Atopic dermatitis is a chronic inflammatory disease that is multifactorial in nature. Allergic contact dermatitis and protein contact dermatitis are allergic conditions that may occur in the context of atopic dermatitis and be the cause of exacerbations. Although the prevalence of allergic contact dermatitis is similar in atopic patients and the general population, these 2 conditions are frequently associated because atopic inflammation disrupts the skin barrier. Skin tests are therefore recommended in atopic individuals. Dupilumab could be useful for treating allergic contact dermatitis if it is mediated by type 2 helper T cells but could exacerbate inflammation if mediated by TH1 cells: further study is needed before conclusions can be drawn. Although the mechanism by which exposure to environmental proteins exacerbates atopic dermatitis remains under discussion, such exacerbations are routinely seen in clinical practice. Prick testing is recommended in symptomatic atopic dermatitis. When prick-test findings are positive, patients should be advised to avoid the culprit substances.
La dermatitis atópica (DA) es una enfermedad inflamatoria crónica multifactorial. La dermatitis de contacto alérgica (DCA) y la dermatitis de contacto por proteínas (DCP) son patologías alérgicas que pueden ser comórbidas a la DA y ser causa de algunas de las exacerbaciones. Aunque la DCA tiene una prevalencia similar en pacientes atópicos que en la población general, debemos considerarla una comorbilidad frecuente en la DA por la disrupción de la barrera cutánea. Por ello, se recomienda la realización de pruebas epicutáneas a los pacientes atópicos. Dupilumab podría ser útil para el tratamiento de la DCA mediada por vía Th2 y exacerbar aquellas que ocurren por vía Th1, aunque se precisan más estudios para establecer conclusiones. El mecanismo por el que la exposición a proteínas ambientales produce exacerbaciones en la DA es controvertido, pero es un fenómeno habitual en la práctica clínica diaria. Se recomienda estudio mediante prick test a pacientes con clínica sugestiva y recomendar conductas evitativas ante pacientes sintomáticos y pruebas positivas.
Atopic dermatitis (AD) is a chronic skin disease comprising a skin barrier defect combined with an impaired immune response in genetically predisposed individuals and in which exposure to external environmental agents play a key role.1,2 Impairment of the skin barrier facilitates the penetration of microbial agents, irritants, and allergens, including both haptens and proteins.
Co-occurrence of AD and irritant contact dermatitis is very frequent and widely accepted. Furthermore, many cases of allergic contact dermatitis (ACD) involve patients with pre-existing irritant contact dermatitis. Following this line of reasoning, patients with AD should be more prone to ACD. However, the opposite has traditionally been stated, namely, that ACD is less common in atopic patients. Given that ACD depends on a mainly type 1 helper T cell (TH1) response, it was believed that the predominance of the TH2 response in atopic patients in some way “protected” them from sensitization to haptens and accounted for the less frequent sensitization observed.3 However, in our daily practice, we see many patients with AD who experience ACD as a complication.
The role of protein allergy in patients with specific atopic diseases, such as asthma, rhinitis, eosinophilic esophagitis, and food allergy, is well known.4 Protein contact dermatitis (PCD) is also widely accepted and is found to be more frequent in atopic patients with chronic hand eczema. It usually occurs in the workplace after exposure to animal or plant proteins. However, the involvement of proteins in exacerbations of AD or the role of immunotherapy as treatment of AD are more controversial topics.
In the present article, we aim to provide an update that covers the latest advances in pathophysiology, symptoms, and treatment that have made it possible to better understand the highly complex relationship between AD, haptens, and proteins.
Atopic Dermatitis and HaptensWorldwide, 15%–20% of children and 1%–10% of adults are affected by AD.1,2 The clinical presentations vary considerably, although they generally include eczema. The clinical manifestations also vary with age and may occur as flare-ups or be persistent. AD is often associated with high levels of serum immunoglobulin (Ig) E, a personal or family history of type 1 hypersensitivity reactions, allergic rhinitis, and asthma. Diagnosis is based on symptoms,5 and diagnostic criteria that could prove useful in more complicated cases have been reported.6
ACD is a delayed hypersensitivity reaction to contact allergens, normally low-molecular-weight substances (haptens) that can cross the skin barrier. Clinically, it presents as eczema, thus hampering differentiation between AD and ACD.7 Histology is not useful for differentiation, since both conditions have a similar histologic pattern. In acute flares, we observed a predominance of spongiosis and vesiculation, whereas in the chronic forms, the predominant pattern is one of acanthosis with hyperkeratosis and, to a lesser extent, spongiosis. Patch testing is the gold standard approach for diagnosis of ACD8,9 and must be performed in the case of a patient with chronic eczema in whom we wish to distinguish between AD, ACD, and AD complicated by ACD.
