered: they exist, they do not exist, or I don’t know if they exist.
Statistical analysis: descriptive statistics were used to evaluate the characteristics of the collected data. Qualitative variables were expressed as relative and absolute frequency distributions. All analyses were performed using STATA statistical software package (Stata Corp. 2021. Stata Statistical Software: version 17. College Station, TX, United States). ArcGIS (ESRI. 2020. Environmental Systems Research Institute: version 10.8. Redlands, CA, United States) was used to create the maps.
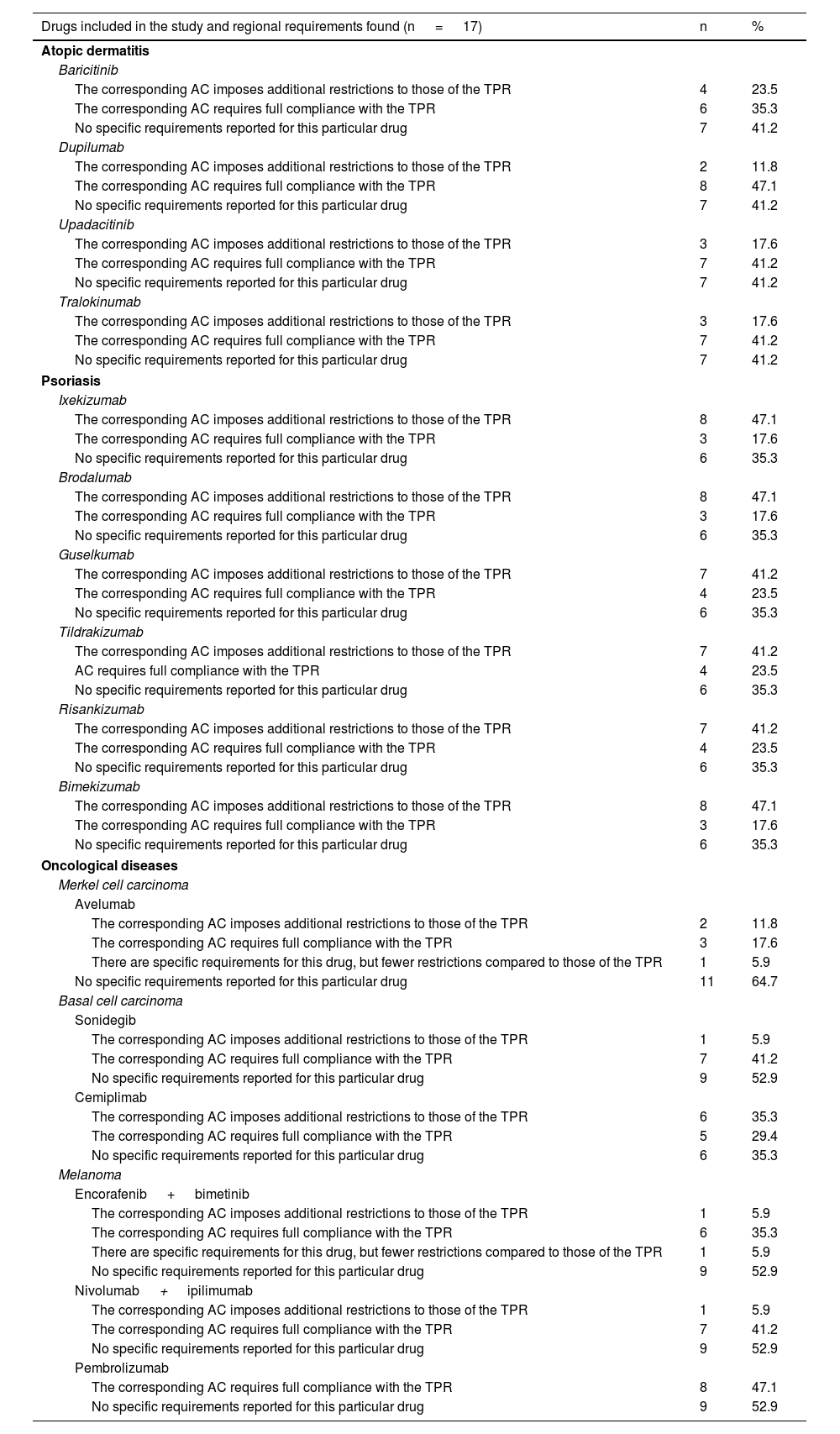
ResultsA total of 34 researchers distributed across the 17 evaluated ACs were contacted. A total of 33 responses were received (97%). One AC (Foral Community of Navarre) was evaluated by 1 researcher only. The professional profile of the surveyed researchers included 66.7% chiefs of staff, 21.2% department heads, and 12.1% specialist physicians. The studied drugs are listed in Table 1.
