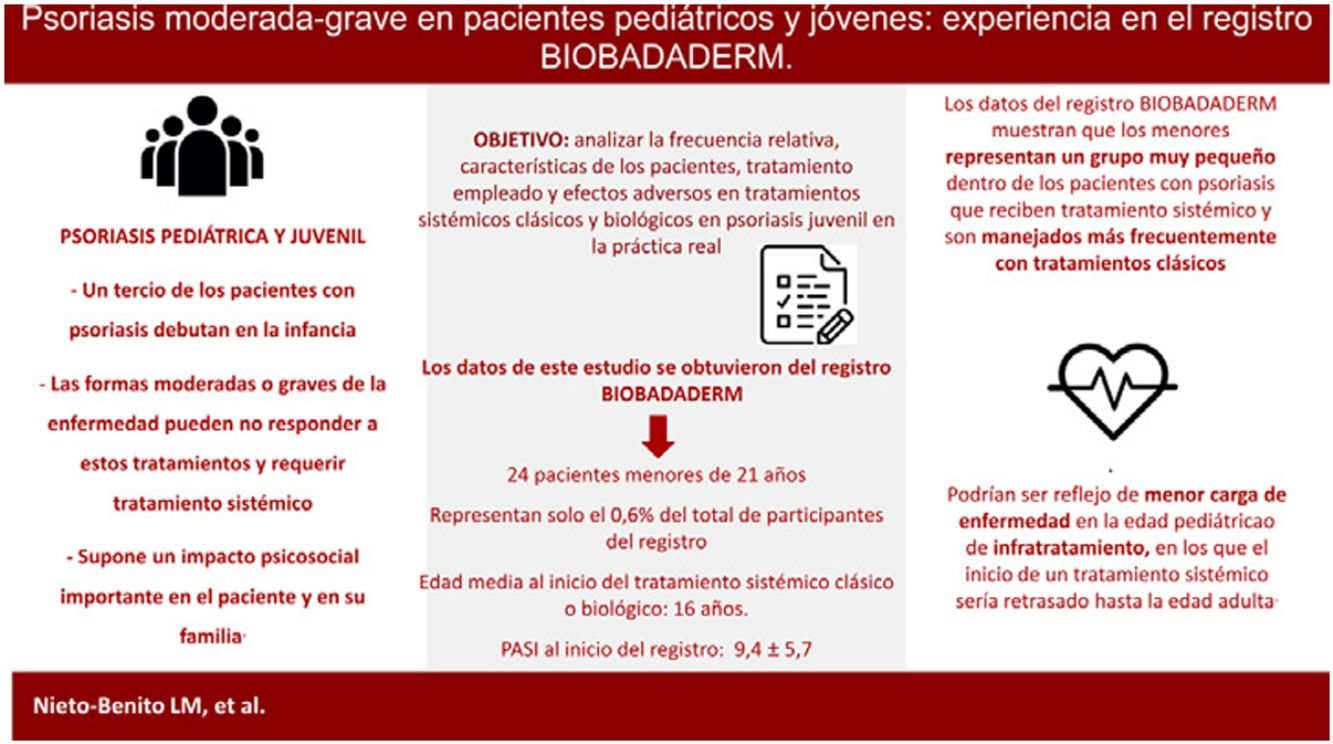
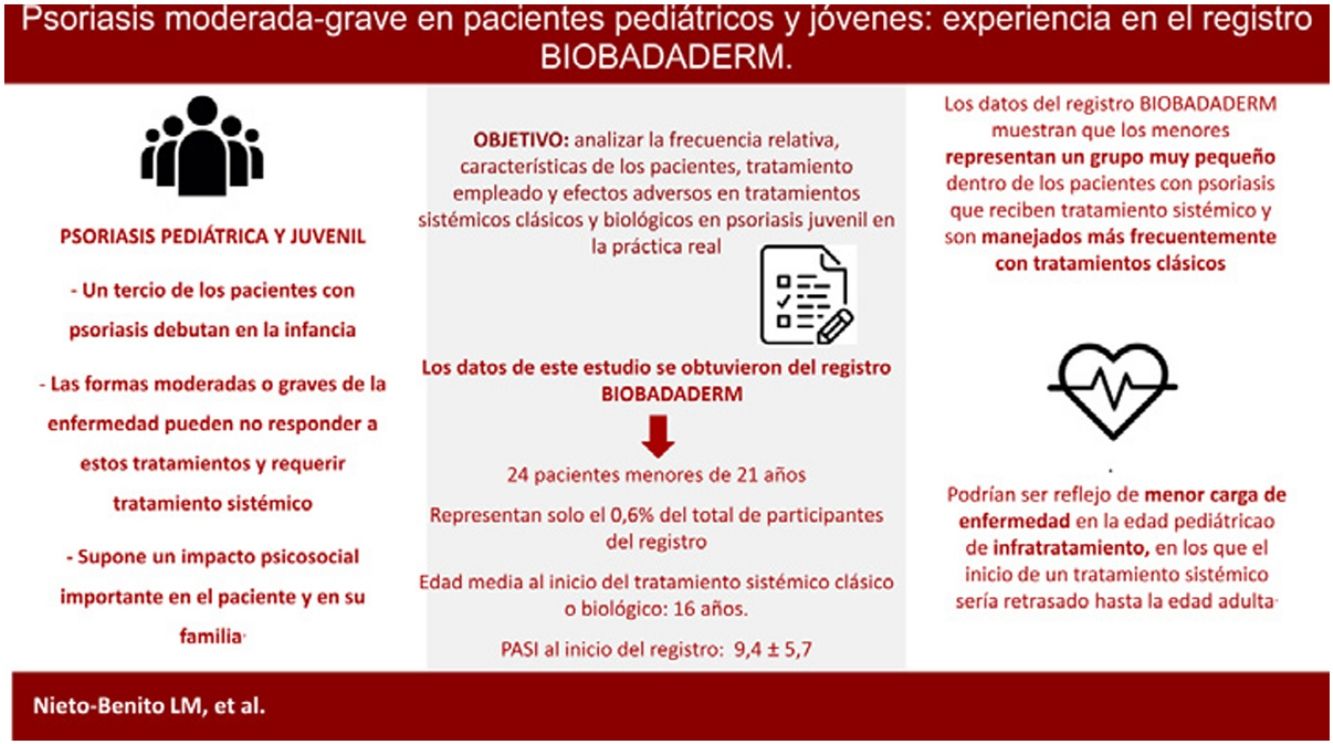
Childhood-onset psoriasis generally follows an indolent course but patients with moderate or severe disease may require systemic treatment. The aim of this study was to determine the relative proportion of children and young people aged up to 21 years with moderate to severe psoriasis in the BIOBADADERM registry and to analyze the characteristics of these patients, treatments used, and adverse events. Of the 3946 patients in the registry, 24 were aged 21 years or younger. The mean age of this group when they started treatment upon registration on Biobadaderm was 16.1 years and the mean Psoriasis Area and Severity Index was 9.4. In 67% the first treatment recorded was with a conventional systemic drug. Treatment was discontinued in 14 patients (58%) due to adverse events or a loss or lack of effectiveness. In conclusion, the BIOBADADERM registry shows that young people account for a small proportion of psoriasis patients receiving systemic treatment, and they are more likely to be treated using conventional systemic drugs.
El comienzo de la psoriasis en la edad pediátrica, aunque generalmente leve, puede requerir tratamiento sistémico en las formas moderadas o graves de la enfermedad. El objetivo de este trabajo es analizar la frecuencia relativa, las características de los pacientes, el tratamiento empleado y los eventos adversos observados a partir del registro BIOBADADERM en niños y jóvenes con psoriasis moderada-grave. Del total de 3.946 pacientes del registro, se incluyen 24 pacientes menores de 21años, con una edad media al inicio del tratamiento en BIOBADADERM de 16,1 años y un PASI medio de 9,4. El 67% de los pacientes estaban en tratamiento sistémico clásico al inicio del registro. Catorce pacientes (58%) suspendieron el tratamiento por pérdida o falta de eficacia o por eventos adversos. En conclusión, los datos del registro BIOBADADERM muestran que los menores representan un grupo muy pequeño dentro de los pacientes con psoriasis que reciben tratamiento sistémico y son manejados más frecuentemente con tratamientos clásicos.
