Cutaneous manifestations are complicated to treat in rare diseases. The main aim of this study was to analyze the impact of compounded drugs prepared by hospital pharmacists on the quality of life of patients with genodermatoses.
Material and methodsWe undertook a cross-sectional study of patients with genodermatoses treated with topical medications compounded and dispensed by the pharmacy at Complejo Hospitalario Universitario in Pontevedra, Spain. We collected demographic data and answers to questionnaires examining generic and disease-specific quality of life, treatment satisfaction, and treatment adherence.
ResultsNine patients were included. We observed a significant improvement in health-related quality of life following treatment with compounded drugs. Satisfaction with the topical medications was 2.8 on a scale of 0 (greatest satisfaction) to 25. Treatment adherence was greater than 89%.
ConclusionsDrug compounding facilitates access to orphan drugs that are not available for many rare diseases. Few studies, however, have analyzed impact on quality of life in this setting. In this series of patients with genodermatoses, topical medications compounded and dispensed by a hospital pharmacy improved health-related quality of life. This preliminary study has given rise to a multicenter study of compounding for ichthyosis. We expect that analysis of a larger sample will confirm our findings.
El abordaje terapéutico de las manifestaciones cutáneas de las enfermedades raras es complejo. El objetivo principal de este trabajo consistió en determinar el impacto de la formulación magistral de dispensación hospitalaria en la calidad de vida de los pacientes con genodermatosis.
Material y métodosSe diseñó un estudio descriptivo transversal. Se incluyeron pacientes con genodermatosis que recibieron tratamientos tópicos elaborados y dispensados por el Servicio de Farmacia Hospitalaria del Complejo Hospitalario Universitario de Pontevedra. Se recogieron datos demográficos, cuestionarios generales y específicos sobre la calidad de vida, y cuestionarios que evaluaban los tratamientos administrados y la adherencia terapéutica.
ResultadosSe incluyeron 9 pacientes. Se observó que, tras la terapia con fórmulas magistrales, hubo una reducción estadísticamente significativa del impacto en la calidad de vida de los pacientes. La satisfacción con los productos fue 2,8 sobre 25 (siendo 0 la mejor puntuación). La adherencia terapéutica superó el 89%.
ConclusionesLa formulación magistral permite el acceso a medicamentos huérfanos y no comercializados para numerosas enfermedades raras. Su impacto en la calidad de vida de los pacientes afectos de estas enfermedades ha sido escasamente estudiado. En la serie de pacientes que se presenta, la elaboración y dispensación hospitalaria de fórmulas magistrales específicas conllevó efectos positivos en su calidad de vida. Este estudio inicial ha derivado en otro trabajo multicéntrico, centrado en las ictiosis, donde previsiblemente aumentará el número de pacientes a incluir y permitirá confirmar nuestros resultados.
In Europe, the term rare disease refers to diseases that affect fewer than 1 in every 2000 individuals.1 The definition is different in the United States, where rare diseases are considered those that occur in fewer than 200 000 individuals throughout the country.2 Most rare diseases are genetic in origin and treatment is complex.
A large number of rare diseases present with skin involvement. To date, no orphan drugs are commercially available for skin involvement in rare diseases. Furthermore, although case reports and case series have demonstrated the effectiveness of some topical treatments, these can only be used off-label.
Drug compounding allows access to these agents and provides the possibility of adapting the drug to the anatomical site to be treated, with selection of the most appropriate vehicle to ensure greatest absorption. Of note is that the compounded drug is subject to quality regulations, as defined by the European Council in their resolution CM/ResAP (2011), dated January 1, 2011,3 and in Spain by Royal Decree 175/2001, dated February 23, 2001.4 However, there are large differences in terms of health coverage and dispensing. There are also other difficulties such as lack of consensus in terms of drug concentration and type of excipient, resulting in a very heterogenous formulation of the final product.
Since 2017, the Hospital Pharmacy of the Complejo Hospitalario Universitario (CHU), Pontevedra, Spain, in collaboration with other centers of the Spanish national health system, has formed part of a project whose objective is to promote the development of compounded drugs for patients with rare diseases.5 The aim is to improve the quality of life of these patients through the constant development of compounded drugs, making the patient an active participant in the development process.
