Methotrexate is one of the most widely used immunosuppressive drugs by dermatologists. Specialists are familiar with both its prescription and recognition of its adverse effects, including drug toxicity. Below, two cases of methotrexate toxicity with different outcomes are described.
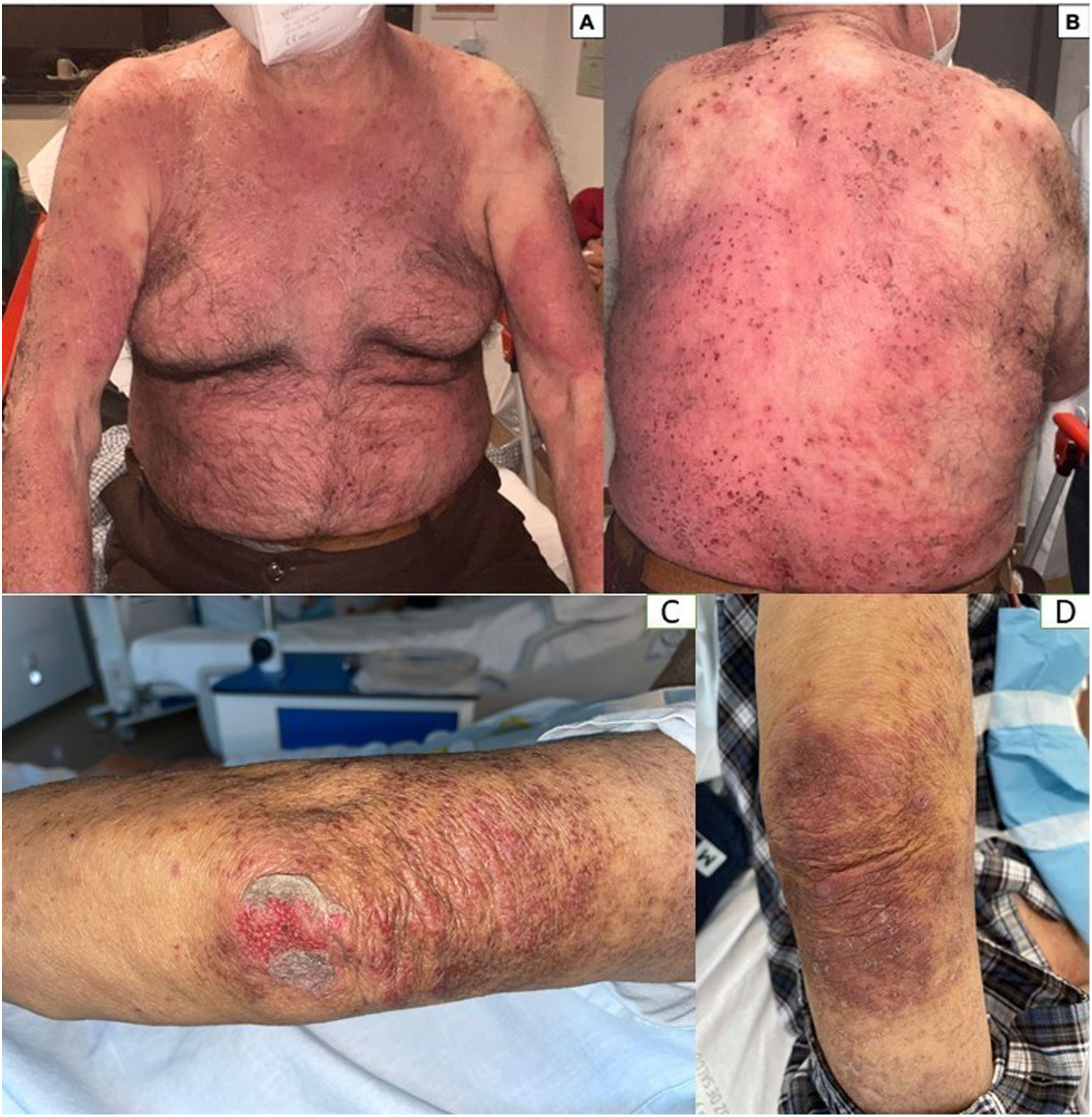
An 86-year-old man, hypertensive and hypothyroid, started on a 10mg/week regimen with oral methotrexate for atopic dermatitis. The patient mistakenly took the prescribed dose for 5 consecutive days. This led to a progressive worsening of skin lesions, which became purplish in color. Additionally, epidermal detachment was observed in previously eczematous lesions. There was no involvement of mucous membranes. Lab test results confirmed acute renal failure associated with bone marrow failure. Plasma levels of methotrexate were within normal parameters (<0.05μM). The patient was admitted to reverse isolation and treated with IV folinic acid 15mg every 6hours, urine alkalinization with bicarbonate, and filgrastim (Neupogen®, G-CSF) 480mcg every 24h. Impetiginization of the lesions was treated with piperacillin 4g/tazobactam 500mg every 8h. Ninety-six hours after admission, the patient died due to severe pancytopenia and systemic multiple organ failure (Fig. 1).
