Livedoid vasculopathy (LV) is a cutaneous thrombotic vascular disease of unknown etiology that is associated with a clotting disorder in approximately 40% of patients.1 It is characterized by painful ulcers on the lower limbs, whitish scars (atrophie blanche), and livedo racemosa. It has a major impact on quality of life, is difficult to treat, and lacks clear treatment guidelines.2 Intravenous immunoglobulins, danazole, corticosteroids, immunosuppressive agents, anticoagulants and antithrombotic agents, and hyperbaric oxygen are some of the treatments used, but response rates are highly variable and many patients relapse.2 A phase II clinical trial (n=25),1 2 small retrospective studies,3,4 and a case series5 all recently showed that rivaroxaban, a modern direct-acting oral anticoagulant, is very effective in LV.2 The vast majority of patients evaluated in these studies, however, were followed for under 6 months (12 weeks in the phase II clinical trial).2 The aim of this study was to evaluate clinical response to rivaroxaban in LV patients with follow-up data spanning at least 12 months.
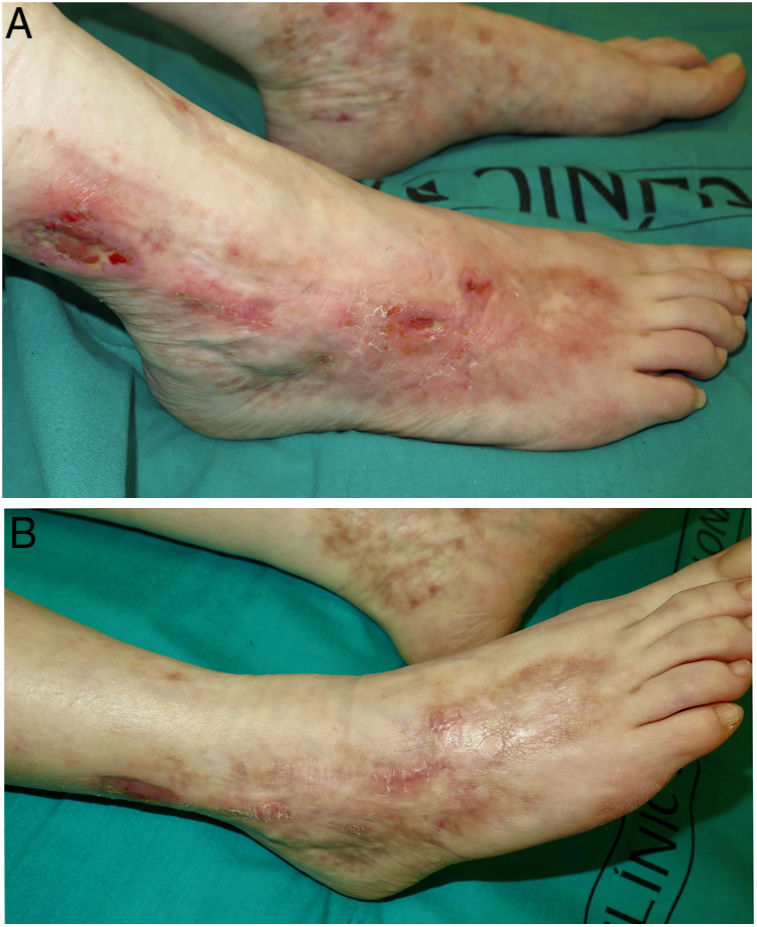
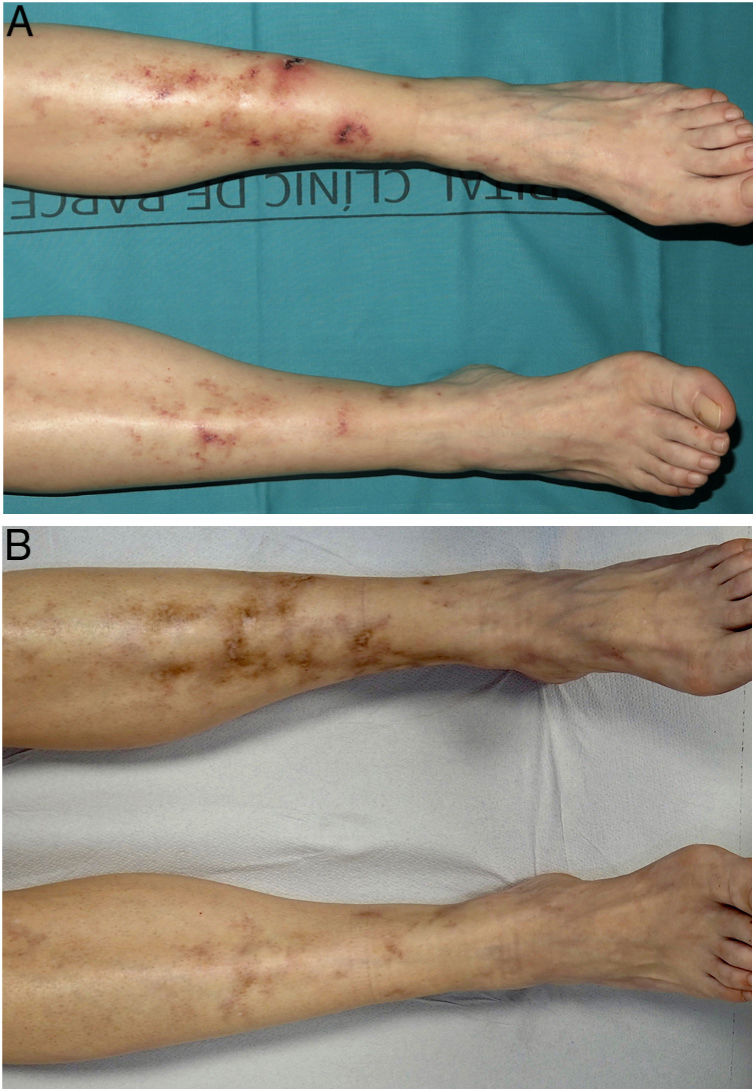
We performed a retrospective study of patients with LV who were treated with rivaroxaban at the dermatology department of a tertiary care hospital in Spain between January 1, 2017 and October 30, 2021. We performed a chart review and collected information on clinical and epidemiological characteristics and treatment responses. Four women aged between 42 and 61 years were included (Table 1). They all had severe leg pain and ulcers and atrophic scars. Coagulation tests and skin biopsies had been performed in all cases. Diagnosis was established by integrating clinical and pathologic findings. The 4 women had shown inadequate responses to multiple previous treatments. Rivaroxaban was started at 10mg/12h. All the patients achieved complete clinical response within 3 months of treatment initiation (Figs. 1 and 2). Maintenance doses ranged from 5 to 10mg/d. Two of the patients (#3 and #4) experienced an LV flare-up following dose reduction, but this was resolved by increasing the dose to 10mg/12h. Following progressive dose reductions, treatment was discontinued in patient #2 after 7 months of treatment. No recurrences were observed over the following 8 months. The other 3 patients continued to receive treatment and maintain complete response. Follow-up times ranged from 12 to 28 months, and no adverse effects were reported.