The most common rare diseases with skin involvement attended by the dermatology department of our hospital are ichthyosis, epidermolysis bullosa (EB), and tuberous sclerosis (TS). The following compounded drugs are available for their treatment: N-acetylcysteine 10% oil/water (O/W) and carbocisteine 10% O/W for ichthyosis,6–10 allantoin 6% O/W for EB,11–13 and rapamycin 0.4% O/W for TS.9,14–18 The selection of these formulations was based on demonstrated efficacy in different published studies, as well as our own experience, where access to these therapies is possible because of personalized compounding and dispensing by the hospital pharmacy.8,10
The main objective of this study was to determine the impact of the individualized compounded formulation and hospital dispensing on the quality of life of patients with genodermatoses. Secondary objectives were to assess patient satisfaction with the organoleptic characteristics of the products and therapeutic adherence.
Material and methodsThis was a cross-sectional descriptive study. Patients with genetic diagnosis of genodermatoses who received topical treatments compounded and dispensed by the hospital pharmacy of the CHU, Pontevedra, Spain, were included. These patients were enrolled between March and December 2019, by the pediatric dermatology department, where they attended a follow-up visit at least once every half year.
Patients who met the inclusion criteria were enrolled consecutively and their demographic data, type of genodermatosis, compounded drug received, and duration of treatment were recorded.
Two different questionnaires were used to evaluate the impact of the individualized compounded drugs on quality of life. One was a generic quality of life measure for dermatology (Dermatology Life Quality Index [DLQI], using the Children's Dermatology Life Quality Index [CDLQI] for pediatric patients between 4 and 16 years) and the other a questionnaire specific to the corresponding dermatosis (Ichthyosis Quality of Life – 32 items [IQoL-32]19 for patients with ichthyosis, Quality of Life evaluation in Epidermolysis Bullosa questionnaire [QOLEB]20 for those with EB, and a modification of the Childhood Atopic Dermatitis Impact Scale21 for those with TS [CADIS-mod]22). Each patient had to respond twice to both questionnaires (generic and specific). One retrospective response corresponded to the period before starting treatment and the second to the time at which the questionnaire was administered with the treatment ongoing. The permission of the authors was obtained for all questionnaires used.
A questionnaire was designed to quantify patient satisfaction with the organoleptic characteristics of the products. The questionnaire comprised 5 questions on smell, color, ease of application, texture, and risk of staining clothing. The score ranged from 0 to 5 for each item with 0 representing the best score and 5 the worst one (with 25 therefore being the worst possible score).
Therapeutic adherence was measured by recording the visits of the patients to the hospital pharmacy where the topical treatment was dispensed and establishing a correlation between the programmed visits and the actual ones.
The study protocol was approved by the Ethics Committee for Clinical Research in Galicia, Spain, and classified by the Agencia Española de Medicamentos y Productos Sanitarios (AEMPS, the Spanish drug approval body) as a “post-authorization study with design other than prospective follow-up.”
The statistical analysis was performed using the R-Statistics program (version R i386 3.4.2). The distribution of frequencies was calculated for qualitative variables, and the mean and standard deviation for quantitative variables. Means were compared using the Student t test. Statistical significance was set at P<.05.
Of note was that this initial study has led to another multicenter study focused on ichthyosis, with a larger sample size in which more robust results may be obtained (registry of patients with ichthyosis treated with compounded drugs and other topical medication in everyday clinical practice, approved by the Ethics Committee for Clinical Research in Galicia, registry code 2020/502).
ResultsNine patients were included (7 of whom were male, with a mean age of 19 years [median 13 years]); 5 had ichthyosis (3 lamellar ichthyosis, 1 recessive X-linked ichthyosis, and 1 ichthyosis vulgaris), 2 EB (1 dystrophic EB pruriginosa and 1 junctional non-Herlitz-type EB) (Table 1).