As for the pathophysiology of ACD, it was initially thought that the patient presented with predominantly TH1-driven inflammatory polarization.10 However, in healthy patients, inflammatory polarization after sensitization has been shown to be diverse, with cytokine-producing effector lymphocytes of various types: type 1 (interferon [IFN] γ, tumor necrosis factor [TNF]), type 17 (interleukin [IL] 17), type 22 (IL-22), and type 2 (IL-4, IL-5, IL-9, IL-13).10 Variability in polarization seems to be associated with the type of allergen and its ability to activate specific pathways in innate immunity, although other factors that have yet to be determined may be involved. For example, nickel is a potent inducer of the TH1, TH17, and TH22 pathways, whereas fragrances and rubbers show more TH2 activity, with less participation of TH1 and TH17.11
Prevalence of Allergic Contact Dermatitis in Patients With Atopic DermatitisDespite the initial proposal of an inverse association between AD and ACD, the co-occurrence of both diseases leads us to believe that there may by a positive relationship between the two. The skin barrier function defect and increased transepidermal water loss leave patients with AD more predisposed to irritant contact dermatitis,12 which increases the likelihood of allergen penetration.13,14
Patients with AD were traditionally thought to experience fewer TH1-mediated type 4 hypersensitivity reactions owing to TH2 polarization. This notion was reinforced by studies showing the inability to sensitize patients with AD after repeated exposure to dinitrochlorobenzene (DNCB).15 Recently, Newel et al.16 compared the clinical and immunological response to sensitization to DNCB in healthy individuals and atopic patients. The authors found that healthy patients had a stronger clinical response to exposure to DNCB, with polarization of the inflammatory response toward TH1. Patients with AD, on the other hand, had a lesser clinical response to exposure to DNCB, with polarization of the inflammatory response toward TH2 and a greater percentage of IL-10 than the controls. In other words, DNCB is in fact capable of producing sensitization in patients with AD, albeit via the TH2 pathway and with less intense symptoms.
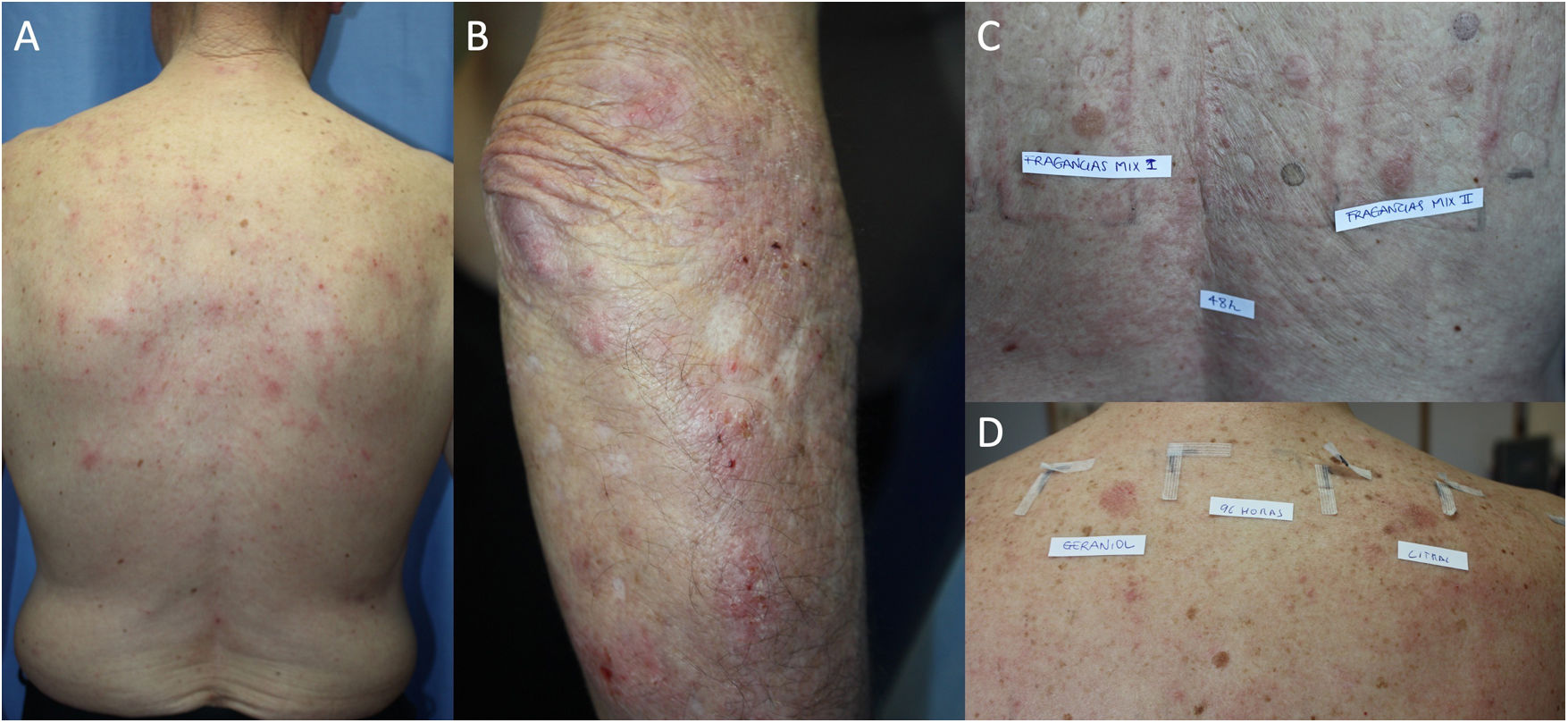
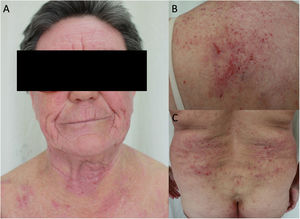