Clinical and Epidemiological Characteristics and Response to Rivaroxaban in Patients With Livedoid Vasculopathy.
| Case | Sex/age, y | Family history | Personal history | Associated clotting disorder | Clinical presentation | Time to diagnosisa | Previous treatments | Rivaroxaban doses | Clinical response | Follow-up time |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F/42 | No | Seronegative arthritisANA+ 1/80Smoking | Yesa | Pain and ulcers on the feet | 28 y | CS, LMWH, ASA, pentoxifylline, sulfasalazine, hyperbaric oxygen, colchicine, anakinra, alprostadil, etanercept | Start: 10mg/12hAfter 5 mo: 10mg/d | Complete | 12 moNo recurrences |
| 2 | F/61 | No | High blood pressure, glaucoma | No | Foot pain and ulcers; livedo and atrophic scars | 1 y | Pentoxifylline, ASA, dipyridamole | Start: 10mg/12hAfter 2 mo: 10mg/d, then 5mg/d. Discontinued after 7 mo | Complete | 7 moNo recurrences |
| 3 | F/42 | Brother with retinal thrombosis | LPPANA+ 1/320 | Yesa | Foot pain and ulcers; livedo and atrophic scars | 9 y | CS, azathioprine, ASA, dipyridamole | Start: 10mg/12hAfter 10 mo: 10mg/db | Complete | 28 moRecurrence after 6 mo of treatmentc |
| 4 | F/49 | No | No | No | Foot pain and ulcers; purpura, atrophic scars | 24 y | ASA, dipyridamole, LMWH, IVIG | Start: 10mg/12hAfter 6 mo: 10mg/d | Complete | 23 moRecurrence on dose reductionc |
Abbreviations: ANA, antinuclear antibodies; ASA, acetylsalicylic acid; CS, oral corticosteroids; F, female: IVIG, intravenous immunoglobulin; LMWH, low-molecular-weight heparin; LPP, lichen planopilaris.
Rivaroxaban is a selective, reversible inhibitor of factor Xa used in the treatment of thromboembolic diseases and the secondary prevention of thromboembolism following hip or knee replacement surgery.2 It is administered orally and does not require coagulation monitoring, giving it an advantage over warfarin, acenocoumarol, and low-molecular–weight heparins. It also has fewer drug–drug interactions compared with conventional oral anticoagulants.2 A recent systematic review evaluating the effectiveness of rivaroxaban in LV in 13 studies involving 73 patients (65 of whom were on rivaroxaban monotherapy) described an effectiveness rate of 82%. The patients had similar clinical responses, regardless of the presence or absence of an associated clotting disorder.2 Most patients responded rapidly, with significant pain relief observed within 11 days of treatment initiation,1,2 although in some patients it took more than a month.3 The most common starting dose was 10–20mg/d, followed by a maintenance dose of 10mg/d.2 In our series, the dose was reduced to 5mg/d after 6 months in one patient and 14 months in another. One of the patients achieved excellent control, but the other experienced disease worsening.
In our review of the literature, we identified just 9 LV patients treated with rivaroxaban who had follow-up data spanning 12 months or more: 4 responded within 12 months,5,6 2 within 14 months,7,8 1 within 18 months,9 and 2 within 23 months.9 All 9 patients were treated with LV 10mg/d. Just 1 experienced a flare-up after exertion at work, but it was managed successfully with wound care and silver dressings. No adverse effects were reported for any of the patients. The 4 patients in our series were followed for more than 1 year, and 2 of them for 2 years or longer. They all responded fully. Two patients relapsed following a dose reduction, but this was resolved by increasing the dose to 10mg/12h. LV flare-ups inadequately controlled by rivaroxaban can be managed by a dose increase and/or the addition of subcutaneous enoxaparin, which has been found to achieve disease control in 95% of cases.1 Enoxaparin was not needed in any of our patients. Patients can often relapse after discontinuation of treatment, with reports showing a mean time to recurrence of 25 weeks and a higher frequency in the summer.3
Rivaroxaban has shown an excellent safety profile in the treatment of LV. Adverse effects are mild and mostly consist of menorrhagia and hypermenorrhea.2 No adverse effects were reported in our series.
Rivaroxaban appears to be a generally well-tolerated treatment for LV, with rapid onset of action, flexible dosage, and excellent long-term response. We recommend its use as a first-line treatment for this disease.
Conflicts of InterestThe authors declare that they have no conflicts of interest.