Characteristics of the 9 Patients Enrolled.
| Sex | Age, ya | Disease | Compounded drug | Treatment duration, mo | DLQI/CDLQI pre-Tx | DLQI/CDLQI post-Tx | |
|---|---|---|---|---|---|---|---|
| Patient 1 | M | 4 | Lamellar icthyosis | N-acetylcysteine 10% O/W | 39 | 15 | 6 |
| Carbocisteine 10% O/W | |||||||
| Patient 2 | M | 6 | Lamellar icthyosis | N-acetylcysteine 10% O/W | 40 | 20 | 9 |
| Carbocisteine 10% O/W | |||||||
| Patient 3 | M | 6 | Lamellar ichthyosis | N-acetylcysteine 10% O/W | 27 | 12 | 2 |
| Carbocisteine 10% O/W | |||||||
| Patient 4 | M | 13 | Recessive x-linked ichthyosis | Carbocisteine 10% O/W | 3 | 28 | 0 |
| Patient 5 | M | 53 | Ichthyosis vulgaris | N-acetylcysteine 10% O/W | 19 | 21 | 10 |
| Carbocisteine 10% O/W | |||||||
| Patient 6 | F | 22 | Dystrophic EB pruriginosa | Allantoin 6% O/W | 2 | 4 | 4 |
| Patient 7 | M | 21 | Non-Herlitz-type flexural EB | Allantoin 6% O/W | 15 | 1 | 1 |
| Patient 8 | F | 38 | TS | Rapamycin 0.4% O/W | 73 | 17 | 0 |
| Patient 9 | M | 7 | TS | Rapamycin 0.4% O/W | 41 | 5 | 5 |
Abbreviations: EB, epidermolysis bullosa; TS, tuberous sclerosis; F, female; M, male; O/W, oil in water; Tx, treatment.
The compounded drugs administered were those described in the introduction (Table 2). Treatment duration ranged from 2 to 73 months (mean 29 months; median 27 months).
Compounded Drugs Used.
| N-acetylcysteine 10%+urea 5% O/W | |
| Ingredients | Quantity |
| N-acetylcysteine | 10.00g |
| Urea | 5.00g |
| Glycerin | 5.00g |
| Sodium hydroxide | 2.00g |
| Sterile water | 51.50mL |
