Localized plaque morphea and eosinophilic fasciitis (EF) are entities included in sclerodermiform syndromes.1–3 Although their pathophysiological mechanism remains unknown to thos date, they have been associated with the consumption of multiple drugs since their description.1,2 There are published cases linking the use of natalizumab—a monoclonal antibody used for the treatment of multiple sclerosis—with the development of these entities.4,5
We describe a case of both signs in a patient on natalizumab who required a change of treatment to ocrelizumab for control.
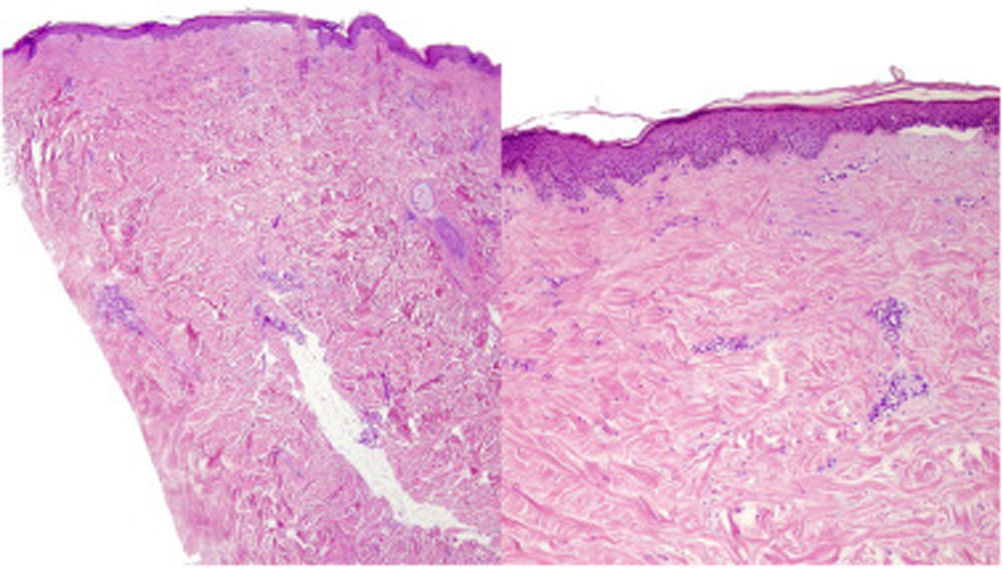
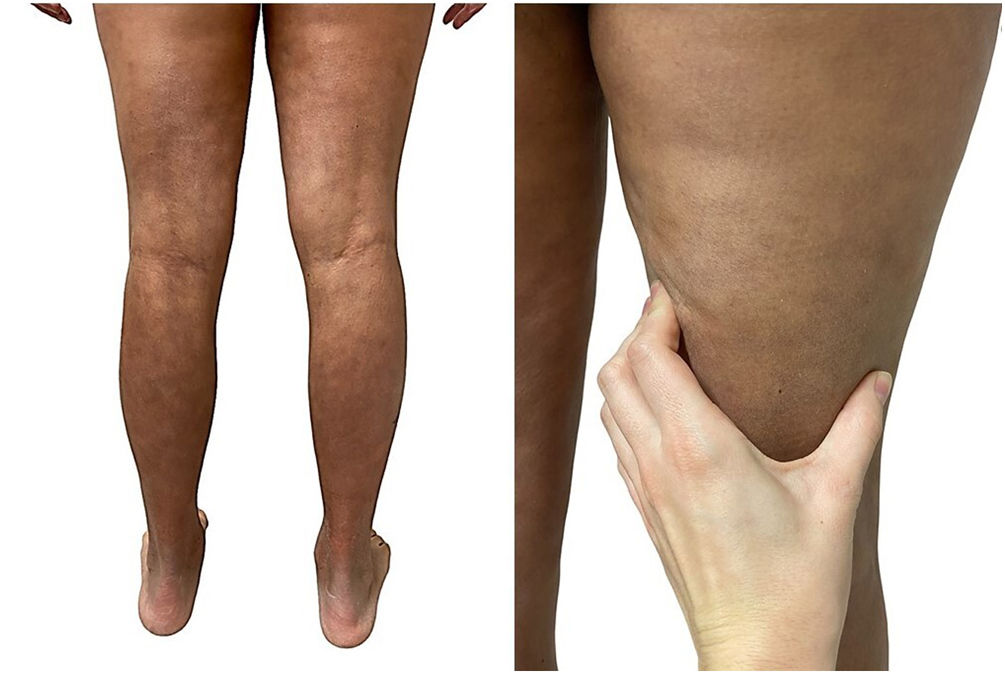
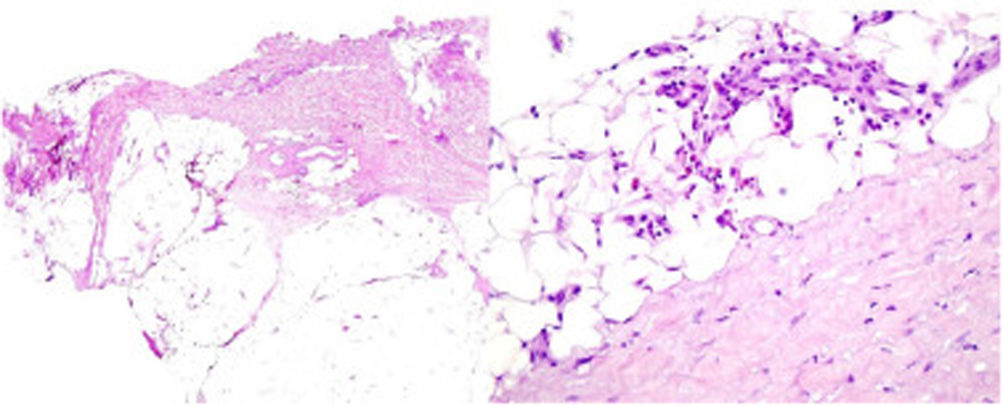
A 39-year-old woman with multiple sclerosis (MS) had been on natalizumab since 2010. She presented to our clinic in 2018 showing the presence of indurated plaques on both abdominal flanks. Suspecting localized plaque morphea, a skin biopsy was performed, which confirmed typical changes including dermal sclerosis and loss of skin appendages (Fig. 1). General laboratory tests and serologies, including for Borrelia burgdorferi, were normal or negative. A 4-month regimen of clobetasol propionate 0.05% was administered, 20 days a month. After observing little improvement, topical photochemotherapy (PUVA) was applied, with complete remission of the lesions after a total dose of 6J/cm2. Three months after finishing treatment, the patient returned, perceiving tension and hardening of the arms and legs. Physical examination revealed generalized induration of all extremities, bilaterally and symmetrically, sparing the trunk, hands, and feet, which prevented pinching and gave an orange peel appearance (Figs. 2A and B). Suspecting EF, a deep biopsy, including the fascia, was performed, which was consistent with the diagnosis, showing superficial and deep cutaneous sclerosis, as well as fascial thickening associated with a mixed infiltrate of lymphocytes, eosinophils, and a few plasma cells (Figs. 3A and B). A complete blood count revealed eosinophilia and elevated acute phase reactants, with persistently negative Borrelia serology.
After reviewing the literature,4,5 natalizumab was recommended to be discontinued, and oral prednisone was down titrated with an initial dose of 1mg/kg/day, and PUVA was administered with partial improvement. However, lesions relapsed after prednisone was down titrated <20mg/day.