Summary of active ingredients approved by the AEMPS from 2016 through 2022 with an indication to treat dermatological diseases and specific regional requirements found in the study.
| Drugs included in the study and regional requirements found (n = 17) | n | % |
|---|---|---|
| Atopic dermatitis | ||
| Baricitinib | ||
| The corresponding AC imposes additional restrictions to those of the TPR | 4 | 23.5 |
| The corresponding AC requires full compliance with the TPR | 6 | 35.3 |
| No specific requirements reported for this particular drug | 7 | 41.2 |
| Dupilumab | ||
| The corresponding AC imposes additional restrictions to those of the TPR | 2 | 11.8 |
| The corresponding AC requires full compliance with the TPR | 8 | 47.1 |
| No specific requirements reported for this particular drug | 7 | 41.2 |
| Upadacitinib | ||
| The corresponding AC imposes additional restrictions to those of the TPR | 3 | 17.6 |
| The corresponding AC requires full compliance with the TPR | 7 | 41.2 |
| No specific requirements reported for this particular drug | 7 | 41.2 |
| Tralokinumab | ||
| The corresponding AC imposes additional restrictions to those of the TPR | 3 | 17.6 |
| The corresponding AC requires full compliance with the TPR | 7 | 41.2 |
| No specific requirements reported for this particular drug | 7 | 41.2 |
| Psoriasis | ||
| Ixekizumab | ||
| The corresponding AC imposes additional restrictions to those of the TPR | 8 | 47.1 |
| The corresponding AC requires full compliance with the TPR | 3 | 17.6 |
| No specific requirements reported for this particular drug | 6 | 35.3 |
| Brodalumab | ||
| The corresponding AC imposes additional restrictions to those of the TPR | 8 | 47.1 |
| The corresponding AC requires full compliance with the TPR | 3 | 17.6 |
| No specific requirements reported for this particular drug | 6 | 35.3 |
| Guselkumab | ||
| The corresponding AC imposes additional restrictions to those of the TPR | 7 | 41.2 |
| The corresponding AC requires full compliance with the TPR | 4 | 23.5 |
| No specific requirements reported for this particular drug | 6 | 35.3 |
| Tildrakizumab | ||
| The corresponding AC imposes additional restrictions to those of the TPR | 7 | 41.2 |
| AC requires full compliance with the TPR | 4 | 23.5 |
| No specific requirements reported for this particular drug | 6 | 35.3 |
| Risankizumab | ||
| The corresponding AC imposes additional restrictions to those of the TPR | 7 | 41.2 |
| The corresponding AC requires full compliance with the TPR | 4 | 23.5 |
| No specific requirements reported for this particular drug | 6 | 35.3 |
| Bimekizumab | ||
| The corresponding AC imposes additional restrictions to those of the TPR | 8 | 47.1 |
| The corresponding AC requires full compliance with the TPR | 3 | 17.6 |
| No specific requirements reported for this particular drug | 6 | 35.3 |
| Oncological diseases | ||
| Merkel cell carcinoma | ||
| Avelumab | ||
| The corresponding AC imposes additional restrictions to those of the TPR | 2 | 11.8 |
| The corresponding AC requires full compliance with the TPR | 3 | 17.6 |
| There are specific requirements for this drug, but fewer restrictions compared to those of the TPR | 1 | 5.9 |
| No specific requirements reported for this particular drug | 11 | 64.7 |
| Basal cell carcinoma | ||
| Sonidegib | ||
| The corresponding AC imposes additional restrictions to those of the TPR | 1 | 5.9 |
| The corresponding AC requires full compliance with the TPR | 7 | 41.2 |
| No specific requirements reported for this particular drug | 9 | 52.9 |
| Cemiplimab | ||
| The corresponding AC imposes additional restrictions to those of the TPR | 6 | 35.3 |
| The corresponding AC requires full compliance with the TPR | 5 | 29.4 |
| No specific requirements reported for this particular drug | 6 | 35.3 |
| Melanoma | ||
| Encorafenib + bimetinib | ||
| The corresponding AC imposes additional restrictions to those of the TPR | 1 | 5.9 |
| The corresponding AC requires full compliance with the TPR | 6 | 35.3 |
| There are specific requirements for this drug, but fewer restrictions compared to those of the TPR | 1 | 5.9 |
| No specific requirements reported for this particular drug | 9 | 52.9 |
| Nivolumab + ipilimumab | ||
| The corresponding AC imposes additional restrictions to those of the TPR | 1 | 5.9 |
| The corresponding AC requires full compliance with the TPR | 7 | 41.2 |
| No specific requirements reported for this particular drug | 9 | 52.9 |
| Pembrolizumab | ||
| The corresponding AC requires full compliance with the TPR | 8 | 47.1 |
| No specific requirements reported for this particular drug | 9 | 52.9 |
TPR, Therapeutic Positioning Report.
