Atypical nevus syndrome has been described as one of the main risk factors for melanoma. The aim of this study was to analyze dermoscopic changes observed in melanocytic lesions over a follow-up period of 5 years in patients with atypical nevus syndrome.
Material and methodsWe conducted a retrospective follow-up study of a cohort of patients seen at a specialized skin cancer and digital body mapping clinic in Medellin, Colombia, between January 2017 and December 2022. We analyzed the dermoscopic changes observed during this period and explored their association with newly diagnosed melanoma.
ResultsA total of 368 patients (187 women) with a median (interquartile range) age of 43 (37-51) years were included. The dermoscopic features observed at 5 years were an atypical network (222 patients, 60.3%), asymmetric globules (163, 44.2%), white-gray regression areas (105, 28.5%), lesion regression (72, 19.5%), a negative pigment network (59, 16%), asymmetric eccentric pigmentation (28, 7.6%), asymmetric projections (21, 5.7%), and asymmetric vascular patterns (8, 2.1%). Melanoma was diagnosed in 12.2% of patients during follow-up. Features significantly associated with a shorter time to melanoma onset were grayish-white areas (P<.001), asymmetric globules (P=.011), asymmetric eccentric pigmentation (P=.047), and a negative pigment network (P=.001).
ConclusionsThe main dermoscopic features of melanocytic lesions in patients with atypical nevus syndrome associated with progression to melanoma were grayish-white areas, asymmetric globules, asymmetric spots, and a negative pigment network.
El síndrome de nevus atípico se ha considerado uno de los factores más importantes para el desarrollo de melanoma. El objetivo de este estudio fue describir los cambios dermatoscópicos de las lesiones melanocíticas en pacientes con diagnóstico de síndrome de nevus atípicos, durante el seguimiento digital en 5 años.
Material y métodosSe realizó un estudio retrospectivo de seguimiento a una cohorte de pacientes atendidos en un consultorio particular, especializado en cáncer de piel y mapeo digital corporal, localizado en Medellín (Colombia), entre enero de 2017 y diciembre de 2022. Se analizaron las características dermatoscópicas encontradas y su relación con el diagnóstico de un melanoma.
ResultadosSe incluyeron 368 pacientes, con una mediana de edad de 43 años RIQ (37-51) de los cuales,187 fueron mujeres. Al finalizar el seguimiento, 222 (60,3%) presentaron red atípica, 163 (44,2%) glóbulos asimétricos, 105 (28,5%) regresión blanco gris, 72 (19,5%) regresión de la lesión, 59 (16%) retículo invertido, 28 (7,6%) pigmento excéntrico asimétrico, 21 (5,7%) proyecciones asimétricas y 8 (2,1%) asimetría en el patrón vascular. A los 60 meses de seguimiento a un 12,2% se les diagnosticó un melanoma. Las áreas blanco-grisáceas, los glóbulos asimétricos, el pigmento excéntrico asimétrico y el retículo invertido fueron las estructuras dermatoscópicas que se relacionaron significativamente con un tiempo menor para la presentación de melanoma (p<0,001, p=0,011, p=0,047 y p=0,001, respectivamente).
ConclusionesEn conclusión, se encontró que las principales características dermatoscópicas de las lesiones melanocíticas en pacientes con nevus displásicos relacionadas con la progresión a melanoma fueron la aparición de áreas blanco-grisáceas, los glóbulos asimétricos, las manchas asimétricas y el retículo invertido.
Atypical nevus syndrome was first described by Clark back in 1978 as a skin phenotype defined by the presence of multiple melanocytic lesions in a familial context where an increased incidence of skin melanoma had been reported.1 In 1985, Elder proposed the theory of sporadic dysplastic nevus as a possible precursor to sporadic melanoma.2 Various studies have confirmed that the presence of dysplastic nevi increases the risk of developing melanoma significantly, indicating that these lesions are not only precursors but also non-neligible risk factors for the development of melanoma.3
Melanoma accounts for approximately 80% of skin cancer-related deaths, despite being representative of only 4% of this type pf cancers.4 Therefore, early diagnosis is of paramount importance since only 14% of the patients with metastatic disease survive >5 years.4,5 Since atypical nevus syndrome has been considered one of the most important factors in the development of melanoma, routine check-ups and studies of these patients are essential.2 However, distinguishing atypical melanocytic lesions from early or emerging melanomas is challenging in today's routine clinical practice.6
The primary support method for the follow-up strategy is digital dermatoscopy, also known as epiluminescence microscopy. This non-invasive diagnostic tool captures dermoscopic images of melanocytic lesions and monitors their evolution over time, and is extremely useful in the early diagnosis of melanoma.7 Total body mapping allows for photographic documentation of the entire body surface, digital dermatoscopy of selected melanocytic lesions, follow-up of the evolution of these lesions over time, while keeping a record of all significant changes of morphology, architecture, and pigmentation that may have occurred to these lesions.6,8
The objective of this study was to describe the dermatoscopic changes reported at the 5-year digital follow-up of melanocytic lesions in patients diagnosed with atypical nevus syndrome, determine the frequency of these structures, establish their relationship with the diagnosis of melanoma, and eventually describe if this patient population had additional risk factors for the development of melanomas.