The prevalence of ACD in patients with AD has been assessed in different populations using retrospective studies, which conclude that atopic patients are equally or more predisposed to ACD than the general population. One systematic review showed a significantly greater prevalence of ACD induced by at least 1 allergen in atopic pediatric patients than in nonatopic pediatric patients (46.6% and 41.7%, respectively).17 Another systematic review and meta-analysis of 74 studies found no statistically significant differences in the prevalence of ACD between patients with and without AD.18 Furthermore, an increased rate of positive results was observed in patients with severe DA; the authors attribute this finding to the fact that it is mainly patients with severe disease who are referred for testing.18 Other studies have shown that the prevalence of ACD is higher in patients with mild AD than in those with severe AD,19,20 and it has been postulated that this phenomenon arises because patients with severe AD have a higher challenge threshold for contact sensitization.18 Also in support of a positive relationship between AD and ACD is the finding that polysensitization (≥3 allergens) is more frequent in atopic patients than in healthy individuals (28.7% vs. 14.5%, P=.002).14 Daily clinical practice shows that both entities are associated, with AD frequently seen to be exacerbated by ACD (Fig. 1).
Adult atopic dermatitis aggravated by contact allergy to fragrances in a 74-year-old man. (A and B) Eczema affecting the posterior trunk and right arm. (C) Results of patch testing with the GEIDAC standard series at 48hours: fragrance mix I+++ and fragrance mix II+++. (D) Patch test results (fragrance series) at 96hours: geraniol++ and citral++. Partial control of AD was achieved with avoidance measures.
Patch testing is highly recommended in patients clinically suspected of having AD, in patients with an atypical or changing distribution of dermatitis, and in therapy-resistant AD. Table 1 shows the recommendations put forward by the Expert Panel of the American Contact Dermatitis Society at their meeting in Denver in 2014.21
When to Consider Patch Testing in Atopic Patients.
| 1. AD that worsens, changes its distribution, does not respond to topical treatment or emollients, or flares up immediately after suspension of topical treatment |
| 2. Atypical distribution of AD lesions or pattern suggestive of ACD: pattern mainly affecting the face and neck, hand and foot involvement, involvement mainly of the eyelids or perioral region/cheilitis |
| 3. Refractory hand eczema in the workplace |
| 4. Onset of AD in adolescents or adults with no childhood history of AD |
| 5. Patients with severe AD before starting immunosuppressive therapy |
Abbreviations: ACD, allergic contact dermatitis; AD, atopic dermatitis.
