The past 5 years have seen a proliferation of new treatments for atopic dermatitis (AD). We analyzed recent drug survival data for cyclosporine in this setting. Because the Spanish National Healthcare system requires patients with AD to be treated with cyclosporine before they can be prescribed other systemic treatments, drug survival for cyclosporine may be shorter than in other diseases.
Material and methodMulticenter, observational, prospective cohort study using data from the Spanish Atopic Dermatitis Registry (BIOBADATOP). Data from the Spanish Registry of Systemic Treatments in Psoriasis (BIOBADADERM) were used to create a comparison cohort.
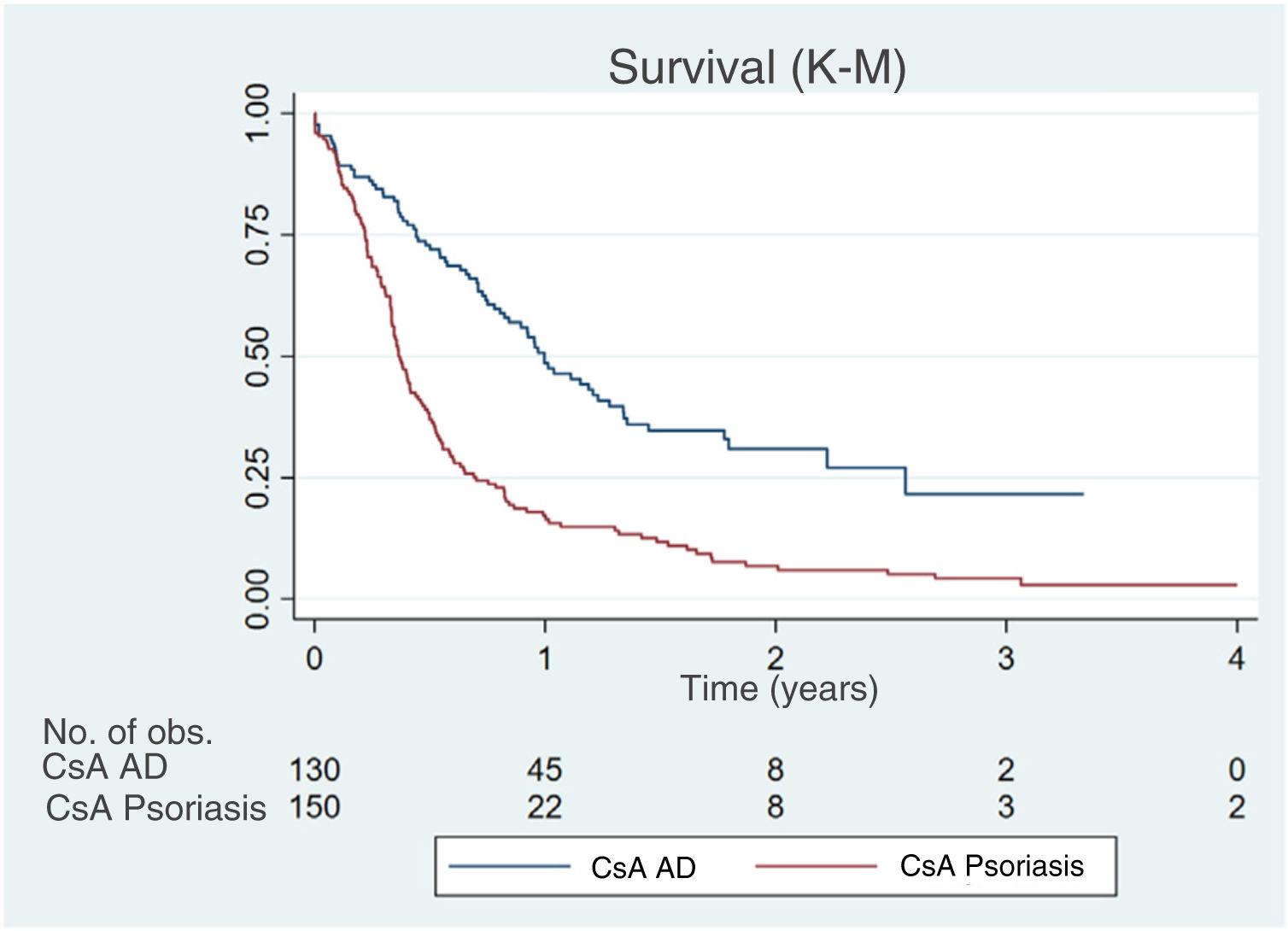
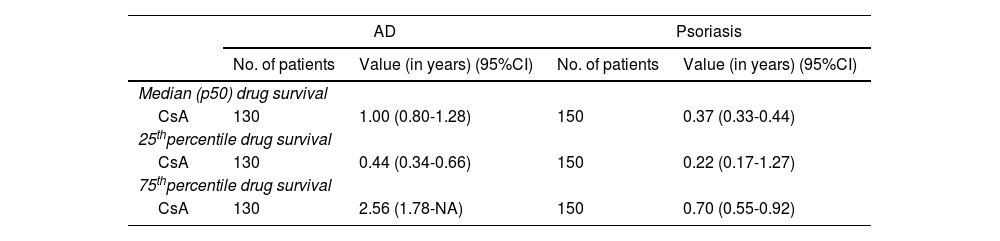
ResultsWe analyzed data for 130 patients with AD treated with cyclosporine (median drug survival, 1 year). Median cyclosporine survival in the psoriasis comparison group (150 patients) was 0.37 years. Drug survival was significantly longer in AD than in psoriasis (P<.001).
ConclusionDrug survival of cyclosporine in the BIOBADATOP registry is similar to that described in other series of patients with AD and longer than that observed in the BIOBADADERM psoriasis registry.
Debido a la eclosión en el último quinquenio de nuevas alternativas terapéuticas para la dermatitis atópica (DA), nos planteamos estudiar la supervivencia actual de la ciclosporina (CsA) en esta patología. La CsA, como paso necesario solicitado por el Sistema Nacional de Salud de España para la autorización de otros tratamientos sistémicos, podría presentar una supervivencia menor que en otras enfermedades.
Material y métodoEstudio multicéntrico, observacional, de cohortes prospectivo para el que se recogieron pacientes incluidos en el Registro Español de Dermatitis Atópica (BIOBADATOP). Como cohorte de comparación se emplearon los datos del Registro Español de tratamientos sistémicos en Psoriasis (BIOBADADERM).