Materials and methodsThis was a health record-based cohort, retrospective, observational, and follow-up trial. Patients were annually monitored since they enterered the digital follow-up program until their last recorded assessment in their health record. The study included patients treated from January 2017 through December 2022. Data were collected from November 2022 through January 2023. The study was conducted with patients from a private clinic specialized in the management of skin cancer and total body mapping (FotoFinder ATBM Classic) from Medellin, Colombia, with a mean annual volume of nearly 2700 patients treated. The research protocol was approved by the Universidad CES Human Research Ethics Committee (code 218). The reporting of this study follows the STROBE recommendations.9
The study included patients with phototypes II, III, and IV who met the following inclusion criteria: 1) diagnosis of atypical nevus syndrome, defined as the presence of >3 of the following criteria: a) presence of >2 clinically atypical nevi, b) >100 nevi in patients between 20 and 50 years old, c) >50 nevi in patients younger than 20 years or older than 50 years, d) >1 nevus on the buttocks or instep, e) nevi on the anterior region of the scalp; 2) histopathological confirmation of some degree of dysplasia or melanoma, before or at the follow-up; 3) aged >20 years. On the other hand, patients with the following parameters were excluded from the study: 1) clinical suspicion of carrying high susceptibility genes for melanoma and other cancer risk conditions, such as patients with medium to giant congenital nevi, immunosuppressed individuals, or patients with genodermatoses (xeroderma pigmentosum, Gorlin-Goltz syndrome); 2) patients without, at least, 1 annual digital follow-up check for, at least, 3 years.
The primary outcome variable was defined as melanoma-free time. The intermediate outcome variable was digital dermatoscopy changes categorized as the presence or absence of each of the following characteristics: increase in size, novelty, regression, atypical network, gray-white regression, asymmetric globules, inverted reticulum, asymmetric projections, asymmetric spot, and asymmetry in vascular pattern. These, along with other characteristics, have been described in the medical literature available as structures specific to melanomas.10,11
Two independent observers took duplicate measurements. In cases of evaluator disagreement, the difference was resolved by a third evaluator. Demographic, and clinical variables were also taken into consideration as possible factors associated with changes in the digital dermatoscopy. The pathology lab test results (mild, moderate, and severe dysplasia, in situ melanoma, invasive melanoma) were obtained, and the past medical history of dysplasia and melanoma was recorded.
Radiation exposure was assessed through patient self-reporting, and by inquiring about the frequency and duration of sun exposure during childhood, adolescence, and adulthood. The number of sunburns throughout the patient's entire life was also discussed. Eventually, the use of tanning beds was characterized as a dichotomous variable.
The family history of melanoma was also considered in first- and second-degree relatives by looking into health records.
Patients were asked at the follow-up on whether they used sunscreen daily.
Information on digital dermatoscopy changes was obtained from clinical and dermoscopic photographic records (FotoFinder ATBM Classic). The remaining variables were obtained from the health records.
Information was obtained by researchers from the Dermatology working group at Universidad CES from health records drafted by an expert female dermatologist in the management of skin cancer and digital dermatoscopy. Information on the confirmation of mild, moderate, and severe dysplasia, in situ melanoma, and invasive melanoma was obtained by the pathology lab test results.
No a priori sample size estimation was ever performed. In the post hoc analysis, with a 95% confidence interval for the log-rank test, it was estimated that the statistical power for HRs >1.4 was >80%.
The descriptive analyses of the categorical variables were expressed as absolute and relative frequencies. A normality test was performed on all quantitative variables using the Kolmogorov-Smirnov test by calculating means and standard deviations, or medians and interquartile ranges. The analysis of digital dermatoscopy changes was performed using the generalized linear model and the binomial family with logarithmic link function, while assessments were considered as repeated measurements. The rates of change with a confidence interval for each year of follow-up were shown too. The melanoma-free survival rate was calculated using the Kaplan-Meier method, the analysis of characteristics associated with dermatoscopy, and other clinical and sociodemographic characteristics of the patients. Additionally, their association with the melanoma-free survival rate was analyzed using Cox regression analyses. Observed and adjusted estimates with confidence intervals and P values<.05 were shown too.