* The requirements described are based on the presence of specific regional guidelines, and do not include possible local restrictions at hospital level.
Discrepancies were found among respondents in 15 out of the 16 ACs that had 2 researchers, which resolved according to the study protocol. The most common reason for the discrepancies was uncertainty on the origin of the restrictions (whether regional or local) since, in multiple centers, hospital-level requirements differed from regional guidelines, or the presence of hospital guidelines confused the existence of regional written regulations, resulting in differences in drug prescription protocols within the same AC.
After resolving the discrepancies found, different access requirements to new dermatology drugs were seen across various ACs. The number of ACs where the existence of autonomous requirements added to those of the TPR varied depending on the drug, ranging from 5.9% for drug combinations such as nivolumab+ipilimumab, or encorafenib+binimetinib to 11.8% for dupilumab, or 17.6% for upadacitinib, with higher rates being reported for psoriasis drugs such as ixekizumab, brodalumab, or bimekizumab (47.1%) (Table 1).
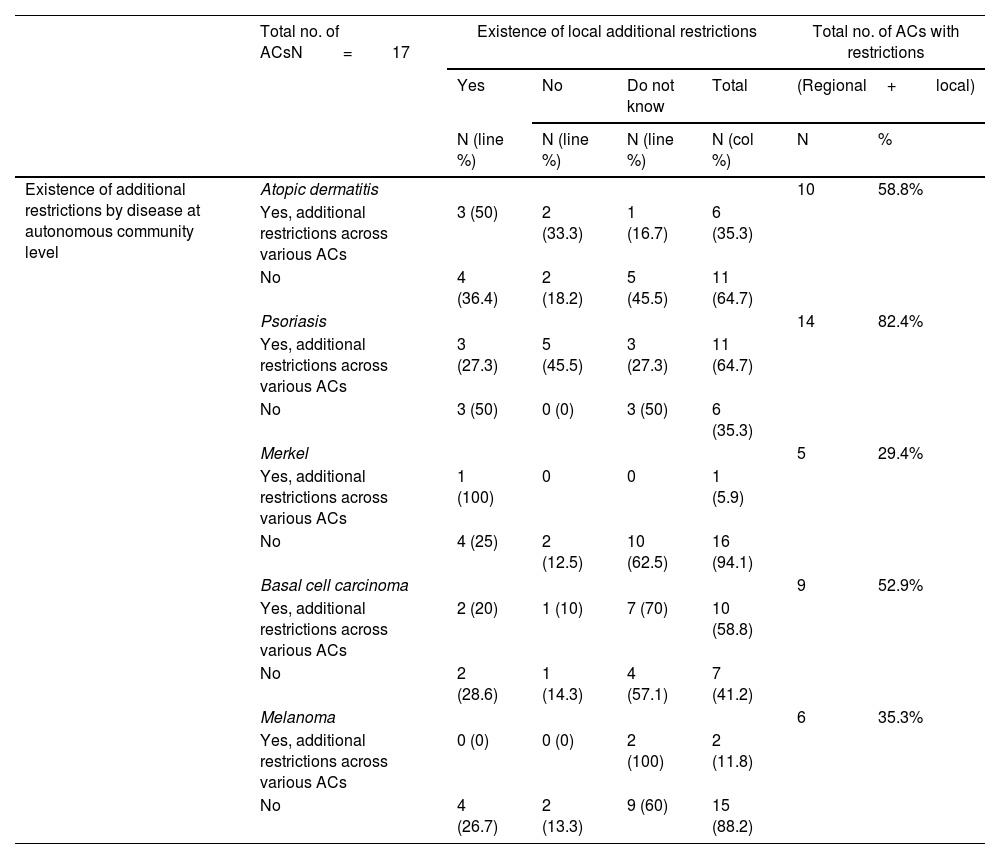
These data were similar to those from the assessment grouped by diseases (Table 2). Psoriasis drugs were the ones most widely affected by autonomous restrictions added to those of the TPR (64.7% of all ACs), with fewer autonomous restrictions for drugs used to treat atopic dermatitis (35.3%), melanoma (11.8%), or Merkel cell carcinoma (5.9%). Detailed data for each AC, and each drug can be found in the supplementary data (tables S1, S2, and S3, annex).
Summary of additional restrictions to the TPR in Spain regarding new drug prescription (both at official regional guidelines level and local hospital-level restrictions).
| Total no. of ACsN=17 | Existence of local additional restrictions | Total no. of ACs with restrictions | |||||
|---|---|---|---|---|---|---|---|
| Yes | No | Do not know | Total | (Regional+local) | |||
| N (line %) | N (line %) | N (line %) | N (col %) | N | % | ||