A 69-year-old man with chronic kidney disease on hemodialysis started on a 10mg/week regimen of subcutaneous methotrexate due to an 8-year history of plaque psoriasis. Two weeks into treatment, he started experiencing worsening of lesions, with increased pain and itching. The purplish coloration of the plaques and the extension of scaling beyond the boundaries of the lesions suggested drug toxicity-related lesions. No signs of ulceration or necrosis were observed. Mucous membranes remained untouched. Additional tests showed normal levels of methotrexate (<0.05μM) and thrombocytopenia of 104×103/μL (normal values at 120–450×103/μL). Treatment with folinic acid 15mg orally every 6h was administered. At 1 week, the patient showed mild worsening of thrombocytopenia, associated with leukopenia of 2.8×103/μL (3.6–10.5×103/μL) and anemia with hemoglobin levels of 12g/dL (13.2–16.6g/dL). Two weeks after symptom onset, complete resolution of all analytical parameters was achieved, allowing hospital discharge without complications.
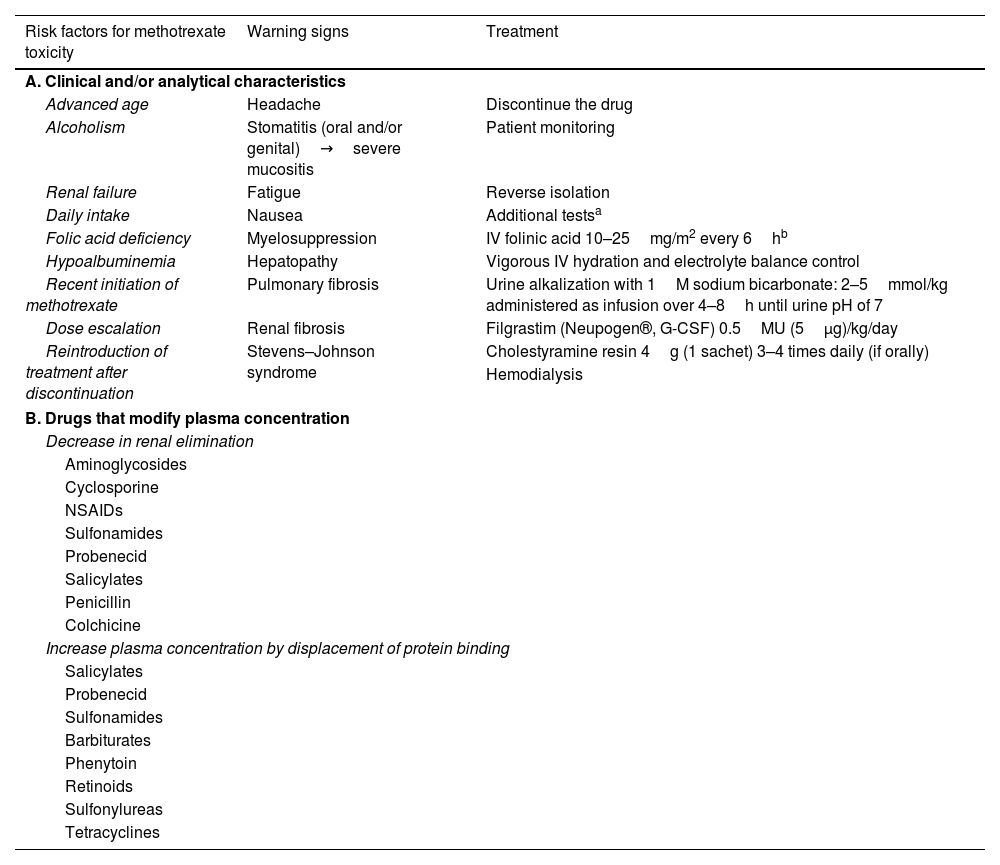
Methotrexate is a folate antagonist that irreversibly inhibits the enzymes dihydrofolate reductase and thymidylate synthetase, interfering with DNA and RNA synthesis and, eventually, with cell proliferation.1,2 The doses most widely used in dermatology range from 7.5mg up to 17.5mg weekly. Despite the low incidence of methotrexate toxicity cases, there are certain risk factors that dermatologists should quickly recognize3 (Table 1).