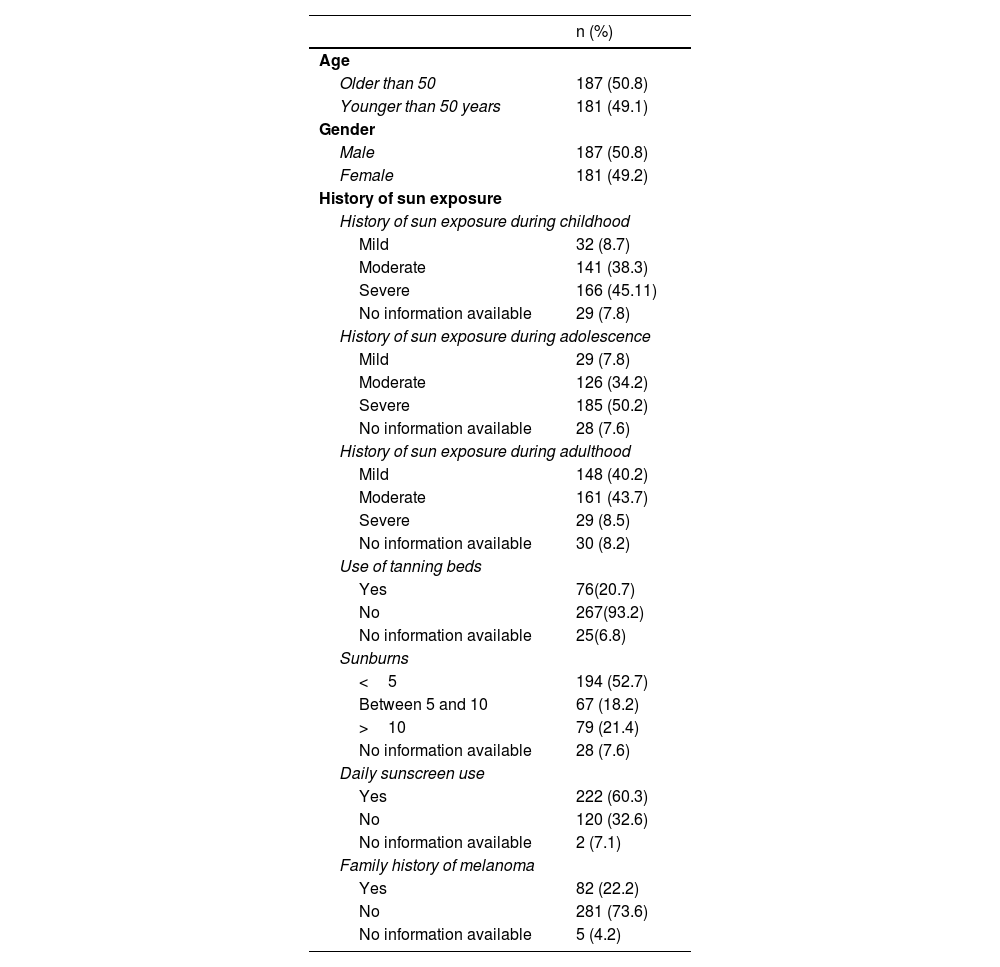
ResultsA total of 989 out of 1357 potentially eligible patients who were under digital dermatoscopy follow-up were excluded for being unable to meet the inclusion criteria. Finally, the study included a total of 368 patients with a median age of 43 years (IQR, 37-51], 187 of whom (50.8%) were women. Regarding the patients’ demographic and clinical characteristics, a history of intense sun exposure during adolescence was reported in 185 patients (50.2%). Also, 76 patients (20.7%) had been previously exposed to tanning beds, and 79 (21.4%) had a past medical history of >10 sunburns. The daily of sunscreen was reported by 222 patients (60.3%), and a family history of melanoma was found in 82 patients (22.2%) (table 1).
Demographic characteristics and past medical history.