In our opinion, patients with AD (both children and adults) are candidates for patch testing at least once in their lifetime. However, in some patients with severe AD, or eczema affecting the arms and back, it is not always possible to perform patch tests under optimal conditions. Performing patch testing in the context of a flare-up of AD can diminish or increase the response (owing to irritation), leading to false-negative or false-positive results.21 In these cases, patch testing should be performed where possible, and the results should be interpreted with caution.22 Readings may also be altered in patients receiving immunosuppressive treatment; therefore, doses should be reduced as much as possible and the readings should be taken late (7–10 days) to avoid false-negatives.21,23
It is important to remember that patients with AD have a greater tendency toward irritative responses that could be interpreted as false positives. Occlusion of patches and application of surgical tape for 48hours, especially in hot regions or during summer, can lead to irritation and folliculitis. In addition, the concentration used in standard series for some allergens, especially metals (chrome and cobalt), fragrances, formaldehyde, and lanolin, can cause irritation in patients with more sensitive skin.21
Furthermore, in patients with AD, the allergic response can be weaker and shorter. The gradual increase in response over a period of days in allergic reactions (crescendo pattern) is not as frequent in atopic patients; therefore, positive results must be observed carefully, even those of weak intensity, in the first reading.21 This altered or attenuated inflammatory response in AD is thought to be associated with differences in the activation pathways of the innate immune system.16 Patients with AD and ACD have a lower number of dendritic cells (CD1a+, CD1C+) and langerin-positive Langerhans cells, thus potentially supporting the theory that antigen presentation is deficient in atopic patients owing to the absence of dendritic cells in the setting of a massive entry of allergens via the skin.24 This altered response can also be explained by the differential expression in the pathways involved in allergic sensitization. Increased expression of the TH17 and TH2 inflammatory pathways in AD could attenuate the TH1 pathway, possibly leading to a less intense allergic response.24 Moreover, increased anti-inflammatory chemokine levels (e.g., IL-10) may also play a role in this altered response.24
In summary, patch testing should be performed in patients with AD, although the timing and interpretation of the tests may be complicated. Table 2 provides a series of points that should be taken into consideration when performing patch tests on affected patients.21
Interpretation of Patch Test Results in Patients with Atopic Dermatitis.
| 1. Irritant reactions are common, especially to metals (chrome and cobalt), fragrances, formaldehyde, and lanolin |
| 2. The patient's own rinse-off products can cause irritant reactions, especially if not appropriately diluted |
| 3. The crescendo pattern between readings is not as common in patients with atopic dermatitis |
| 4. Applying the patches during a flare-up of atopic dermatitis can diminish the response, leading to false negatives. The risk of increased skin irritation is also higher |
| 5. Immunosuppressive treatment can induce false negatives; therefore, testing should be performed without the drug or at the smallest dose possible. If the drug cannot be suspended, testing should be repeated at a later date, when the disease is controlled and the drug can be suspended |
| 6. Greater susceptibility to changes in climate, with the possibility of more irritant responses during hotter periods |
Most of the allergens involved in ACD in atopic patients (both children and adults) are present in their topical medication or in their cosmetic or personal care products,44 many of which are classed as hypoallergenic.18,25,26 The risk factors for becoming sensitized to one's own products are onset of AD before 6 months of age, high IgE value, and moderate-severe AD.26
Many studies report a higher frequency of sensitization to allergens such as fragrances, plants, antiseptics, corticosteroids, topical antibiotics, and surfactants than in the healthy population.26–36Tables 3 and 4 show the most common allergens in adults and children with AD.26,35,36 Nickel sulfate is the most common allergen in patients with AD and in the general population, although various studies have reported sensitization rates that are lower than, higher than, or similar to those reported for the healthy population.18,35,37 Many studies have found an increase in sensitization to sesquiterpene lactones in atopic patients, although in most cases the relevance of this finding is unknown.35
Most Common Allergens in Adults With Atopic Dermatitis.
| Nickel sulfate |
| Fragrance mix I |
| Methyisothiazolinone and methylchloroisothiazolinone |
| Balsam of Peru |
| Thiomersal |
| Cobalt chloride |
| Potassium dichromate |
| Ethylenediamine |
| Paraphenylenediamine |
| Formaldehyde |
| Neomycin sulfate |
| Colophony |
| Thiuram mix |
| Budesonide |
| Black rubber mix |
| Benzocaine |
| 4-tert-Butylphenol |
| Paraben mix |
| Quaternium 15 |
| Mercapto mix |
Most Frequent Allergens in Pediatric Patients With Atopic Dermatitis.