| Neo PCL O/Wa | 25.00g |
| Essence of rosemary | 1.50mL |
| Carbocisteine 10% + Urea 5% O/W | |
| Ingredients | Quantity |
| Urea | 5.00g |
| Sterile water | 44.00mL |
| Neo PCL O/Wa | 25.00g |
| Carbocisteine | 10.00g |
| Glycerin | 15.00g |
| Sodium hydroxide | 1.00g |
| Allantoin 6% O/W | |
| Ingredients | Quantity |
| Allantoin | 6.00g |
| Glycerin | 5.00g |
| Neo PCL O/Wa | 25.00g |
| Sterile water | 64.00mL |
| Rapamycin 0.4% O/W | |
| Ingredients | Quantity |
| Rapamycin | 400.00g |
| Glycerin | 0.50g |
| Cream O/W | 100.00g |
Abbreviation: O/W, oil in water.
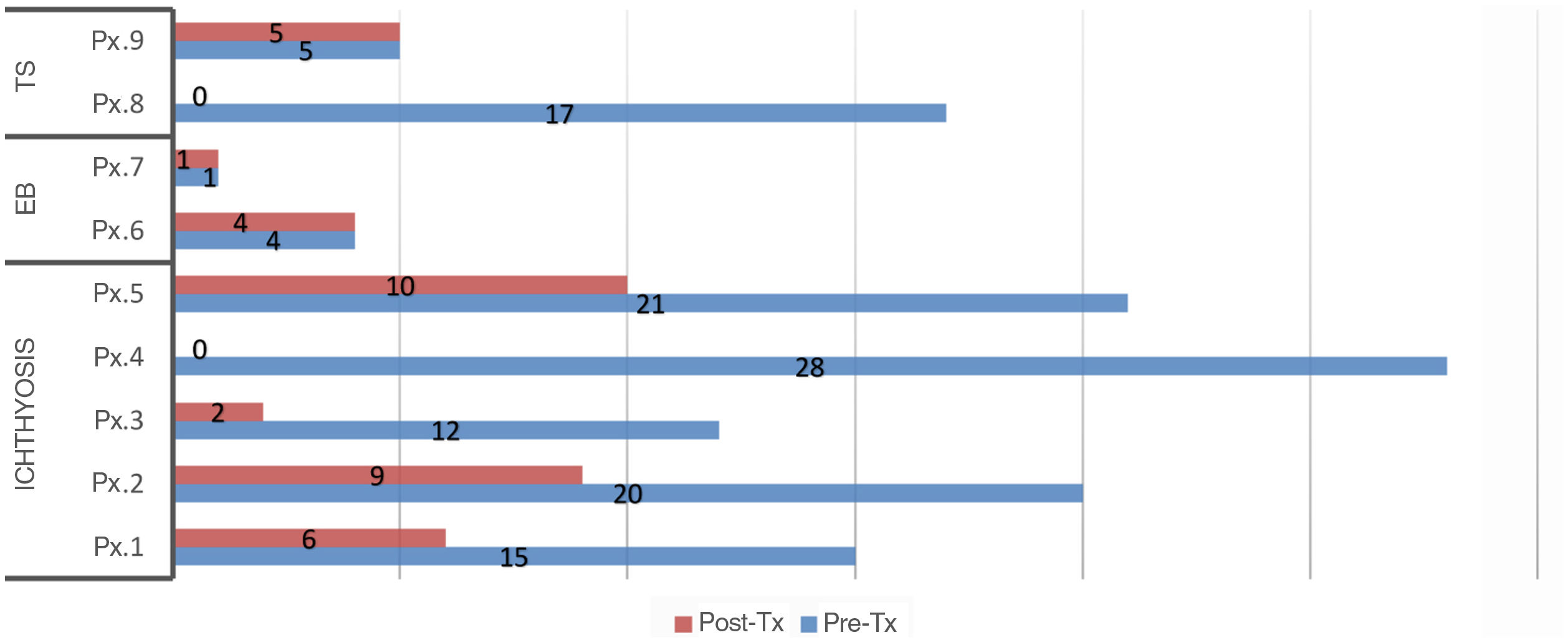
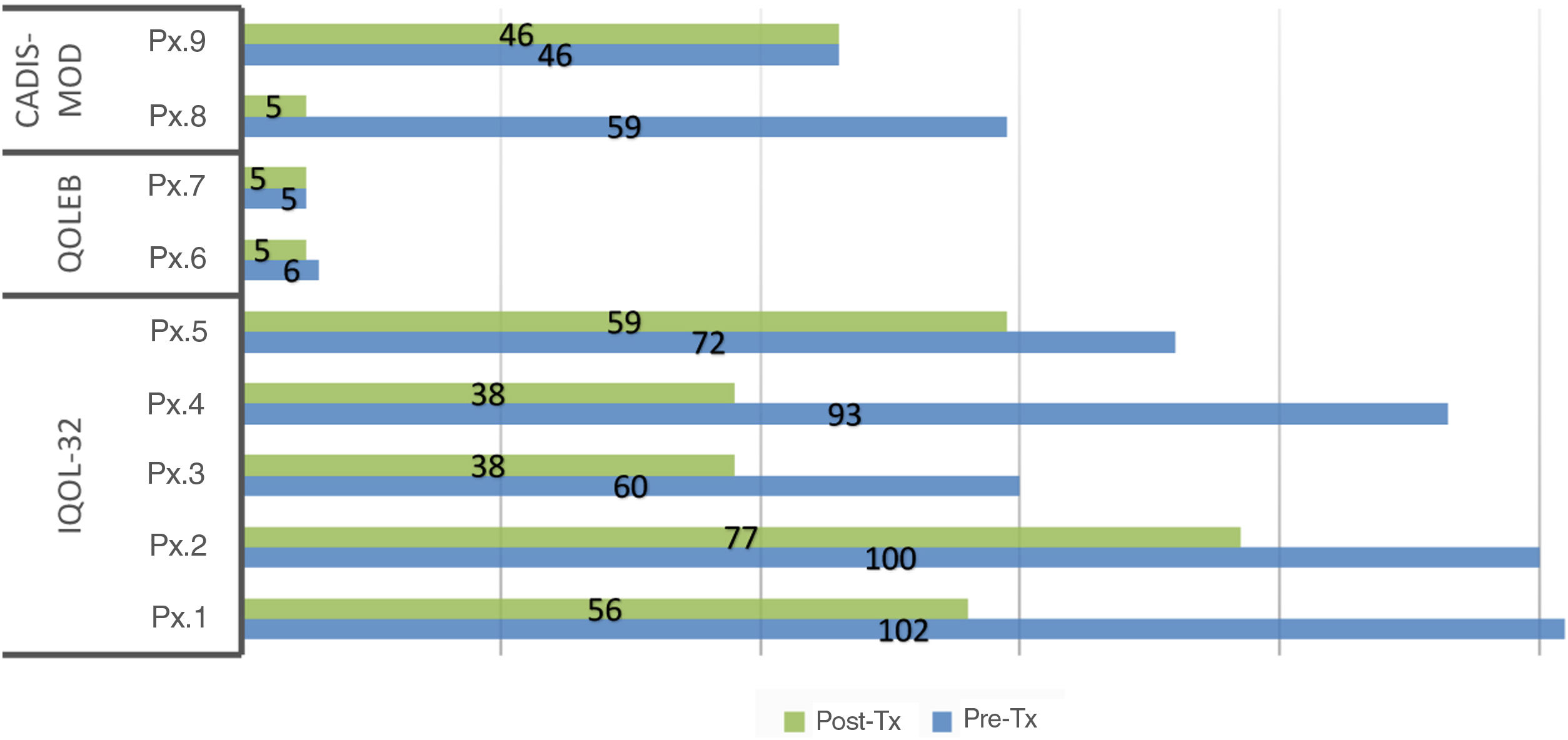
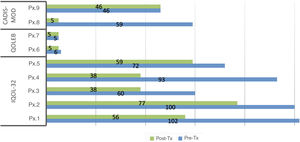
A decrease in the mean scores on both the generic and specific quality-of-life questionnaires was observed after administration of compounded drugs (Table 3). This decrease was statistically significant for DLQI/CDLQI and for IQoL-32. In 55% of the patients, the impact on quality of life decreased by at least 2 levels of the DLQI/CDLQI after administration of the corresponding topical treatment. In all patients with ichthyosis, there was a decrease in the scores on the quality of life, both with the generic and specific questionnaires. However, there were no changes in the generic questionnaire in patients with EB and only 1 of the patients improved by a point on the specific questionnaire. In the case of TS, only 1 of the 2 patients improves their score on the generic and specific questionnaires after treatment. The individual scores on each of the questionnaires are shown in Figs. 1 and 2.