The neurology service discontinued natalizumab and changed it for ocrelizumab, which was started 6 months later, without evidence of relapses of her neurological disease in this interval.
After the second dose of ocrelizumab, there was progressive improvement in the skin condition, allowing the discontinuation of PUVA and oral prednisone 8 months after starting it. Since then, the patient has remained asymptomatic both cutaneous and neurologically.
EF is characterized by acute or subacute hardening of the skin of the extremities bilaterally and symmetrically, sparing the hands and feet.1,2 It starts with a sensation of pain or tightness in the skin that progresses into overt hardening, resulting from the development of sclerotic bands between the deep dermis and fascia, giving the clinical appearance of “orange peel”.1–3 It has been reported that up to 30% of patients present with typical localized plaque morphea lesions, which can appear before, synchronously, or months after EF.6 Known triggers include strenuous exercise, trauma, insect bites, and multiple drugs.1–3
There are 2 articles in the literature linking natalizumab, a humanized recombinant antibody used in MS treatment, with the development of EF.4,5 These are 2 isolated cases where the clinical condition developed within the first year of treatment, with rapid improvement after discontinuation, but no relapse after reintroduction. According to updated WHO causality criteria,7 both in these cases and ours, the association between the drug and the disease would only be possible. Specifically, in our case, the timing and lack of improvement upon discontinuing natalizumab lead us to consider other possible triggers, and in this regard, we find a well-documented association in the literature between EF and other autoimmune diseases, including another case of MS treated with fumarates.8
Ocrelizumab is a humanized anti-CD20 monoclonal antibody approved by the FDA in 2017 for the treatment of MS, with a similar mechanism of action to rituximab. Since there are 8 cases in the literature of refractory EF with a good response to rituximab,9,10 we expected disease control with this drug, being, to date, the first case ever reported of EF responding to ocrelizumab described in the literature. We believe it is important to highlight the efficacy of this drug and consider it in refractory cases.
Conflicts of interestNone declared.