ResultadosSe incluyeron 130 pacientes diagnosticados de DA que habían recibido CsA (mediana de supervivencia de CsA: 1 año). En el grupo comparador se incluyeron 150 pacientes psoriásicos que habían recibido CsA (mediana de supervivencia: 0,37 años). Observamos una mayor supervivencia de la CsA en los pacientes con DA en comparación con los pacientes psoriásicos (p<0,001).
ConclusiónLa supervivencia de la CsA en BIOBADATOP es similar a la descrita en otras series de pacientes con DA, y superior a la observada en los pacientes con psoriasis en el registro BIOBADADERM.
Atopic dermatitis (AD) is a chronic immune-mediated disease believed to affect 20% and 8% of the pediatric and adult populations, respectively.1 Moderate and severe forms of the disease can be detrimental to the quality of life of the patients and their surroundings.2 Itching, sleep disturbances, and changes to social interaction behaviors are among the factors that negatively impact these patients’ quality of life, which can lead to a higher risk of psychiatric comorbidity, specifically a higher prevalence of anxiety, depression, and suicidal ideation.2,3 Additionally, the economic burden of this disease on the patients and their families is significant.4
The management of moderate-to-severe AD is complex and continues to be challenging for clinicians. Due to the emergence of new therapeutic alternatives to treat AD in recent years, including biologic drugs (dupilumab and tralokinumab) and Janus kinase inhibitors (upadacitinib, baricitinib, and abrocitinib), we decided to study the current survival of cyclosporine (CsA) in the management of this condition. Specifically, we hypothesized that the fact that CsA treatment is a mandatory step for the Spanish National Health System to be authorized to use other systemic treatments for AD 5 may be associated with a lower survival rate compared to other diseases.