| Nickel sulfate |
| Fragrance mix I |
| Balsam of Peru |
| Bacitracin |
| Formaldehyde |
| Cocamidopropyl betaine |
| Propylene glycol |
| Wool alcohols |
| Lanolin |
| Bronopol |
| Neomycin sulfate |
| Quaternium 15 |
| Colophony |
| Tixocortol-21-pivalate |
| Methylisothiazolinone and methylchloroisothiazolinone |
| Cobalt |
| Fragrance mix II |
| Potassium dichromate |
| Compositae mix |
| Parthenolide |
Atopic patients are less frequently sensitized to potent allergens (methylchloroisothiazolinone, cobalt, and potassium dichromate) than the general population27; in contrast, they are more frequently sensitized to weak allergens (e.g., propylene glycol, eugenol, vanilla, parabens).28 Weak allergens are thought to be capable of producing more frequent sensitization in AD owing to the disruption of the skin barrier, which favors penetration and immunologic imbalance.28 Potent allergens are capable of sensitizing healthy patients via the TH1 pathway, although the immunologic imbalance in AD seems to induce “hyporeactivity”, resulting in a diminished response.24,28 Greater sensitization to haptens via the TH2 pathway (e.g., fragrances and rubbers) than via the TH1 pathway could also account for these differences in sensitization in AD.
Patch tests should include, at least, the standard Spanish or European series (TRUE test may be insufficient) and the patient's own products (i.e., both personal care products and topical treatments).21 Patch testing should not be performed with rinse-off products owing to the irritation they can induce.5
Dupilumab: Efficacy in the Treatment of ACD and Effect on Patch TestingDupilumab was recently approved for the treatment of moderate to severe AD.38 It has also been used for the treatment of ACD, although the results have been inconsistent. A recent systematic review of 47 patients with ACD treated with dupilumab found clearance of ACD in 9 cases, partial improvement in 31, no improvement in 4, and worsening in 3.39 It has been postulated that dupilumab could be useful for the treatment of ACD caused by TH2-mediated allergens, since improvements in the symptoms of ACD caused by allergens such as fragrances, rubbers, and textile dyes have been observed.39–41 In contrast, dupilumab could exacerbate ACD caused by allergens that act via the TH1 pathway, with reports of cases involving methylisothiazolinone, formaldehyde releasers, and phenylguanidines.42 Other studies found no statistically significant differences in the response to dupilumab in atopic patients with and without ACD.43,44
In summary, there is considerable variation in the response to dupilumab in ACD, and outcomes that are independent of the allergen and the TH pathway are thought to be involved. The variability in the results found in clinical practice cannot be explained solely by the different inflammation patterns generated by the allergens, as argued by some authors.45 The rigid division of allergens into those that sensitize via the TH1 pathway or the TH2 pathway may be insufficient. In the polarization toward a specific pathway, in addition to the characteristics of the allergen itself, patient-related factors may play a role, as seen in the more marked tendency toward TH2-mediated reactions in atopic patients. It may be that the immunologic context in which sensitization occurs (e.g., during a flare-up of AD) plays a role in polarization toward TH1 or TH2. Furthermore, the improvement in the skin barrier brought about by dupilumab could improve symptoms irrespective of the pathway involved. Dupilumab could be proposed as an option in cases where it is impossible to avoid the allergen and the dermatitis is severe.46 In addition, it should be considered in atopic patients with concomitant ACD owing to its effect on the underlying disease.46
The effect of dupilumab on the results of patch tests is also being assessed. A study analyzing the results of patch tests before, during, and after treatment with dupilumab found that of the 144 allergens tested before and after, 17 lost positivity after treatment, including fragrances and balsam of Peru, which are known to polarize toward TH2.39 In contrast, a recent retrospective study in which patch tests were performed before and after treatment found that positive results persisted in 51.2% of patients (64/125) and a loss of positivity in only 10.4% (13/125).43 The allergens for which positivity was lost included emulsifiers, fragrances, metals, sunscreens, medications, resins, and preservatives. Almost three-quarters (73.1%) of the positive results with fragrances before treatment remained unchanged in the posttreatment tests. The authors concluded that dupilumab did not seem to have a major impact on the results of the tests.43 However, since the results were inconclusive in 38.4% of cases, we believe that the drug's ability to modify the results of patch tests remains doubtful.
Atopic Dermatitis and ProteinsProteins are a recognized allergen in type 1 hypersensitivity reactions, which manifest on the skin as wheals, angioedema, or both. Traditionally, proteins were not associated with eczema lesions, since their high molecular weight meant that they were not considered capable of crossing the skin barrier and causing a type 4 reaction. PCD was first described some decades ago as a condition comprising both type 1 and type 4 reactions. The condition facilitated our understanding of how food and environmental proteins play a role in specific exacerbations of AD, in both children and adults.
