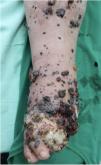
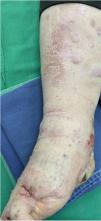
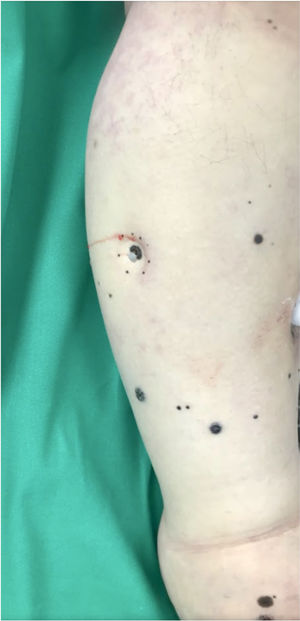
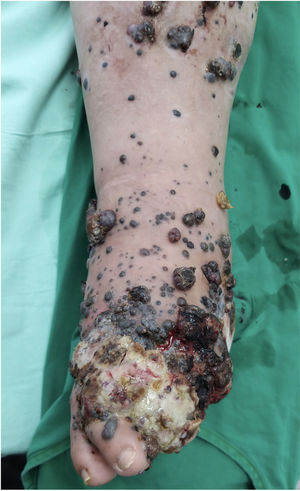
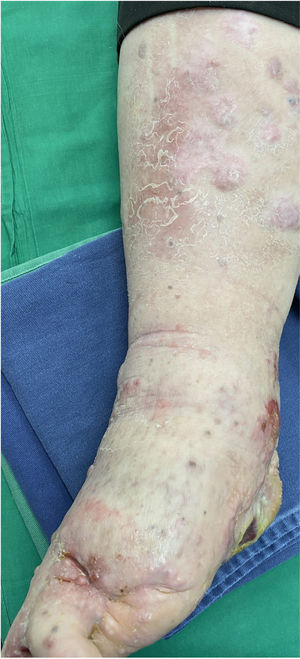
A 62-year-old man was diagnosed with an ulcerated acral lentiginous melanoma (Breslow thickness, 10mm; 6mitoses/mm2, BRAF-positive) on the fifth toe of the left foot in 2016. Treatment included amputation of the fourth and fifth toes of the left foot and left inguinal lymph node dissection, which showed 8 of 10 lymph nodes to be affected. The patient was diagnosed with stage IIID disease (pT4b pN3b M0, according to the 8th edition of the American Joint Committee on Cancer Cancer Staging Manual). He was started on adjuvant interferon therapy and received 20 sessions up to February 2017, when the treatment was suspended due to neutropenia. He experienced locoregional progression in 2018 and was treated with dabrafenib plus trametinib (Fig. 1). The disease, however, progressed rapidly in 2019 (Fig. 2). The tumor committee decided to amputate the lower left limb and start the patient on nivolumab. The patient, however, refused amputation, and it was decided to combine nivolumab with cryosurgery in an attempt to achieve regional control. Clinical response was satisfactory: the in-transit cutaneous metastases disappeared and the patient regained function of his limb after 18 cycles of nivolumab and 26 sessions of cryosurgery (Fig. 3). We report this case because cryosurgery is an affordable adjuvant treatment for locally advanced melanoma that shows very good results in patients who adhere well to treatment.
Cryosurgery has conventionally been used in melanoma as a palliative treatment or to achieve local tumor destruction in patients with associated comorbidities and high surgical risk.1 Temperatures ranging from −4°C to −7°C have been shown to destroy melanoma cells. At the National Cancer Institute in Bogotá, Colombia, we have developed a single-cycle cryotherapy technique applied under local anesthesia with lidocaine and 1% epinephrine in which tumors are frozen for approximately 10minutes using a cryoprobe and then thawed for 20minutes. This cycle is repeated monthly until resolution of lesions. The mechanism of action involves direct cytolysis caused by the formation of intracellular and extracellular crystals and vascular endothelial disruption, leading to thrombosis and ischemia and ultimately coagulation necrosis.1,2 There have also been reports of immune stimulation following cryotherapy marked by increased activation of T helper cells and peripheral cells (in particular CD3+ and CD4+ cells), increased production of cytokines (e.g., gamma interferon, tumor necrosis factor, interleukin [IL] 6, and IL-12 [IL-10 has not been observed]), and generation of autoantibodies against both healthy and tumor tissue.1,3 It is important to mention that the successful outcome observed in the present case is not due to cryosurgery alone but to the synergistic effects of cryosurgery and systemic immunotherapy, as animal studies have shown that anti-cytotoxic T lymphocyte-associated protein 4 blockade combined with cryosurgery increases tumor-specific T responses in models expressing melanoma.4 Similar findings have been observed with anti-CD4 and anti-CD25 monoclonal antibodies.5 It is important to recall that the purpose of cryosurgery is to achieve local disease control; it does not prevent the spread of melanoma through the lymphatic system or bloodstream. In a case series of 20 patients with cutaneous locoregional metastatic melanoma treated with monthly cryosurgery combined with daily application of topical imiquimod 5%, 40% of patients experienced complete resolution of metastatic lesions; 35%, however, did not respond. None of the patients received adjuvant systemic immunotherapy.6
In the present case, we believe that the PD1 inhibitor nivolumab boosted the immune system. By generating an inflammatory response in the tumor area, it will have resulted in a more effective immune response and a more favorable clinical response. At the time of writing, the patient has returned to his normal activities and regained quality of life. We believe that adjuvant cryosurgery should be proposed as an alternative for achieving locoregional control of melanoma in patients being treated with PD1 inhibitors.
FundingThe authors declare that they have not received any funding.
Conflicts of InterestThe authors declare that they have no conflicts of interest.