The objective of this study is to describe the survival of CsA in the management of AD, with the hypothesis that CsA—prescribed as a necessary step requested by the Spanish National Health System prior to the use of innovative treatments—may be administered temporarily, resulting in lower survival.
MethodThis was a multicenter, observational, and prospective cohort study of the patients enrolled in the Spanish Registry of Atopic Dermatitis (BIOBADATOP) who would have started CsA therapy from March 2020 (the registry starting date) through January 2023.6
The aim of the study was to determine the survival time of CsA in moderate-to-severe AD. Survival time was defined as the time between the initiation of the drug and its discontinuation for any reason. Demographic variables, those associated with the disease and the drug, and the reason for its discontinuation were collected.
As a comparison cohort, data from the Spanish Registry of Systemic Treatments in Psoriasis (BIOBADADERM) were used.7 The reason for using the BIOBADADERM registry as a comparator was that CsA is also indicated to treat psoriasis, and in this condition, it is not necessary to prescribe it as a step prior to biologic treatments, so its survival should not be impacted by this factor.
Statistical analysis was performed to obtain the median survival, as well as the 25th and 75th percentiles of survival. Similarly, Kaplan-Meier survival curves were used to show the survival of both drugs. The log-rank test was used for the comparative analysis of drug survival.
Statistical analysis was conducted using the Stata 17 statistical software package (StataCorp. 2021). P values <.05 were statistically significant.
Both studies were approved by a research ethics committee (BIOBADATOP: CEIC Aragon no. 22/2018, BIOBADADERM: CEIC Hospital Universitario Doce de Octubre no. 216/07), and all patients gave their prior written informed consent to participate in the study.
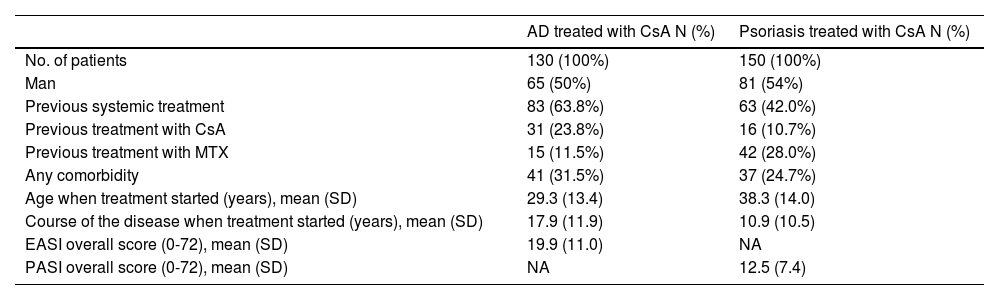
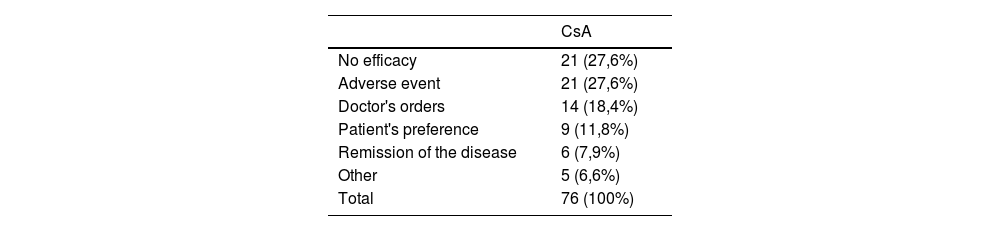
ResultsA total of 130 patients diagnosed with AD treated with CsA were included (50% men; mean age, 29.3 years). The baseline EASI (Eczema Area and Severity Index) of those who started CsA treatment was 19.9, and the mean course of the disease when treatment started, 17.9 years. A total of 63.8% of the patients on CsA had previously received treatment with some kind of systemic drug (Table 1). The CsA median survival in AD was 1 year (95%CI, 0.80-1.28) (Table 2). The most frequent reasons for discontinuing CsA were lack of drug efficacy (27.6%), and the presence of adverse events (27.6%) (Table 3).