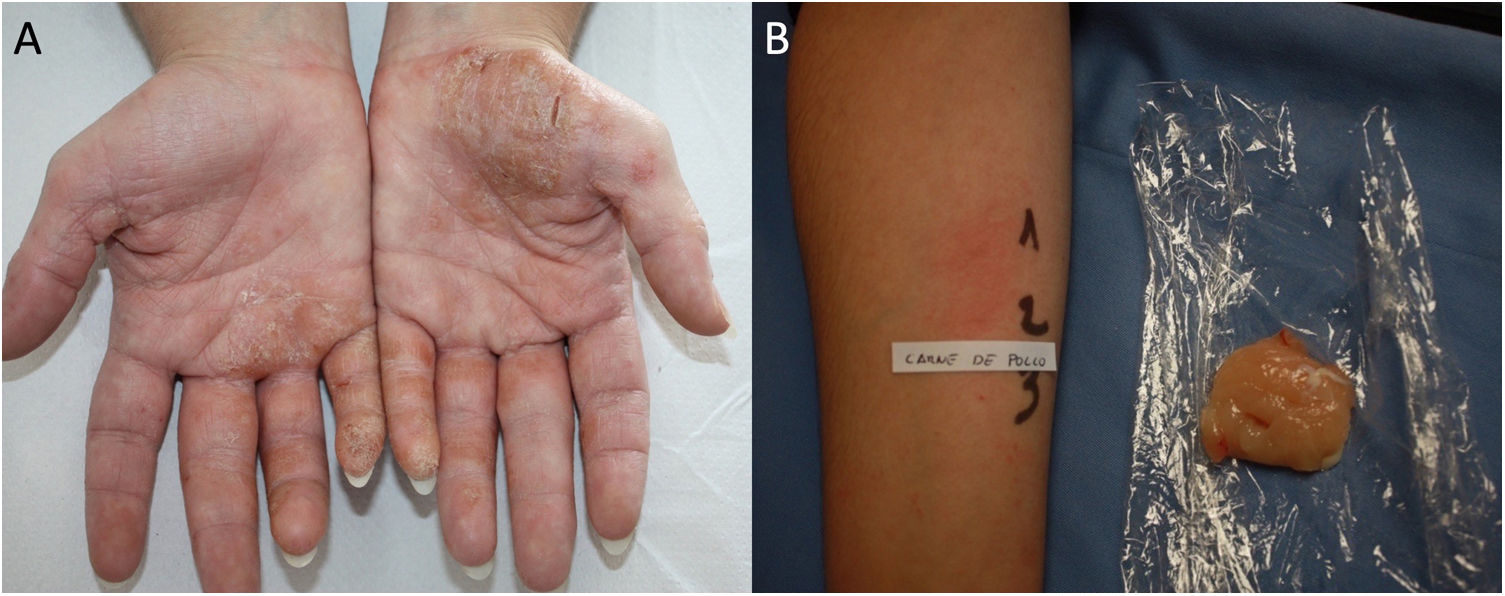
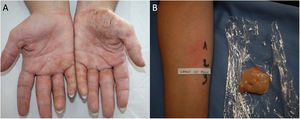
Protein Contact DermatitisPCD is a type 1 and type 4 hypersensitivity reaction. Patients present with pruritus immediately after exposure to the allergen and subsequently develop eczematous lesions. The conditions can manifest as contact urticaria-type lesions, followed by eczematous lesions at the same site.47 The lesions most commonly affect the hands and forearms. Patients generally have chronic hand eczema and are atopic (Fig. 2). Most cases are occupational and involve contact with plants or animals (workers who handle foodstuffs). It is important to rule out oral symptoms on contact with the foods (oral allergy syndrome), since patients could experience anaphylaxis on ingestion.48
The diagnosis of PCD is based on prick testing, which involves applying a drop of the study allergen on the forearm and puncturing the skin with a lancet. A positive histamine control is always recommended (this should produce a wheal measuring≥3mm), with a negative saline control. The reading is taken at 15minutes, and a reaction is considered positive if it generates a wheal measuring≥3mm.49 Since application of previously prepared food extracts usually generates false negatives, prick-prick testing should be performed with fresh foods.22 Given that the reaction is a type 1 and type 4 hypersensitivity reaction, late readings should be taken.
In our opinion, the term PCD could also be used for generalized exacerbations or in those with an airborne pattern of AD in patients sensitized to proteins (with a positive prick test result).