Mean (SD) scores on the quality of life questionnaires.
| Pretreatment | Post-treatment | P | |
|---|---|---|---|
| DLQI/CDLQI | 13.7±9.0 | 4.1±3.7 | .007 |
| IQoL-32 | 85.4±18.5 | 53.6±16.3 | .008 |
| QOLEB | 5.5±0.7 | 5.0±0 | .25 |
| CADIS-mod | 52.5±9.2 | 25.5±29.0 | .25 |
Abbreviations: CADIS-mod, modified Childhood Atopic Dermatitis Impact Scale Caregiver Quality of Life; CDLQI, Children's Dermatology Life Quality Index; DLQI, Dermatology Life Quality Index; IQoL-32, Ichthyosis Quality of Life – 32 items; QOLEB, Quality of Life in Epidermolysis Bullosa.
Scores on the generic quality of life (DLQI/CDLQI) questionnaires before and after treatment. Abbreviations: CDLQI: Children's Dermatology Life Quality Index; DLQI: Dermatology Life Quality Index; EB: epidermolysis bullosa; Pre-Tx: pretreatment; Post-Tx: post-treatment; Px: patient; TS: tuberous sclerosis (DLQI/CDLQI score: 0–30; degrees: no effect, small effect moderate effect large effect extremely large effect).
Scores on the specific quality-of-life questionnaires before and after treatment.* Abbreviations: CADIS-mod: modified Childhood Atopic Dermatitis Impact Scale Caregiver Quality of Life (score: 0–100, 100 worst effect; adult version: 0–84, 84 worst effect); IQoL-32: Ichthyosis Quality of Life – 32 items (score: 0–128, 128 worst effect); Pre-Tx: pretreatment; post-Tx: post-treatment; Px: patient; QOLEB: Quality of Life in Epidermolysis Bullosa (score: 0–51, 51 worst effect). *A non-validated translation of the validated QOLEB was used.