| Existence of additional restrictions by disease at autonomous community level | Atopic dermatitis | 10 | 58.8% | ||||
| Yes, additional restrictions across various ACs | 3 (50) | 2 (33.3) | 1 (16.7) | 6 (35.3) | |||
| No | 4 (36.4) | 2 (18.2) | 5 (45.5) | 11 (64.7) | |||
| Psoriasis | 14 | 82.4% | |||||
| Yes, additional restrictions across various ACs | 3 (27.3) | 5 (45.5) | 3 (27.3) | 11 (64.7) | |||
| No | 3 (50) | 0 (0) | 3 (50) | 6 (35.3) | |||
| Merkel | 5 | 29.4% | |||||
| Yes, additional restrictions across various ACs | 1 (100) | 0 | 0 | 1 (5.9) | |||
| No | 4 (25) | 2 (12.5) | 10 (62.5) | 16 (94.1) | |||
| Basal cell carcinoma | 9 | 52.9% | |||||
| Yes, additional restrictions across various ACs | 2 (20) | 1 (10) | 7 (70) | 10 (58.8) | |||
| No | 2 (28.6) | 1 (14.3) | 4 (57.1) | 7 (41.2) | |||
| Melanoma | 6 | 35.3% | |||||
| Yes, additional restrictions across various ACs | 0 (0) | 0 (0) | 2 (100) | 2 (11.8) | |||
| No | 4 (26.7) | 2 (13.3) | 9 (60) | 15 (88.2) | |||
ACs, autonomous communities.
The restrictions added to those of the TPR found for new dermatology drugs at AC level were of 2 types mainly. Firstly, the most widely described additional requirement by researchers was that of a prior prescription order (tables S1, S2, annex), meaning that patients had to receive certain treatments before being eligible to receive the drug, even if this was not anticipated by the TPR. This was reported in 47.1% and 29.4% of all ACs for psoriasis and atopic dermatitis drugs, respectively. In the case of atopic dermatitis, the prescription order varied across various ACs (table S1, annex). Regarding psoriasis, the most common requirement was the use of biosimilar drugs or anti-TNF drugs in the first place (table S2, annex). Secondly, the following requirements were reported: not being able to prescribe the drug under any circumstances whatsoever, which occurred in up to 3 ACs for certain psoriasis drugs such as bimekizumab, 2 ACs for certain atopic dermatitis drugs such as tralokinumab (table S1, annex), or the fact that the prescription was limited to a different specialty such as medical oncology for oncology drugs (reported in up to 35.2% of all ACs for cemiplimab).