Proteins and Exacerbations of Atopic DermatitisIn patients with AD, skin barrier defects enable proteins to penetrate the epidermis despite their high molecular weight. Here, they interact with local inflammatory cells to trigger a type 1 and type 4 hypersensitivity reaction.50 Antigen-presenting cells in the epidermis and dermis display higher surface expression of the high-affinity IgE receptor (FcɛRI). After capturing proteins, they migrate to the lymph nodes, where they induce a TH2- and B cell-specific lymphocyte response, with production of specific IgE.51 This would explain both the hypersensitivity reactions and the co-occurrence of urticaria-like and eczematous lesions.
Foods are the most common allergens in atopic pediatric patients. Children are sensitized during the first months of life, when foods are introduced into their diet. Food penetrates the body percutaneously in the context of perioral eczema.52 Therefore, both pediatricians and parents should be taught to treat perioral eczema appropriately until it is fully controlled with topical corticosteroids. Up to one-third of atopic children experience worsening of their dermatitis because of exposure to food, with the most common culprits being egg, milk, and wheat.53 PCD to foods should be suspected in children who experience generalized or severe flare-ups of their AD triggered by exposure to food. Therefore, a full history should be taken with the parents.
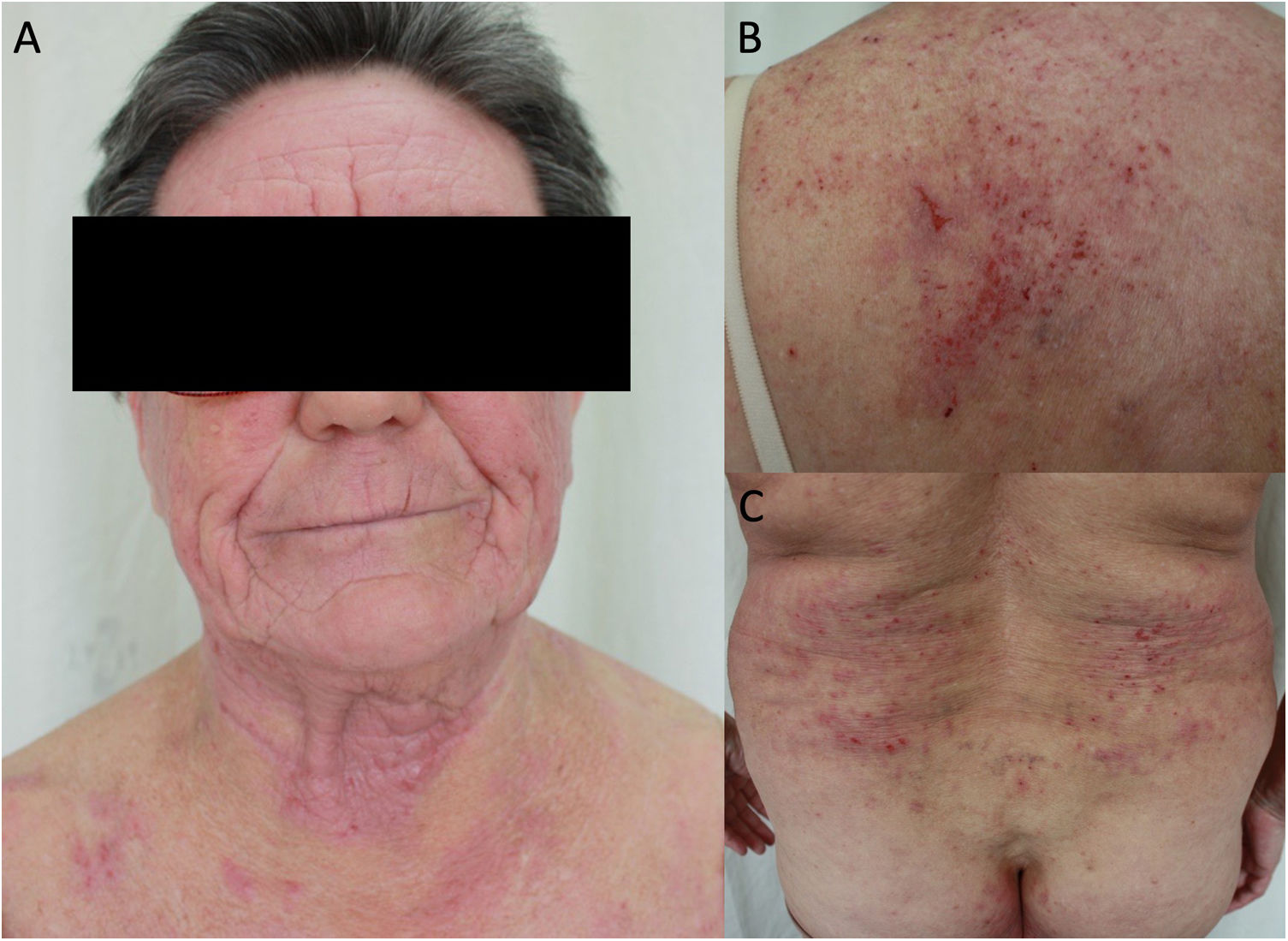
Aeroallergens are more frequent in adults, with the most common being dust mites, animal dander, pollens, and cockroach.50 Aeroallergens can also worsen AD through their inherent proteolytic ability, which aggravates alteration of the barrier function (they also act as irritants or pseudoallergens).50 PCD should be suspected in atopic adults who develop eczema with an airborne pattern in areas such as the face (involvement of the eyelids), neck (involvement of the retroauricular area), and exposed areas of the upper limbs and skin folds (involvement of the axillae and cubital fossa) (Fig. 3).22 In adults, PCD induced by food is less common, although some allergens, such as carrot, hazelnut, and celery, are involved in cross-reactions with aeroallergens and can trigger flare-ups in pollen-sensitized patients.22
Diagnosis of PCD in atopic patients should be based on the prick test when there is clinical suspicion. However, this approach should not be used indiscriminately, and clinical relevance should always be established, since many patients are sensitized to aeroallergens and foods. In other words, sensitization is not synonymous with involvement.22 The atopy patch test was initially put forward as a useful diagnostic method, although it does not currently form part of the recommendations set out in clinical practice guidelines.21,22 The test involves applying proteins such as foods, dust mite, animal dander, or pollen on the skin, followed by an assessment of the eczematous reaction between 24 and 72hours. It has not proven possible to standardize the test, which makes it difficult to differentiate between results indicating irritation and results indicating allergy (especially to dust mites). Determination of serum specific IgE may also be useful, although a negative result does not rule out the diagnosis.48