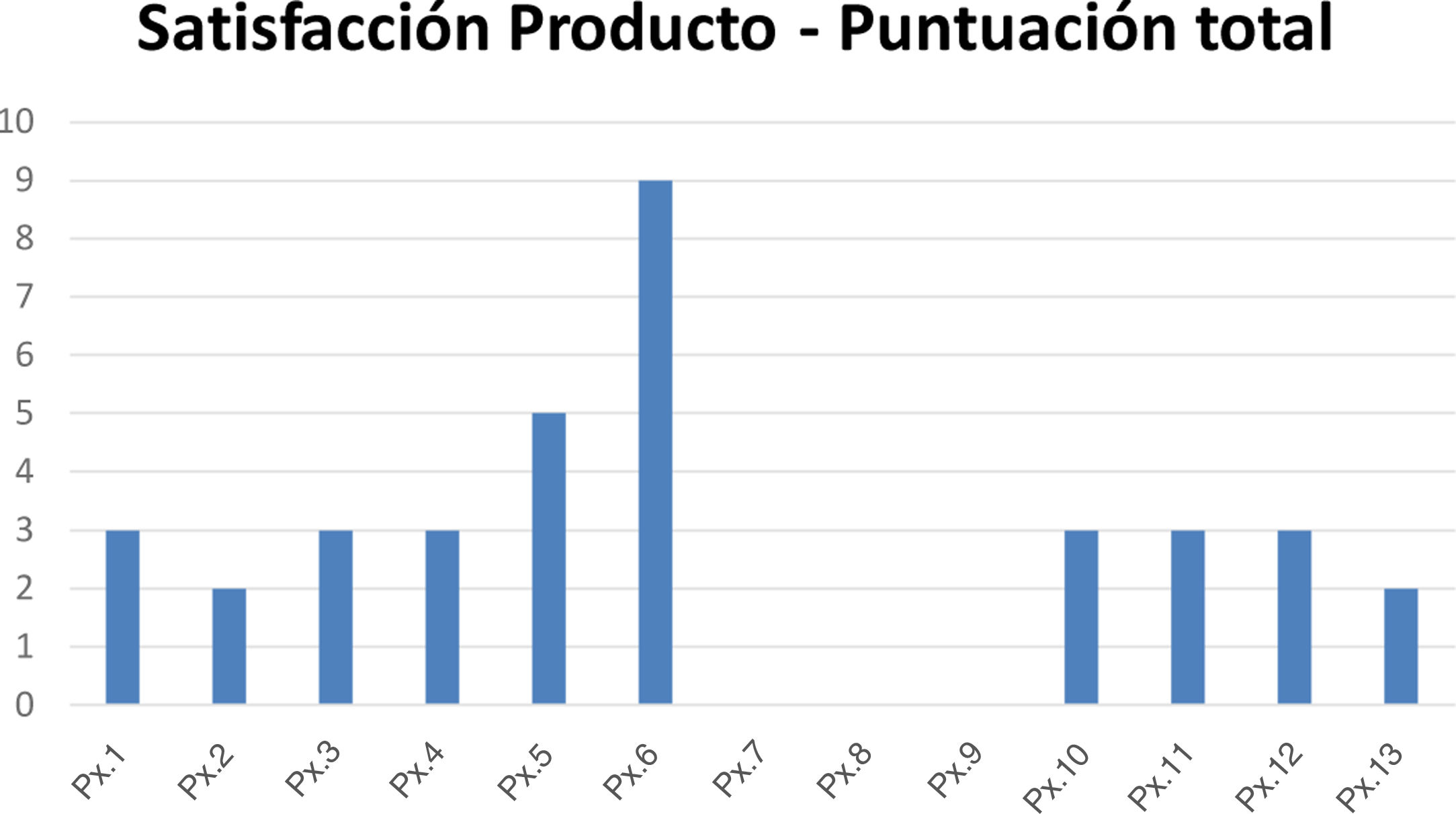
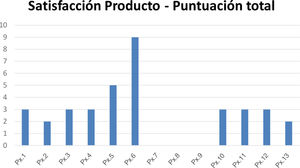
The mean score on the questionnaire on satisfaction with the organoleptic characteristics of the products was 2.8, with a median of 3 (Fig. 3).
Score on the questionnaire on satisfaction with the applied product. The questionnaire comprised 5 items (smell, color, ease of application, texture, and risk of staining clothing). Each item has a score of 0–5, with 5 corresponding to the worst score. The total can vary from 0 to 25, with 25 being the worst score. Abbreviation: Px, patient.
The correlation between planned visits for collecting treatment and the actual visits was greater than 89% in all cases.
DiscussionThis study showed an improvement in quality of life for most patients with genodermatosis treated with compounded drugs.