In one-third of patients, psoriasis first develops in childhood,1 with a mean age at diagnosis of 9 years. Pediatric psoriasis is generally mild and it can usually be controlled with topical treatments and/or phototherapy.2 Moderate to severe forms of the disease may not, however, respond to these therapies and may require systemic treatment.1,2
The onset of psoriasis in childhood and adolescence has significant psychological and social repercussions on patients and their families.2 Early onset is also associated with greater cardiovascular and metabolic comorbidity. Prompt intervention is vital, particularly in severe cases, to modify the inflammatory course of the disease and prevent complications and sequelae in later life.3,4 Given the vulnerability of this age group, research is needed to generate long-term data on safety and, ideally, to inform the use of weight-adjusted dosing regimens in these young patients.2,4
The aim of the present study was to analyze the relative frequency of pediatric psoriasis, the characteristics of the patients, and the treatments used, and to characterize the adverse effects associated with systemic therapy in pediatric psoriasis in real-life clinical practice.
Material and MethodsThe data for this study were obtained from the BIOBADADERM registry set up by the Psoriasis Group of the Spanish Academy of Dermatology and Venereology (AEDV) to study the safety of systemic therapy in psoriasis. A description of the registry has been published previously.5,6
We collected the following demographic and clinical data for all the patients under 18 years of age on the registry as of November 2020: clinical form, Psoriasis Area Severity Index (PASI) score, comorbidities, prior therapy, number of prior therapies, adverse events, and disease course. We collected the same set of variables for the patients aged between 19 and 21 years.
BIOBADADADERM was approved by the Clinical Research Ethics Committee of the Hospital Doce de Octubre (216/07, Madrid) and is conducted in accordance with the Declaration of Helsinki. Written consent is obtained from all the patients included in the registry or, in the case of minors, from a parent or legal guardian.
A descriptive analysis of the data collected was carried out. Continuous variables were expressed as mean (SD) or median (interquartile range). Qualitative variables were expressed as percentages and absolute values. The statistical analysis was performed with Stata® (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX. StataCorp LLC).
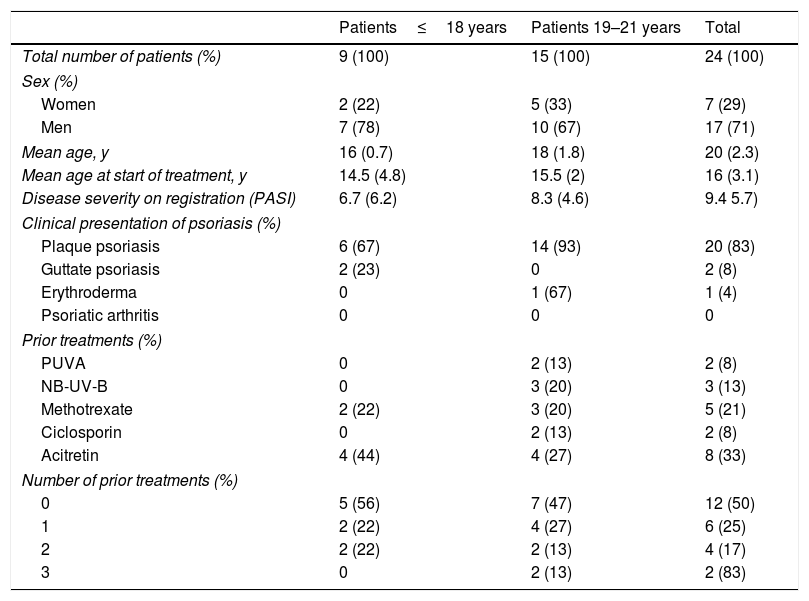
ResultsOf the 3946 patients on the registry, 9 were under 18 years of age and 15 were aged between 19 and 21 years. In this group of 24 patients, mean (SD) age at diagnosis was 16 (3.1) years and 71% of the patients were male. The mean interval between diagnosis and start of treatment was 5.5 (4.8) years and the mean PASI score on registration was 9.4 (5.7). The only patients in this group with comorbid conditions were 2 patients in the 19-21 year cohort: 1 with arterial hypertension and 1 with HIV infection.