Risk factors, warning signs, and treatment of methotrexate toxicity.
| Risk factors for methotrexate toxicity | Warning signs | Treatment |
|---|---|---|
| A. Clinical and/or analytical characteristics | ||
| Advanced age | Headache | Discontinue the drug |
| Alcoholism | Stomatitis (oral and/or genital)→severe mucositis | Patient monitoring |
| Renal failure | Fatigue | Reverse isolation |
| Daily intake | Nausea | Additional testsa |
| Folic acid deficiency | Myelosuppression | IV folinic acid 10–25mg/m2 every 6hb |
| Hypoalbuminemia | Hepatopathy | Vigorous IV hydration and electrolyte balance control |
| Recent initiation of methotrexate | Pulmonary fibrosis | Urine alkalization with 1M sodium bicarbonate: 2–5mmol/kg administered as infusion over 4–8h until urine pH of 7 |
| Dose escalation | Renal fibrosis | Filgrastim (Neupogen®, G-CSF) 0.5MU (5μg)/kg/day |
| Reintroduction of treatment after discontinuation | Stevens–Johnson syndrome | Cholestyramine resin 4g (1 sachet) 3–4 times daily (if orally) |
| Hemodialysis | ||
| B. Drugs that modify plasma concentration | ||
| Decrease in renal elimination | ||
| Aminoglycosides | ||
| Cyclosporine | ||
| NSAIDs | ||
| Sulfonamides | ||
| Probenecid | ||
| Salicylates | ||
| Penicillin | ||
| Colchicine | ||
| Increase plasma concentration by displacement of protein binding | ||
| Salicylates | ||
| Probenecid | ||
| Sulfonamides | ||
| Barbiturates | ||
| Phenytoin | ||
| Retinoids | ||
| Sulfonylureas | ||
| Tetracyclines | ||
Methotrexate toxicity usually results from therapeutic errors due to overdosage (daily instead of weekly intake of the drug), mainly if the prescription comes in tablets as it occurred in case #1, or by initiating or continuing the drug in the presence of pre-existing renal, hepatic, or bone marrow dysfunction as it occurred in the case #2.4 Clinical signs are heterogeneous. We should mention that the development of erosions and/or ulcers on the lesions of the previous dermatosis (dermatitis or psoriasis) are considered an early cutaneous sign of pancytopenia, along with mucositis. In patients with psoriasis, pain on the plaques is usually disproportionate, especially in lesions of acral location. As it occurred in case #1, depression of the bone marrow increases the risk of neutropenia, sepsis, and multiple organ failure followed by hemorrhages, heart, respiratory, renal, and hepatic failure.5
Monitoring plasma levels of methotrexate is controversial and seems to be of little use, as its half-life in healthy individuals is approximately 6–8h, with concentrations usually <0.01μM 24h after the last administration.6 Multiple drugs can impact the bioavailability of the drug (Table 1). Hospitalization is mandatory. The initiation of IV folinic acid until normalization of the blood count and the resolution of skin lesions is key to the patient's prognosis. Marrow toxicity can be treated with filgrastim (Neupogen®, G-CSF), a granulocyte colony-stimulating factor that acts on hematopoietic cells by stimulating proliferation and differentiation, thereby accelerating myeloid recovery.7 Urine alkalinization (pH>7.5) increases drug excretion. The use of cholestyramine resin decreases GI absorption by inhibiting the enterohepatic circulation of methotrexate. In case #2, where the patient remained on hemodialysis, the outcome was favorable, suggesting that it should be considered in cases associated with methotrexate toxicity.
In conclusion, methotrexate toxicity is an event of low incidence but of a severe nature, where suspicion and early initiation of treatment will eventually favor the patient's prognosis.
FundingNone declared.
Conflicts of interestNone declared.