| n (%) | |
|---|---|
| Age | |
| Older than 50 | 187 (50.8) |
| Younger than 50 years | 181 (49.1) |
| Gender | |
| Male | 187 (50.8) |
| Female | 181 (49.2) |
| History of sun exposure | |
| History of sun exposure during childhood | |
| Mild | 32 (8.7) |
| Moderate | 141 (38.3) |
| Severe | 166 (45.11) |
| No information available | 29 (7.8) |
| History of sun exposure during adolescence | |
| Mild | 29 (7.8) |
| Moderate | 126 (34.2) |
| Severe | 185 (50.2) |
| No information available | 28 (7.6) |
| History of sun exposure during adulthood | |
| Mild | 148 (40.2) |
| Moderate | 161 (43.7) |
| Severe | 29 (8.5) |
| No information available | 30 (8.2) |
| Use of tanning beds | |
| Yes | 76(20.7) |
| No | 267(93.2) |
| No information available | 25(6.8) |
| Sunburns | |
| <5 | 194 (52.7) |
| Between 5 and 10 | 67 (18.2) |
| >10 | 79 (21.4) |
| No information available | 28 (7.6) |
| Daily sunscreen use | |
| Yes | 222 (60.3) |
| No | 120 (32.6) |
| No information available | 2 (7.1) |
| Family history of melanoma | |
| Yes | 82 (22.2) |
| No | 281 (73.6) |
| No information available | 5 (4.2) |
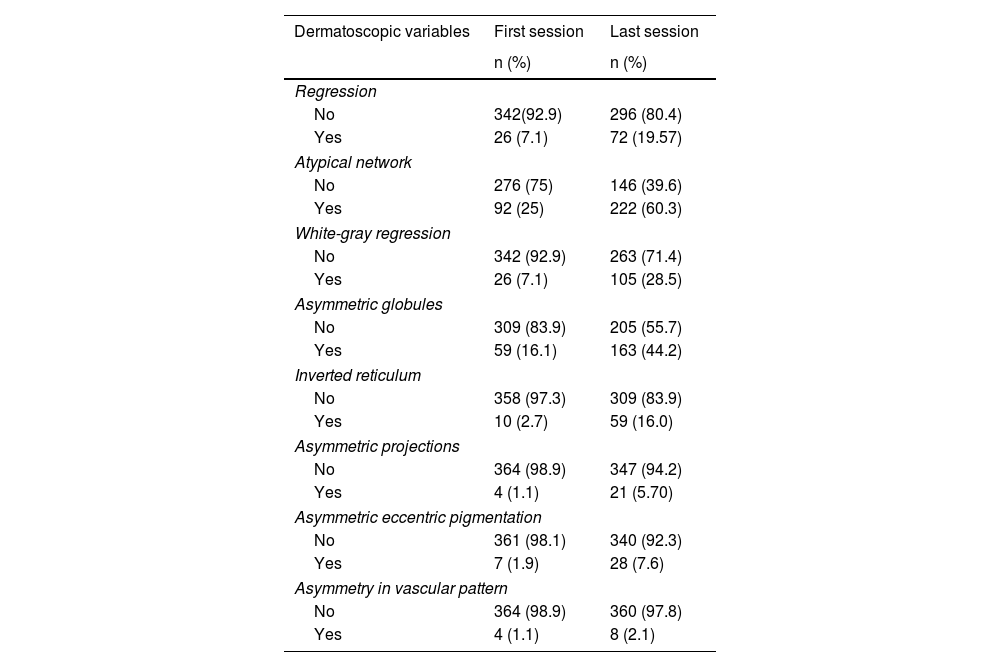
In the initial findings, 92 patients (25%) showed atypical networks, 59 (16.1%) asymmetric globules, 26 (7.1%) white-gray regression, 26 (7.1%) lesion regression, 10 (2.7%) inverted reticulum, 7 (1.9%) asymmetric blotch, 4 (1.1%) asymmetric projections, and 4 (1.1%) asymmetry in vascular pattern. At the end of the follow-up, a total of 222 patients (60.3%) showed atypical networks, 163 (44.2%) asymmetric globules, 105 (28.5%) white-gray regression, 72 (19.5%) lesion regression, 59 (16%) inverted reticulum, 28 (7.6%) asymmetric eccentric pigmentation, 21 (5.7%) asymmetric projections, and 8 (2.1%) asymmetry in vascular pattern (table 2).
Findings in the dermatoscopic variables.
| Dermatoscopic variables | First session | Last session |
|---|---|---|
| n (%) | n (%) | |
| Regression | ||
| No | 342(92.9) | 296 (80.4) |
| Yes | 26 (7.1) | 72 (19.57) |
| Atypical network | ||
| No | 276 (75) | 146 (39.6) |
| Yes | 92 (25) | 222 (60.3) |
| White-gray regression | ||
| No | 342 (92.9) | 263 (71.4) |
| Yes | 26 (7.1) | 105 (28.5) |
| Asymmetric globules | ||
| No | 309 (83.9) | 205 (55.7) |
| Yes | 59 (16.1) | 163 (44.2) |
| Inverted reticulum | ||
| No | 358 (97.3) | 309 (83.9) |
| Yes | 10 (2.7) | 59 (16.0) |
| Asymmetric projections | ||
| No | 364 (98.9) | 347 (94.2) |
| Yes | 4 (1.1) | 21 (5.70) |
| Asymmetric eccentric pigmentation | ||
| No | 361 (98.1) | 340 (92.3) |
| Yes | 7 (1.9) | 28 (7.6) |
| Asymmetry in vascular pattern | ||
| No | 364 (98.9) | 360 (97.8) |
| Yes | 4 (1.1) | 8 (2.1) |
The inter-observer agreement percentage was >97%.