The clinical form of psoriasis and the prior treatments received by the patients in both age groups are shown in Table 1.
Epidemiological and Clinical Characteristics of the Patients Studied and Prior Treatments Received.
| Patients≤18 years | Patients 19–21 years | Total | |
|---|---|---|---|
| Total number of patients (%) | 9 (100) | 15 (100) | 24 (100) |
| Sex (%) | |||
| Women | 2 (22) | 5 (33) | 7 (29) |
| Men | 7 (78) | 10 (67) | 17 (71) |
| Mean age, y | 16 (0.7) | 18 (1.8) | 20 (2.3) |
| Mean age at start of treatment, y | 14.5 (4.8) | 15.5 (2) | 16 (3.1) |
| Disease severity on registration (PASI) | 6.7 (6.2) | 8.3 (4.6) | 9.4 5.7) |
| Clinical presentation of psoriasis (%) | |||
| Plaque psoriasis | 6 (67) | 14 (93) | 20 (83) |
| Guttate psoriasis | 2 (23) | 0 | 2 (8) |
| Erythroderma | 0 | 1 (67) | 1 (4) |
| Psoriatic arthritis | 0 | 0 | 0 |
| Prior treatments (%) | |||
| PUVA | 0 | 2 (13) | 2 (8) |
| NB-UV-B | 0 | 3 (20) | 3 (13) |
| Methotrexate | 2 (22) | 3 (20) | 5 (21) |
| Ciclosporin | 0 | 2 (13) | 2 (8) |
| Acitretin | 4 (44) | 4 (27) | 8 (33) |
| Number of prior treatments (%) | |||
| 0 | 5 (56) | 7 (47) | 12 (50) |
| 1 | 2 (22) | 4 (27) | 6 (25) |
| 2 | 2 (22) | 2 (13) | 4 (17) |
| 3 | 0 | 2 (13) | 2 (83) |
Abbreviations: COPD, chronic obstructive pulmonary disease; PASI: Psoriasis Area Severity Index; NB-UV-B, Narrowband UV-B; PUVA, psoralen-UV-A.
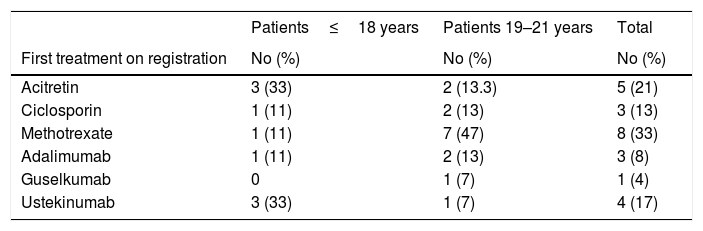
Most of the patients in this study (67%) received a non-biologic systemic drug as their first treatment after their inclusion on the registry: 5 received acitretin, 3 ciclosporin, and 8 methotrexate (Table 2). Among the patients who started with biologic therapy, ustekinumab was the most frequently prescribed agent, followed by adalimumab, and then guselkumab. In the group of patients under 18 years of age, acitretin was the systemic treatment most frequently prescribed during follow-up; by contrast, in the 19–21 years age group, methotrexate was the drug most often prescribed. The overall follow-up time for all patients was 38 person-years.