As for treatment, allergen avoidance has been shown to improve symptoms, although it is insufficient to stop exacerbations of AD owing to the multifactorial nature of the disease.54 In fact, only half of adults sensitized to ≥1 food see an improvement in their symptoms by removing the food from their diet.22 In children, removal of the food that causes flare-ups of AD may lead to a clinical improvement in the skin lesions, although it can induce IgE-mediated allergy.55
Specific immunotherapy is indicated for the treatment of allergic asthma, allergic rhinoconjunctivitis, and hymenoptera venom allergy. It is not currently indicated for treatment of AD. No satisfactory results have been obtained with respect to preventing flare-ups of AD in protein-sensitized patients,22,54 probably because immunotherapy was started with the aim of preventing all the exacerbations the patient experiences and not only to obtain a partial improvement in AD. The main allergen studied is dust mite.56 The results of different trials and meta-analyses are very inconsistent, concluding that scientific evidence in favor of immunotherapy is insufficient to recommend it as a general approach in the treatment of atopic patients.57 However, European AD guidelines recommend considering immunotherapy in selected patients, namely, those with proven sensitization (prick test or serum specific IgE) and flare-ups of AD triggered by exposure to the allergen (mainly dust mite, birch pollen, and grasses).57–59 In contrast with European guidelines, American guidelines do not recommend immunotherapy for treatment of AD.60 Before starting immunotherapy, it is recommended to wait until AD is controlled. It should not be indicated if the patient is receiving immunosuppressive therapy, although it seems that it can be introduced if the patient is receiving dupilumab or tralokinumab.61
Omalizumab is approved for treatment of allergic asthma, chronic rhinosinusitis with nasal polyps, and chronic spontaneous urticaria.62 Its use in AD has not yielded sufficiently satisfactory results.63 However, it is currently being investigated for use in the treatment of food allergy, in which it seems to be effective when administered in monotherapy and in combination with oral immunotherapy.62,64 Ligelizumab is also under study for the treatment of food allergy.65
ConclusionAD is a complex disease with a multifactorial nature. ACD and PCD are allergic diseases that can co-occur with AD and lead to exacerbations. Clinicians should be aware of both conditions and the appropriate diagnostic procedure in order to avoid causative allergens and improve control of the underlying disease. New treatments for AD with specific targets on the TH2 pathway could prove useful for treating specific cases of associated ACD, although they could also modify the results of diagnostic tests.
FundingThe authors declare that no funding was received for the present article.
Conflicts of InterestThe authors declare they have no conflict of interest.