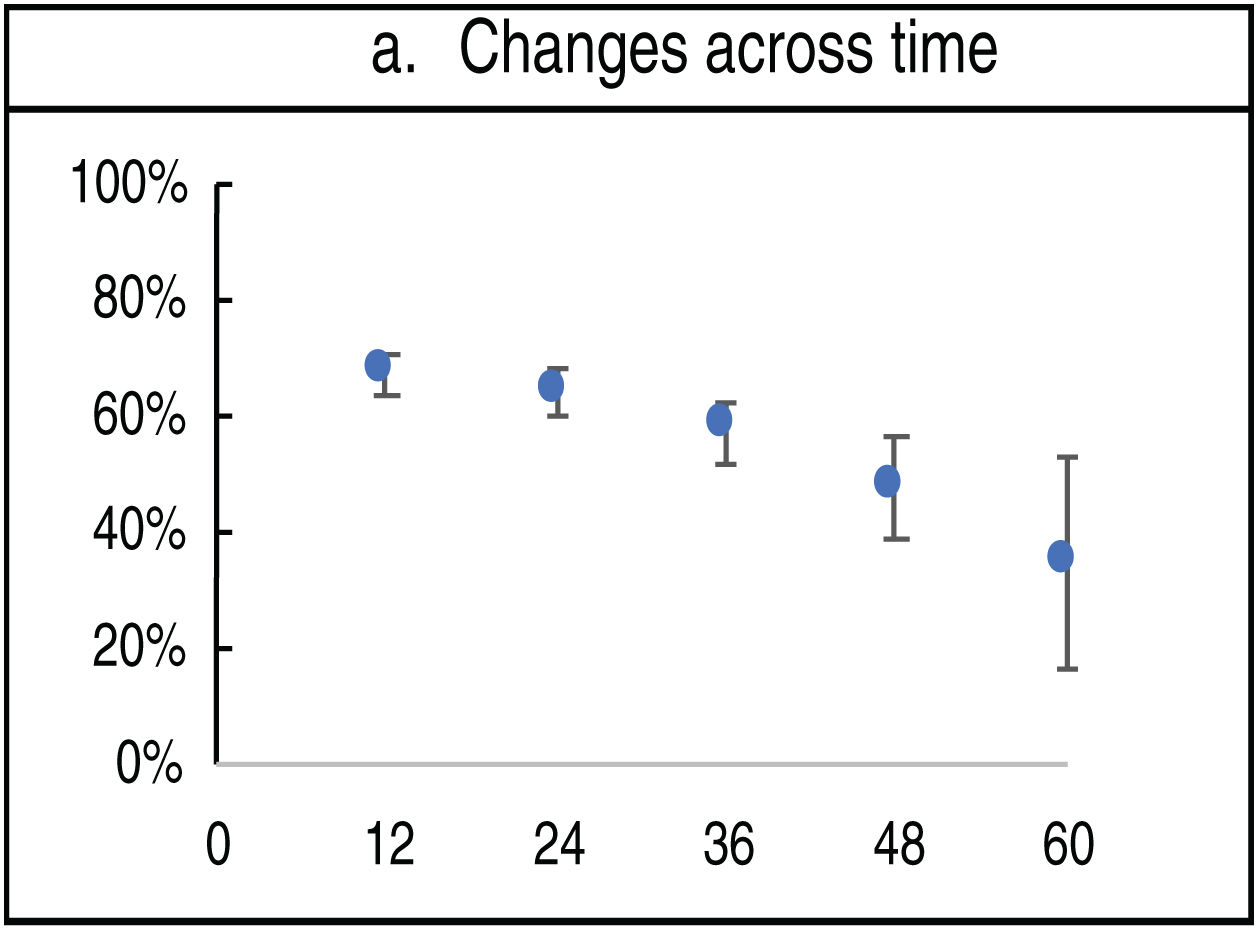
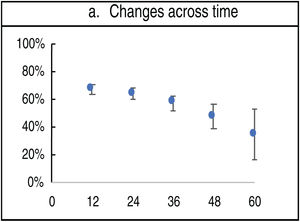
The appearance of changes on the dermatoscopy decreased over time (12 months vs 60 months) (67% vs 34%) (fig. 1).
The median follow-up for all patients included in the study was 24.60 months (IQR, 20-72).