The patients with ichthyosis included in the study showed a significant improvement in their quality of life, as reflected by both the generic and specific questionnaires. N-acetylcysteine and urea are effective in the treatment of congenital ichthyosis.6–9 However, the main disadvantage of N-acetylcysteine is its bad smell, hindering adherence to treatment.6–9 Carbocisteine is a similar molecule that does not have this problem and has shown similar efficacy.9,10 To date, studies have not been conducted that analyze the impact of these therapies on quality of life. Dreyfus et al.23 and Mazereeuw-Hautier et al.24 investigated the factors that influence the quality of life of patient with ichthyosis. These studies showed that there is a more vulnerable population for whom the impact of the disease is greater. The vulnerable population essentially comprises female patients with symptoms of pain or impaired mobility, signs such as severe scaling, deterioration in interpersonal relationships, and impact on daily life due to the economic cost of the disease. The authors suggest that these patients should be the main target for improvements in therapeutic management.23 As shown by the present study, personalized treatment without direct costs for the patient could be very beneficial.
In the case of EB, there are several articles that have analyzed its impact on quality of life, and several scales have been developed to measure this effect.20,25–29 Allantoin has been designated by the European Commission and the United States Food and Drug Administration as an orphan drug for the treatment of EB.13 Although studies on its efficacy and safety have been published recently,11,12 we have not found any that investigate the drug's impact on the quality of life. In the present study, only 1 of the 2 patients with EB experienced a slight quality of life improvement after administration of allantoin. In patient 6, allantoin 6% cream improved healing of lesions, but its effectiveness was not correlated with improved DLQI scores, with only a 1-point improvement in QOLEB. Patient 7 had dystrophic EB pruriginosa, a type of EB that does not usually present erosions, and this may explain the lack of therapeutic response.
Regarding the use of topical rapamycin for TS, most literature reports suggest that it is an effective and safe treatment for managing and preventing facial angiofibromas.9,14–18 The benefit is greater if it is applied at early ages.14 It is usually a well-tolerated treatment, but often causes local irritation, particularly when applied as a solution. Crall et al.22 suggested that the presence of facial angiofibromas, as well as the limited therapeutic options, significantly impact the quality of life of patients with TS and their caregivers. They also suggested that, although topical rapamycin is effective, access to the drug is difficult.22 In the present study, application of the drug to the adult patient (patient 8) achieved an excellent response and a notable improvement in quality of life. In contrast, no changes were observed in CDLQI or CADIS-mod in the pediatric patient (patient 9). This could be due to the lower number of lesions and their more incipient nature in the child.
In all cases, patient satisfaction with the organoleptic characteristics of the products was less than 10, and was greater than 3 in only 2 patients (possible score of 0–25, with 25 representing the worst value). This reflects a general satisfaction with compounded drugs, demonstrating the benefits of a personalized compounding and dispensing. Dispensing of drugs by the hospital pharmacy, regular visits to the dermatology service, and continuous communication between the 2 contributed to an improved relationship with the patients and a greater awareness of their satisfaction with the organoleptic characteristics of the products. This feedback enabled a continuous improvement and adaptation of the compounded drugs to the specific needs of each patient, empowering the patient and making them participants in the final product. This is also reflected in a greater treatment adherence by the patients in the study.
Further prospective studies are needed with a larger number of patients to confirm the effects of these therapies on the quality of life of the patients with genodermatoses.
LimitationsOne of the main limitations of our study is the low number of patients enrolled, all with very different genodermatoses. Likewise, there is a possibility of recall bias, as one of the copies of each of the quality-of-life questionnaires was completed retrospectively. Of note is that the questionnaire used to assess satisfaction with the product characteristics had not been validated.
ConclusionsDrug compounding allows access to orphan drugs that are not available on the market but that are nevertheless effective. In the population studied, drug compounding significantly improved the quality of life of patients with ichthyosis. Individualized elaboration and direct dispensing in hospitals had a positive impact on therapeutic adherence and empowerment of patients in the management of their disease. There is a strong case for extending the hospital drug compounding to other diseases to increase the number of patients who benefit.
Conflicts of interestThe authors declare that they have no conflicts of interest.
The authors would like to thank Jennifer Huang, Sarah L. Chamlin, John Frew, Isabelle Dreyfus, and Dedee F. Murrel for their permission to use the questionnaires on the impact on quality of life, as well as for the use of the DLQI/CDLQI questionnaires.