Treatments Administered and Adverse Effects Reported: Data from the BIOBADADERM Registry.
| Patients≤18 years | Patients 19–21 years | Total | |
|---|---|---|---|
| First treatment on registration | No (%) | No (%) | No (%) |
| Acitretin | 3 (33) | 2 (13.3) | 5 (21) |
| Ciclosporin | 1 (11) | 2 (13) | 3 (13) |
| Methotrexate | 1 (11) | 7 (47) | 8 (33) |
| Adalimumab | 1 (11) | 2 (13) | 3 (8) |
| Guselkumab | 0 | 1 (7) | 1 (4) |
| Ustekinumab | 3 (33) | 1 (7) | 4 (17) |
| Treatment cycles | No (%) | No (%) | No (%) |
|---|---|---|---|
| Acitretin | 3 (23) | 3 (9) | 6 (13) |
| Ciclosporin | 1 (8) | 5 (15) | 6 (13) |
| Methotrexate | 1 (8) | 14 (42) | 15 (33) |
| Dimethyl fumarate | 0 | 1 (3) | 1 (2) |
| Adalimumab | 2 (15) | 5 (3) | 7 (15) |
| Guselkumab | 0 | 1 (3) | 1 (2) |
| Risankizumab | 0 | 1 (3) | 1 (2) |
| Secukinumab | 1 (8) | 1 (3) | 2 (4) |
| Ustekinumab | 5 (39) | 2 (6) | 7 (15) |
| Total | 13 | 33 | 46 |
| Reason for withdrawal | No (% of all patients) | No (% of all patients) | No (% of all patients) |
|---|---|---|---|
| Lack or loss of efficacy | 2 (15) | 10 (30) | 12 (26) |
| Adverse event | 2 (15) | 0 | 2 (4) |
| Remission | 1 (8) | 8 (24) | 9 (20) |
| Preventive withdrawal due to COVID-19 | 0 | 1 (3) | 1 (2) |
| Other | 0 | 4 (12) | 4 (9) |
Of the 46 treatment cycles administered, 28 (61%) were discontinued. In 14 (28%) cases, the reason for withdrawal was a lack or loss of efficacy, or adverse events. Of these, 4 were patients aged under 18 years. The adverse events leading to withdrawal of treatment were diarrhea and nausea associated with headache.
Of the 27 adverse events recorded, 5 were in children under 18 years of age (none considered serious) and 22 were in patients aged 19-21 years. In the older group, 2 patients experienced infectious adverse events that did not lead to withdrawal of treatment: acute otitis media in a patient treated with acitretin and acute appendicitis in a patient treated with risankizumab.
DiscussionIn this study, we collected data from the BIOBADADADERM registry on 24 patients with psoriasis aged under 21 years, a subgroup that represents only 0.6% of the patients on the registry. In the subgroup of patients aged under 18 years, mean age at start of conventional or biologic systemic therapy was 14.5 years.
These findings may reflect the lower burden of disease in patients in this age group, in whom psoriasis can usually be controlled with topical therapy.1,2 They may, however, be an indication of under-treatment, that is, a tendency to postpone systemic treatment until the patient reaches adulthood.4,7
Most of the evidence available on the treatment of moderate to severe psoriasis in pediatric patients treated with conventional and biologic systemic therapies is based on experience in adults.4 Nevertheless, the data on the safety and efficacy of biologic therapies in a pediatric setting has increased considerably in recent years.8–17 There are currently 5 systemic biologic drugs approved by the European Medicines Agency (EMA) for the treatment of pediatric psoriasis: etanercept, ustekinumab, secukinumab, ixekizumab, and adalimumab. The first 4 of these are indicated for the treatment of moderate to severe plaque psoriasis in pediatric patients older than 6 years in whom classic systemic treatments and/or phototherapy are contraindicated or have failed. Adalimumab is indicated in patients older than 4 years and as a first-line treatment in severe psoriasis.16
The characteristics and management of patients under 18 years of age with moderate to severe psoriasis have been analyzed in several retrospective studies. Charbit et al.18 analyzed data from 154 patients in a retrospective multicenter study. In that study, the mean age of onset of psoriasis was 7.7 years and the mean age at the start of systemic treatment was 10.3 years. Acitretin, the most frequently used therapy (55.2%), was mainly prescribed as the first line of treatment (76.6%). In that series, 34 patients required biologic therapy. Of the 261 treatments used, 15 were discontinued due to adverse events. Ciclosporin was the drug most frequently associated with severe adverse events that led to withdrawal of treatment (myalgia, gastrointestinal disorders, hypertrichosis).