Baseline characteristics of patients diagnosed with AD and psoriasis on CsA.
| AD treated with CsA N (%) | Psoriasis treated with CsA N (%) | |
|---|---|---|
| No. of patients | 130 (100%) | 150 (100%) |
| Man | 65 (50%) | 81 (54%) |
| Previous systemic treatment | 83 (63.8%) | 63 (42.0%) |
| Previous treatment with CsA | 31 (23.8%) | 16 (10.7%) |
| Previous treatment with MTX | 15 (11.5%) | 42 (28.0%) |
| Any comorbidity | 41 (31.5%) | 37 (24.7%) |
| Age when treatment started (years), mean (SD) | 29.3 (13.4) | 38.3 (14.0) |
| Course of the disease when treatment started (years), mean (SD) | 17.9 (11.9) | 10.9 (10.5) |
| EASI overall score (0-72), mean (SD) | 19.9 (11.0) | NA |
| PASI overall score (0-72), mean (SD) | NA | 12.5 (7.4) |
AD, atopic dermatitis; CsA, cyclosporine; MTX, methotrexate; NA, not applicable; SD, standard deviation.
Survival of CsA in AD and psoriasis.
| AD | Psoriasis | |||
|---|---|---|---|---|
| No. of patients | Value (in years) (95%CI) | No. of patients | Value (in years) (95%CI) | |
| Median (p50) drug survival | ||||
| CsA | 130 | 1.00 (0.80-1.28) | 150 | 0.37 (0.33-0.44) |
| 25thpercentile drug survival | ||||
| CsA | 130 | 0.44 (0.34-0.66) | 150 | 0.22 (0.17-1.27) |
| 75thpercentile drug survival | ||||
| CsA | 130 | 2.56 (1.78-NA) | 150 | 0.70 (0.55-0.92) |
AD, atopic dermatitis; CsA, cyclosporine; NA, data unavailable due to insufficient follow-up.
In the group of the patients with psoriasis used as a comparator, a total of 150 patients treated with CsA were included (54% men; mean age, 38.3 years). The baseline PASI (Psoriasis Area and Severity Index) was 12.5, and the mean course of the disease when treatment started, 10.9 years (Table 1). The median survival of CsA in this group of patients was 0.37 years (4 months) (95%CI, 0.33-0.44) (Table 2).
In the Kaplan-Meier curve shown in figure 1, the survival of CsA in AD and psoriasis can be observed, being the survival of CsA significantly higher in the AD than in the psoriasis group (log-rank test; P <.001).
DiscussionAD is one of those diseases that has experienced a significant growth in the development of therapeutic alternatives in recent years, which has led to a therapeutic paradigm shift. Currently, the Spanish National Health System has established criteria for the funding of advanced systemic therapies based on a severity level measured by the EASI score> 21, and previous use of CsA, except for cases contraindicated.5 These access conditions could be impacting the prescription patterns, potentially resulting in lower CsA survival compared to other diseases. Based on this hypothesis, we studied the survival of CsA in the Spanish registry of systemic treatments of AD from the Spanish Academy of Dermatology and Venereology (AEDV), and then compared it with that of psoriasis.8 Contrary to the hypothesis, we saw a higher CsA survival rate in the AD group.
Survival studies show therapeutic management in the real-world routine clinical practice for diseases in which efficacy, safety, and physician preferences are beyond the ideal clinical trial conditions.9 In recent years, several trials have been published on the survival of classic systemic drugs and biologics to treat AD.1,10–17
The CsA survival data we found after 1 year of treatment are slightly better than those described in the current scientific medical literature available. For example, Van der Schaft et al. reported a 1-year CsA survival rate of 34% (compared to 48.6% from our study), while Law Ping Man et al. reported a median CsA survival of 8 months (compared to 1 year in our study).10,17 In a retrospective Italian series of 95 patients with AD on CsA, only 21.02% of the patients were still on this drug on week 72.11
Recently, the survival of CsA has been compared to dupilumab, with the latter showing higher survival rates.1,11,13,14,16 Organ toxicity, drug interactions, the need for monitoring, and the availability of other alternatives with better efficacy and safety profiles are factors that significantly limit the survival of classic immunosuppressors compared to biologic drugs.11,12,14,16,18
To interpret drug survival results, it is essential to consider the reasons for their discontinuation. Regarding CsA in the management of AD, the presence of adverse events accounts for 20% to 50% of all discontinuations,13,15–17 which are similar rates to the findings made in our study where we reported a 27.6% discontinuation rate due to adverse events.