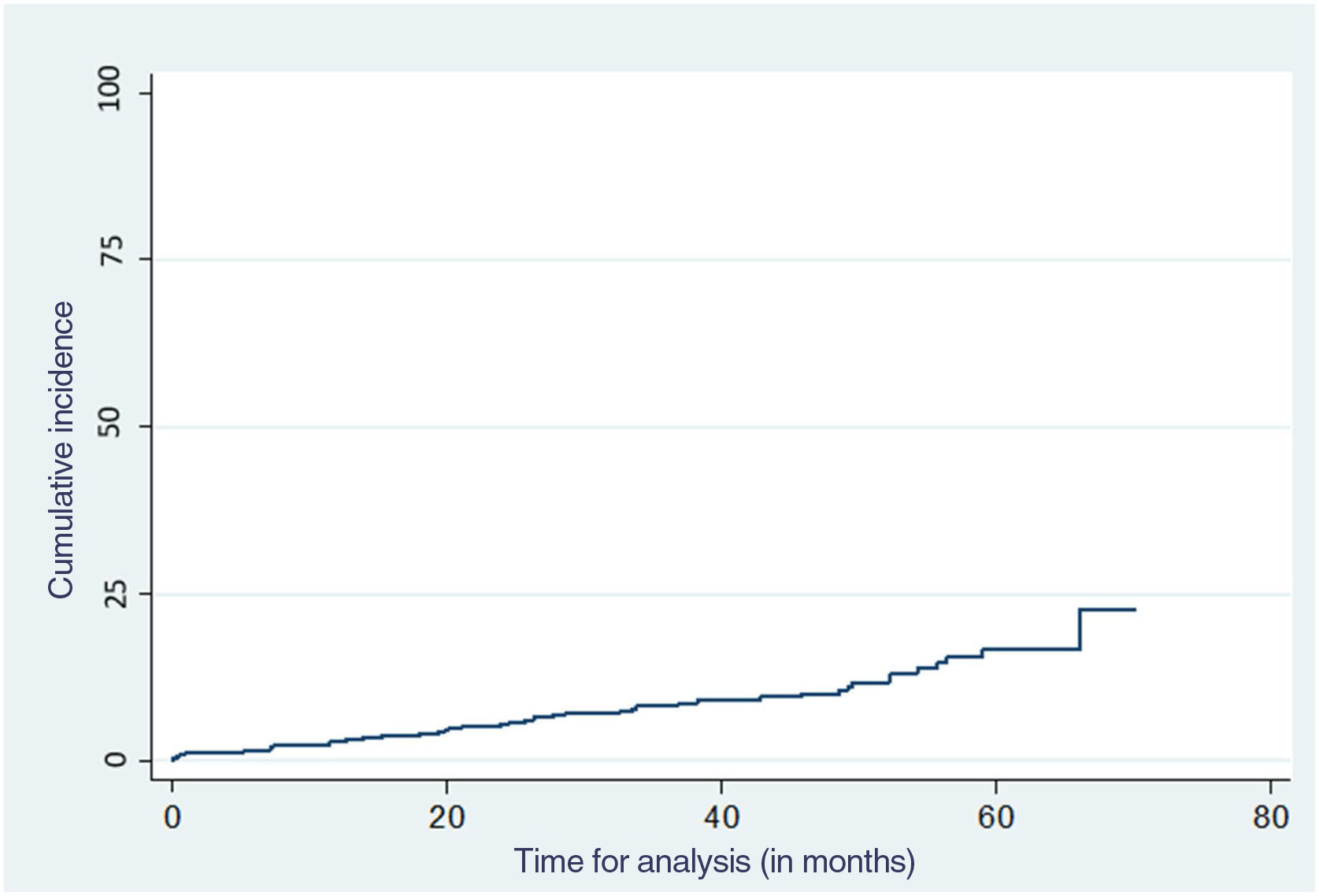
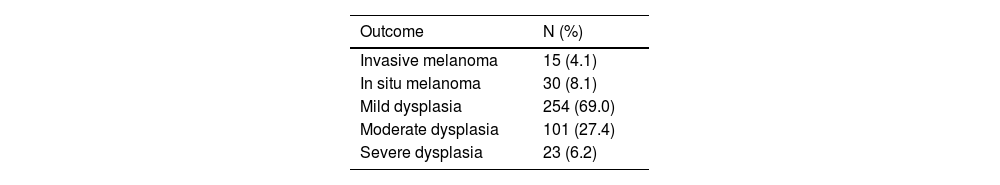
At the end of the follow-up, 45 patients (12.2%) developed melanomas, 15 (4.0%) invasive melanomas, and 30 (8.2%) in situ melanomas. Three of the patients who developed invasive melanoma also had in situ melanomas. Regarding dysplasia, 254 patients (69%) had mild dysplasia, 101 (27.5%) moderate dysplasia, and 23 (6.3%) severe dysplasia at the end of the follow-up (table 3).