Another study analyzed data from 51 patients who received conventional systemic treatment and biologic therapy alone and/or in combination.19 Methotrexate, administered as single-drug therapy or in combination with a biologic, was the most frequently used non-biologic treatment (27.5%) and only 1 patient received acitretin. The biologic agent most frequently prescribed was etanercept (23 patients), followed by adalimumab and ustekinumab. In the 80 treatment cycles studied, 29 adverse events were reported. Combination treatment regimens were not associated with a higher number of adverse events, although the statistical power to detect differences is low in this subgroup. The most frequent adverse event in the patients treated with methotrexate was gastrointestinal intolerance; the drug was administered subcutaneously in only 1 patient.
A Spanish study analyzed data from a group of 40 younger patients, with a mean age of 10.2 years at diagnosis and of 13.4 years at start of systemic treatment.20 Of the 63 treatment cycles studied, 36 were phototherapy. Excluding those, acitretin was the most commonly used first-line treatment and methotrexate the most frequently used overall. Etanercept and acitretin were the drugs used for the longest periods. Adverse events were reported in 11% of the patients in that study, none of which led to withdrawal of treatment.
With respect to biologic therapy, Bronckers et al.8 conducted a retrospective study of 390 patients, with a mean age of 11 years, who underwent 482 treatment cycles. The biologic agent most frequently prescribed in that study was etanercept, followed, in order of frequency, by adalimumab, ustekinumab, and infliximab. Of the 270 patients treated with methotrexate, 48.1% experienced adverse events, which were considered serious in 3 cases, leading to discontinuation of the treatment. Three patients receiving fumaric acid esters and 2 on adalimumab also discontinued treatment due to severe adverse events. No adverse events were observed in the 2 patients treated with infliximab.
Etanercept was also the most prescribed biologic agent in a series of 10 patients with severe psoriasis reported by Ollech et al.4 In that series, the mean age at the start of biologic therapy was 12.6 years. A second-line treatment was necessary in 6 cases and a third line in 3. In the 2 patients who received infliximab, treatment was withdrawn due to a hypersensitivity reaction to the drug.
In conclusion, the data from the BIOBADADADERM registry show that children and young adults represent a very small proportion of patients with psoriasis who require systemic therapy and that the condition is more often managed with non-biologic treatments in this setting. It is, however, not possible to establish whether this is due to undertreatment or to a lower burden of severe disease in this age cohort.
FundingThe BIOBADADADERM project is an initiative of the Fundación Piel Sana and the Spanish Academy of Dermatology and Venereology. It receives financial support from theSpanish Agency of Medicines and Medical Devices (AEMPS) and a number of pharmaceutical companies (Abbott/AbbVie, Amgen, Almirall, Janssen, Novartis, and UCB). The following companies have collaborated in this project in the past: Leo Pharma, Lilly, MSD, and Pfizer. The collaborating pharmaceutical companies were not involved in the design and conduct of the present study. Neither did they have any input regarding the following: the collection, management, analysis, or interpretation of the data; the preparation, review, or approval of the manuscript; the decision to submit the manuscript for publication.
Conflicts of InterestDr. Baniandrés-Rodríguez has served as a consultant and/or speaker for Janssen-Cilag, AbbVie, Pfizer, Novartis, Lilly, Celgene, Leo Pharma, and Almirall.
Dr. Carretero has served as a consultant and/or speaker for Janssen, AbbVie, Novartis, Pfizer, MSD, and Celgene.
Dr. Vilar-Alejo has served on advisory boards for Janssen, Novartis, AbbVie, Almirall, and Celgene.