Comparison of CsA survival in the management of atopic dermatitis and psoriasisRegarding the comparison between the two diseases, we should mention that CsA had higher survival in the management of AD compared to psoriasis. We found twice as many patients still on this drug after 1 year of treatment in the AD group (48.6%) vs the psoriasis group (17.2%). Several factors may contribute to this difference, being the main one the lower number of approved therapeutic alternatives to treat AD. For example, from 2017 through 2021, dupilumab, CsA, and corticosteroids were the only systemic treatments approved by the European Medicines Agency to treat severe AD. Also, we should mention that patients with AD and EASI scores <21 included in the BIOBADATOP registry currently do not have access to funded therapeutic alternatives such as biologic drugs or JAK inhibitors, which irrevocably leads to prolonged CsA courses of treatment.5,19 Another factor that may contribute to these findings is the different CsA prescription practices seen in these two conditions.
Limitations and advantagesAs limitations of our study, we should acknowledge the small sample size and potential differences in the characteristics of the patients with AD and psoriasis. Other significant and inevitable limitations have to do with the use of drug survival as an indirect measurement of efficacy, safety, or both, which is impacted by multiple external factors such as the availability of other drugs or dosing intervals.20 However, we should mention the value of this prospective study based on data from numerous centers, since most survival studies published to date are retrospective trials. Also, this study reflects the use of CsA in the management of AD in the real-world routine clinical practice, at a time when the treatment of this condition is at a turning point.
ConclusionsIn conclusion, we observed a median survival of CsA to treat AD of 1 year, which was higher compared to that of patients with psoriasis. The requirement by the Spanish National Health System for prior CsA use in patients with moderate-to-severe AD to access innovative therapies does not seem to negatively impact survival. Access requirements to advanced systemic therapies could change in the future, likely modifying CsA survival in the management of AD.
FundingThe BIOBADATOP project has been promoted by the Piel Sana Foundation of the Spanish Academy of Dermatology and Venereology (AEDV), and received financial support from pharmaceutical companies such as Sanofi, Abbvie, Pfizer, and Leo-Pharma. We should mention that the collaborating pharmaceutical companies were not involved in the study design or execution; data curation, management, analysis or interpretation; manuscript preparation, review or approval; or in the decision to submit the manuscript for publication.
Conflicts of interestC. Couselo-Rodríguez has participated as a sub-investigator or speaker in projects sponsored by Abbvie, Sanofi, Leo-Pharma, Lilly, UCB, Novartis, Pierre Fabre, and Janssen. A. Batalla has been involved in training activities and attended courses and conferences sponsored by Abbvie, Celgene, Faes Pharma, Isdin, Janssen, Leo-Pharma, Lethipharma, Lilly, Mylan, Novartis, Pierre Fabre, and Sanofi. Also, she has been a sub-investigator in clinical trials sponsored by Abbvie, Celgene, Leo-Pharma, Lilly, Novartis, Pfizer, and Sanofi. She has also engaged in consulting activities for Abbvie and Sanofi. J.M. Carrascosa has participated as a principal investigator/sub-investigator and/or received honoraria as a speaker and/or member of expert, or steering committees for Abbvie, Novartis, Janssen, Lilly, Sandoz, Amgen, Almirall, BMS, Boehringer Ingelheim, Biogen, and UCB. P. Chicharro has participated in consultations, colloquia, and clinical trials organized by the Janssen Pharmaceuticals, Almirall, Sanofi Genzyme, Lilly, Abbvie, Novartis, Leo-Pharma, and Pfizer-Wyeth. A. González Quesada has participated as a