Regarding the use of drugs vs cutaneous oncological processes, although most ACs did not have specific written requirements for most of the studied drugs (table S3, annex), the practical prescription of these drugs fell exclusively within the scope of Medical Oncology, making it virtually impossible for dermatologists—the specialists who treat patients when the tumor is localized exclusively at skin level and are involved in all stages of the disease at the follow-up. We do not know if this inability to prescribe is backed by any official autonomous or local documents.
Another requirement at AC level found in the study was the need to keep spending per patient-year within certain limits in some ACs (table S2, annex). In this regard, the choice of the drug would be left to the physician's discretion as long as the overall cost of the drug remained within the range required, which was not publicly accessible in official documents.
Researchers acknowledged the existence of local AC-based requirements in some hospitals in 41.2% of all ACs for atopic dermatitis, 35.3% for psoriasis, 29.4% for Merkel cell carcinoma, and 23.5% for drugs vs basal cell carcinoma and melanoma. The rate of researchers who did not know whether there were local additional requirements or not was higher in oncological diseases than in psoriasis and atopic dermatitis (Table 2).
Overall, considering both the autonomous requirements and those expressed by researchers at local level, the existence of additional restrictions beyond those imposed by the TPR was reported in up to 82.4% of all ACs for psoriasis drugs (Table 2) (Fig. 1). This rate was lower for drugs approved vs atopic dermatitis (58.8%), basal cell carcinoma (52.9%), melanoma (35.3%), and Merkel cell carcinoma (29.4%) (Table 2).
DiscussionWhile the TPR defines the requirements for the use and reimbursement of innovative drugs in dermatology, this study showed significant heterogeneity in its application across various ACs. Consequently, a significant number of ACs have specific written provisions added to those of the TPR, or specific requirements for the use of these drugs (Table 1) (Fig. 1). In many cases, local requirements implemented by the management of each center complicates the situation even more.
In ACs with specific regional requirements on drug prescription added to those already existing in the TPR, the most widely observed requirement was the order of prescription, prioritizing the use of biosimilar drugs, or drugs that had been in market for a longer period of time. However, the order of prescription was not the same across all ACs. Considering that the goal of the prescription order is likely to contain drug spending, it seems reasonable to believe that these measures should be based on studies that assess the efficacy and safety profile of such prescription orders. Otherwise, the recommendations made could eventually lack pharmacoeconomic impact and even worsen it. Without evidence supporting these additional restrictions, alternative methods of drug spending management should be implemented to facilitate greater prescription freedom based on scientific data without losing sight of efficacy, Therefore, requirements such as mean patient-year spending, pay-for-results, dose optimization promotion, or national-level procurement systems should be in place.
It is striking how, regardless of the existence of regional requirements, the common presence of additional in-hospital local requirements has been reported in several ACs. (table S2, annex). These data are consistent with what Rodríguez-Lescure et al. reported regarding access to oncologic drugs in Spain.7 They showed that decisions on access to drugs were made on a local basis in over half of the cases. In this case no documented evidence was found either that would justify these decisions. We could not find any more studies close to our specialty on the subject matter of this publication.
The geographical description of access to hospital prescription innovation in dermatology shows great heterogeneity, not only at regional but at local level too. Guidelines and requirements, often without a well-established scientific basis, often lack the transparency and accessibility that would be expected. In these circumstances, individual solutions are sometimes implemented In, at least, 3 ACs, it was reported that, due to additional local restrictions at hospital level, there were patients who changed their place of residence, or requested referral to a different hospital only to have access to a drug they wouldn’t have access to at a different center in the same city because of these restrictions. Several researchers also expressed their concern that, in the development of these protocols that implement additional local restrictions, the dermatologists’ opinions—the experts on how to manage the disease and treat the patients—are often left behind.
The main limitation of this study was the inability to collect data on a center-by-center basis, which is why the study focused on data curation at regional level only. Therefore, it is likely that the variability and inequities existing at local level—which could be even greater than those actually found—were underrepresented. Additionally, the difficulty in accessing protocols in some communities may have introduced biases. Finally, as health policies change over time, the data collected represent only the moment of data mining. Regardless of this, they serve the goal of the study, which was to evaluate the existence of inequities regarding access to new drugs in dermatology and, if reported, give a description of these inequities.