Dr. Rivera has served as a consultant and/or speaker for and/or participated as principal investigator (PI) in trials sponsored by AbbVie, Almirall, Celgene, Janssen, Leo Pharma, Lilly, Novartis, MSD, and Pfizer-Wyeth.
Dr. Carrascosa has served as an advisor and/or speaker for Celgene, Janssen, Lilly, Novartis, Leo Pharma, Pfizer, MSD, AbbVie, Biogen, and Amgen.
Dr. Dauden has served as a consultant for Abbott, Amgen, Astellas, Centocor Ortho Biotech Inc, Galderma, Glaxo, Jansenn-Cilag, Leo Pharma, Novartis, Pfizer, MSD, and Celgene, has received honoraria from Abbott, Amgen, Janssen-Cilag, Leo Pharma, Novartis, Pfizer, MSD, Celgene, has served on speaker's bureau for Abbott, Pfizer, MSD, and Janssen, and has received grants from Pfizer, Abbott, Janssen, and MSD.
Dr. Herrera-Acosta has served as a consultant and/or speaker for Leo Pharma, Novartis, Janssen, Lilly, Celgene, and AbbVie.
Dr. Sahuquillo has received honoria as a consultant and/or speaker for and/or has participated in clinical trials sponsored by companies that manufacture drugs used in the treatment of psoriasis, including AbbVie, Celgene, Janssen-Cilag, LEO Pharma, Lilly, Novartis, and Pfizer.
Dr. de la Cueva has served as a consultant for Janssen-Cilag, AbbVie, MSD, Pfizer, Novartis, Lilly, Almirall, UCB, Biogen, Celgene, Amgen, Sandoz, Sanofi, and Leo-Pharma.
Dr. López-Estebaranz has served on advisory boards and/or received grants from Janssen, AbbVie, MSD, Lilly, Novartis, Leo Pharma, and Pfizer.
Dr. Belinchón has served as a consultant and/or speaker for and/or participated in clinical trials sponsored by companies that manufacture drugs used in the treatment of psoriasis, including Janssen Pharmaceuticals Inc, Almirall SA, Lilly, AbbVie, Novartis, Celgene, Biogen Amgen, Leo-Pharma, UCB, Pfizer-Wyeth, and MSD.
Dr. Ferran-Farrés has served as a speaker and advisor for Janssen, Lilly, Novartis, Pfizer, MSD, Abbvie, Celgene, and Almirall.
Dr. Rodríguez Fernández-Freire has served as a consultant and speaker for Janssen-Cilag, AbbVie, MSD, Pfizer, Novartis, Lilly, Almirall, Celgene, and Leo-Pharma.
Dr. García-Donoso has served on advisory boards for AbbVie and Almirall and as a speaker for Janssen, Lilly, and Celgene.
Dr. Llamas-Velasco has served as a consultant and speaker and has participated in clinical trials sponsored by Janssen-Cilag, AbbVie, Celgene, Pfizer, Novartis, Lilly, Almirall, and Leo-Pharma.
Dr. Herrera-Ceballos has served as a consultant and/or speaker for and/or has acted as PI in clinical trials sponsored by companies that manufacture drugs used in the treatment of psoriasis, including AbbVie, Janssen-Cilag, LEO Pharma, Lilly, Novartis, and Pfizer.
Dr. Botella-Estrada has received honoraria as a consultant and/or speaker for and/or has participated in clinical trials sponsored by companies that manufacture drugs used in the treatment of psoriasis, including AbbVie, Celgene, Janssen-Cilag, LEO Pharma, Lilly, Novartis, and Pfizer.
Dr. Ruiz-Genao has received honoraria from Pfizer, Janssen, Celgene, AbbVie, Novartis, and LEO Pharma for consultancy and speaking services.
Dr. Riera-Monroig has received travel grants to attend conferences from AbbVie, Almirall, Janssen, LEO-Pharma, and Novartis.
Dr. García-Doval has received grants to offset the cost of travel to conferences from AbbVie, MSD, and Pfizer.
None of the other authors declare conflicts of interests.
The authors wish to thank all the medical professionals and patients who participate in the BIOBADADERM registry.