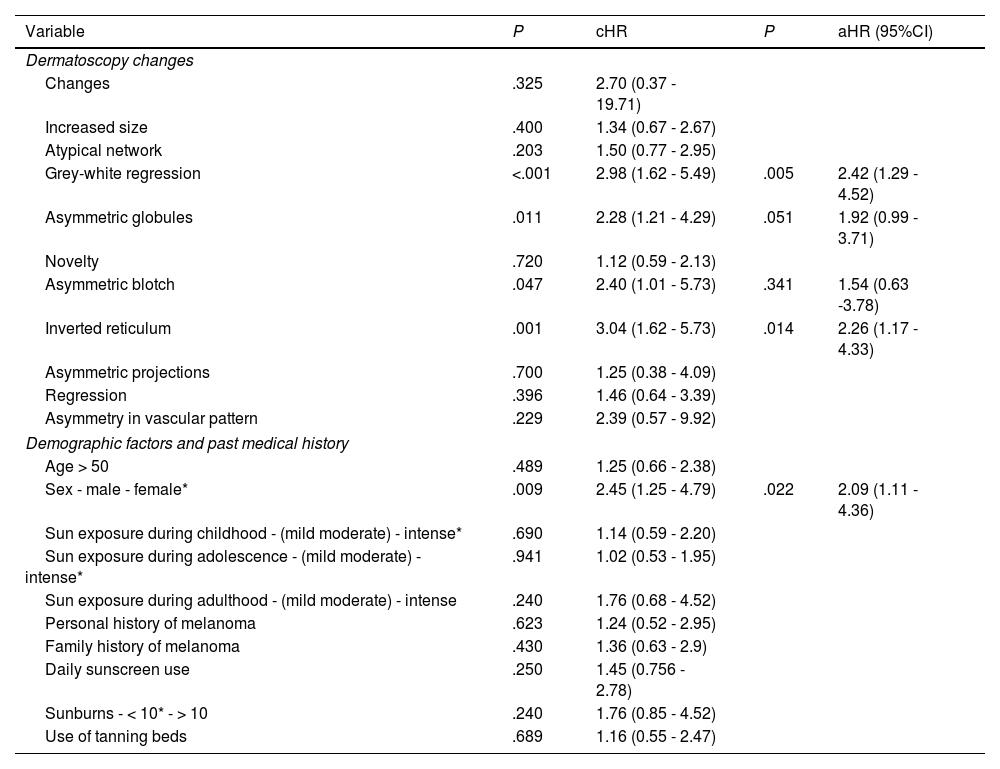
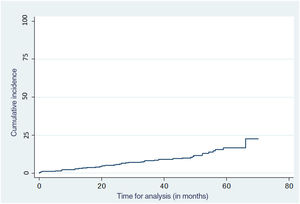
A total of 12.2% of the patients included developed some type of melanoma at the 60-month follow-up (fig. 2). White-gray regression, asymmetric globules, asymmetric eccentric pigmentation, and inverted reticulum were the dermoscopic structures more significantly associated with a shorter time to melanoma presentation (P<.001, P=.011, P=.047, and P<.001, respectively). Among the sociodemographic factors, male sex was a risk factor for the development of melanoma (P=.009) (table 4).
Factors associated with the development of melanoma.
| Variable | P | cHR | P | aHR (95%CI) |
|---|---|---|---|---|
| Dermatoscopy changes | ||||
| Changes | .325 | 2.70 (0.37 - 19.71) | ||
| Increased size | .400 | 1.34 (0.67 - 2.67) | ||
| Atypical network | .203 | 1.50 (0.77 - 2.95) | ||
| Grey-white regression | <.001 | 2.98 (1.62 - 5.49) | .005 | 2.42 (1.29 - 4.52) |
| Asymmetric globules | .011 | 2.28 (1.21 - 4.29) | .051 | 1.92 (0.99 - 3.71) |
| Novelty | .720 | 1.12 (0.59 - 2.13) | ||
| Asymmetric blotch | .047 | 2.40 (1.01 - 5.73) | .341 | 1.54 (0.63 -3.78) |
| Inverted reticulum | .001 | 3.04 (1.62 - 5.73) | .014 | 2.26 (1.17 - 4.33) |
| Asymmetric projections | .700 | 1.25 (0.38 - 4.09) | ||
| Regression | .396 | 1.46 (0.64 - 3.39) | ||
| Asymmetry in vascular pattern | .229 | 2.39 (0.57 - 9.92) | ||
| Demographic factors and past medical history | ||||
| Age > 50 | .489 | 1.25 (0.66 - 2.38) | ||
| Sex - male - female* | .009 | 2.45 (1.25 - 4.79) | .022 | 2.09 (1.11 - 4.36) |
| Sun exposure during childhood - (mild moderate) - intense* | .690 | 1.14 (0.59 - 2.20) | ||
| Sun exposure during adolescence - (mild moderate) - intense* | .941 | 1.02 (0.53 - 1.95) | ||
| Sun exposure during adulthood - (mild moderate) - intense | .240 | 1.76 (0.68 - 4.52) | ||
| Personal history of melanoma | .623 | 1.24 (0.52 - 2.95) | ||
| Family history of melanoma | .430 | 1.36 (0.63 - 2.9) | ||
| Daily sunscreen use | .250 | 1.45 (0.756 - 2.78) | ||
| Sunburns - < 10* - > 10 | .240 | 1.76 (0.85 - 4.52) | ||
| Use of tanning beds | .689 | 1.16 (0.55 - 2.47) | ||
As it has already been reported for several years, one of the most important risk factors for the development of melanoma is te atypical nevus syndrome, because this population has been found to be more prone to develop it due to the large number of dysplastic nevi.12 This entity can be sporadic or hereditary and show an autosomal dominant pattern with variable expressivity and incomplete penetrance. However, to date, no susceptibility gene has ever been identified.13
Atypical nevus syndrome affects men and women alike, and is often diagnosed in young adults <40.3
In this study, the mean age of the patients was higher than that described in other reports from the medical literature available.3,14 Also, the gender ratio was 1:1.