consultant, speaker, and been involved in clinical trials for Abbvie, Pfizer, Novartis, Sanofi, Boehringer, Bristol-Meyer, Leo-Pharma, Janssen. P. de la Cueva has participated as an advisor, and/or investigator, and/or speaker with Abbvie, Almirall, BMS, Boehringer, Celgene, Janssen, Leo-Pharma, Lilly, MSD, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi, and UCB. A.M. Giménez-Arnau has served as a medical advisor for Uriach Pharma / Neucor, Genentech, Novartis, FAES, GSK, Sanofi-Regeneron, Amgen, Thermo Fisher Scientific, Almirall, Celldex, and Leo-Pharma. Also, she has participated as a researcher in projects sponsored by Uriach Pharma, Novartis, Instituto Carlos III-FEDER, and been engaged in training activities sponsored by Uriach Pharma, Novartis, Genentech, Menarini, Leopharma, GSK, MSD, Almirall, Sanofi, and Avene. Y. Gilaberte has served as an advisor for Isdin, Roche Posay, and Galderma; given lectures for Almirall, Sanofi, Avene, Rilastil, Lilly, Uriage, Novartis, Cantabria Labs; and participated in research projects for Almirall, Sanofi, Pfizer, Abbvie, and Leo-Pharma. M. Rodríguez-Serna has participated as a speaker and consultant for Novartis, Sanofi, Abbvie, Pfizer, Lilly, and Leo-Pharma; she has been the principal investigator in clinical trials sponsored by Novartis, Abbvie, and Leo-Pharma. T. Montero-Vilchez has participated as a consultant, in colloquia, and in clinical trials for Abbvie, Almirall, Incyte, Leo-Pharma, Lilly, Novartis, Sanofi, Pfizer-Wyeth, and UCB Pharma and Instituto de Salud Carlos III. M. Elosua-González has been a researcher and/or speaker for Abbvie, Lilly, Galderma, Leo-Pharma, Pfizer, UCB Pharma, and Sanofi Genzyme. J.F. Silvestre-Salvador declared no conflicts of interest regarding this article. M. Munera-Campos has received fees for scientific advisory, presentations, or other related activities from Abbvie, Leo-Pharma, Janssen, Sanofi, and Galderma. She has also served as a principal investigator and sub-investigator in clinical trials sponsored by Lilly, Leo-Pharma, Novartis, Janssen, Sanofi, Pfizer, Abbvie, Almirall, UCB, and Galderma. J. Sánchez-Pérez declared no conflicts of interest regarding this article. G. Carretero declared no conflicts of interest regarding this article either. C. Mauleón-Fernández has participated in clinical trials on atopic dermatitis sponsored by Sanofi and Leo-Pharma. L. Curto-Barredo received speaker fees and advisory fees from Novartis, Sanofi, Abbvie, Lilly, Leo-Pharma, and Menarini. A. Ballano-Ruiz declared no conflicts of interest regarding this article. R. Botella-Estrada participated as a consultant, speaker, or investigator in clinical trials sponsored by Pfizer, Abbvie, Almirall, Novartis, Janssen, Leo-Pharma, Lilly, Celgene, Roche, and SunPharma. S. Arias-Santiago declared no conflicts of interest regarding this article. F.J. Navarro-Triviño declared no conflicts of interest regarding this article. G. Roustán-Gullón received fees for scientific advisory and courses from Sanofi, Abbvie, Lilly, Pfizer, and Leo-Pharma. I. Betlloch declared no conflicts of interest regarding this article. E. del Alcázar participated as a speaker and investigator in clinical trials sponsored by Amgen, Almirall, Janssen, Lilly, Leo-Pharma, Novartis, UCB, and Abbvie. M.T. Abalde-Pintos declared no conflicts of interest regarding this article. I. García-Doval received travel expenses and training at scientific congresses sponsored by Abbvie, MSD, Pfizer, and Sanofi. M.Á. Descalzo declared no conflicts of interest regarding this article. Á. Flórez conducted clinical trials and acted as a speaker and consultant for Abbvie, Almirall, Amgen, Janssen, Leo-Pharma, Lilly, Novartis, Pfizer, Sanofi, and UCB Pharma.
We wish to thank all the researchers of BIOBADATOP and BIOBADADERM, for making an extra effort in clinical practice regarding data curation, and the patients and/or their legal representatives for agreeing to be included in the respective registry.