Some of the strengths of this study were that all researchers involved in management at their centers from all ACs nationwide participated in the study, and data were collected through 2 different sources with subsequent correction of discrepancies. For future reference, it would be interesting that forthcoming studies should include data on equity regarding access, not only to novel drugs but also to dermatology consultations.
In conclusion, data reflect a situation of inequality in access requirements for new drugs in dermatology across various ACs, with multiple criteria at both regional and local levels. It is not uncommon to find additional restrictions beyond those established by the TPR; restrictions rarely based on clear decision-making methods showing how to implement the scientific evidence available. This situation can lead to differences in access to innovation in dermatology and have an impact on the patients’ prognosis and quality of life based on their place of residence.
In the context of new prescription drugs in dermatology, the coordination effort made by multiple managing authorities stands as a priority to develop access criteria consistent with the TPR based on methodologically sound and accessible pharmacoeconomic studies, and receive support and advice from professionals involved in the management of these diseases. We understand that, among other factors, restrictions can be associated with different budgets and funding across various ACs, or different prioritization of spending. In any case, the existence of these requirements creates individual inequalities in the management of prevalent diseases such as psoriasis or atopic dermatitis, and oncologic diseases nationwide, something that needs to be fixed as a top priority. Interregional coordination and greater transparency are essential to equalize access to new drugs across different areas of the country, which should be considered a top priority of health policies.
Conflicts of interestManuel Sánchez Díaz received conference fees from Sanofi, Lilly, Novartis, and Almirall.
Ángeles Flórez has received fees or support for educational activities, and acted as a speaker or consultant, conducted clinical trials, or participated in research projects on behalf of Abbvie, ACELYRIN Inc, Alcedis GmbH, Almirall, Amgen, BMS, Celgene, Galderma, Incyte Corporation, Janssen-Cilag, Kyowa Kirin, Leo Pharma, Lilly, Novartis, Pfizer, Roche Farma, Sanofi-Aventis, Sun Pharma, Takeda, and UCB Biosciences GmbH.
Mariano Ara-Martín: speaker and consultant for Abbvie, Novartis, Janssen, Lilly, Almirall, Leo Pharma, Amgen, UCB, Sanofi, Pfizer.
Pablo Coto-Segura: speaker, and consultant. Also, he declared to have received congress support, or research funds from Abbvie, Almirall, Amgen, Bristol Meier Squib, Leo Pharma, UCB pharma, and Sanofi.
Ana Martín-Santiago has served as a consultant, or received speaker fees for her participation in educational events organized by AbbVie, Amgen, Janssen, LEO Pharma, Leti, Lilly, Mylan, Novartis, Pierre-Fabre, Pfizer, Sanofi-Genzyme, UCB, and Viatrix.
Pedro Mercader-García received conference fees from Lilly and Sanofi, and speaker fees from Leo Pharma, Abbvie, and Sanofi.
Jaime Notario has received fees for participating in consultations, or speaker fees, or fees for being involved in clinical trials sponsored by AbbVie, Almirall, Amgen, Boehringer Ingelheim, Eli Lilly, Janssen, LEO Pharma, MSD, Novartis, Pfizer, and UCB Pharma.
Lucía Palacio Aller received conference fees from Lilly, Janssen, and Sanofi.
Ignacio García-Doval received conference fees from Abbvie, MSD, Pfizer, and Sanofi.
Mercè Grau-Pérez received conference or seminar fees from Abbvie, Almirall, Janssen, Sanofi, Lilly, and Novartis.
José-Manuel Carrascosa has received fees or support for educational activities, and acted as a speaker or consultant, conducted clinical trials, or participated in research projects on behalf of Abbvie, Almirall, Amgen, BMS, Celgene, Galderma, Janssen-Cilag, Leo Pharma, Lilly, Novartis, Pfizer, Sanofi-Aventis, UCB, Sandoz, and Boehringer Ingelheim.
This study was possible thanks to the Juan de Azúa Grant awarded by Piel Sana Foundation attached to the Spanish Academy of Dermatology and Venereology.