On the contrary, it has been reported that melanoma mainly occurs in older white men,15 This was confirmed in this study that found a significant correlation between progression into melanoma and male gender.
Specific structures have been identified in regard to the diagnosis of melanoma due to its high specificity and OR. These structures include atypical network, asymmetric globules, angulated lines, negative network, eccentric asymmetric pigmentation, pseudopods, whitish-blue veil, white-gray regression, structure-less areas, vascular pattern polymorphism, and shiny white structures.10,16 However, some of these structures can also be present in atypical or dysplastic nevi, mainly the atypical network and the asymmetric globules, and to a lesser extent, the vascular pattern polymorphism and eccentric asymmetric pigmentation.17,18 Our study analyzed 7 of these melanoma-specific tructures, since they were the ones most widely found at the repeated follow-ups of our study population. We found that the predominant structures in these patients both at the beginning and the end of the follow-up were the atypical network and asymmetric globules, followed by white-gray regression, lesion regression, inverted reticulum, eccentric asymmetric pigmentation, asymmetric projections, and vascular pattern polymorphism, respectively.
Our study also analyzed which of these structures were significantly associated with the diagnosis of melanoma (both in situ and invasive). We found that white-gray regression, asymmetric globules, inverted network, and eccentric asymmetric pigmentation mainly appeared in patients who developed this neoplasm at the follow-up. This was a novel contribution to the current medical literature since no additional studies have ever been published to this date in a population of patients like these with findings like the ones we reported.
Today, it is well-known that melanoma is a multifactorial entity due to an interaction between genetic susceptibility and environmental exposure.19 Some of the most significant risk factors triggering melanomas are the personal and family history of melanoma. However, in this study, we did not find any significant associations regarding these variables.
Sun exposure has also been reported as an important environmental factor following its genotoxic effect. Intermittent sun exposure and a past medical history of sunburns, especially during childhood, have been suggested as possible determinants for the development of some types of melanoma.20,21 Conversely, a continuous pattern of sun exposure is more associated with actinic damage and non-melanoma skin cancer.22
It has also been reported that artificial exposure to UV radiation from tanning beds is associated with the development of this type of skin cancer,23 due to the deliberate emission of UVA radiation from these beds that damages DNA, thus triggering immunosuppression.24
Our study examined whether a history of sun exposure and use of tanning beds were additional factors involved in the diagnosis of melanoma in this population. However, no statistically significant relationship was found with either one of them. This may be due to the limited sample size of our study and self-report bias since this information was obtained from personal accounts from each patient.
Regarding the use of sunscreen and its relationship with melanoma, the evidence collected to date remains inconclusive, as most studies have not been able to demonstrate that the use of daily sunscreen reduces the risk of developing melanoma.24 In our study, the use of daily sunscreen was not seen as a protective factor against this type of neoplasm either.
As we saw in our study, 10% of the population developed melanoma by the end of the follow-up (most of them in situ melanomas). This confirms how important and useful digital follow is in these patients and how the impact it has on the early detection of this type of skin cancer.
In a recent study conducted among high-risk patients, 31.2% of melanomas were found with the help of total body mapping.25,26 In our study, a smaller percentage of patients developed melanoma, which could be explained by the size of the sample. Therefore, larger studies are needed to determine the real utility of this tool in the early diagnosis of melanoma.
ConclusionsIn conclusion, as far as we know, this is the first study ever conducted reporting on the main dermatoscopic characteristics of melanocytic lesions in patients with dysplastic nevi associated with disease progression into melanoma. Therefore, should these structures be present, the dermatologist needs to be briefed on these findings so he can eventually consider removing the lesion. Although only 10% of the patients developed melanoma, most were diagnosed at an early stage, which proves the utility of this tool for the follow-up of high-risk patients.
FundingNone whatsoever.
Conflicts of interestNone declared.
We wish to express our gratitude to Dr. Sebastián Bedoya Mejía, MD, and Dr. Diego Fernando Rojas Gualdrón, MD for their support and contribution to this study.